Abstract
The purpose of this study was to determine whether responders (minimum 4‐mm Hg reduction of systolic blood pressure [BP]) at 24 weeks) to a 52‐week lifestyle intervention had greater changes in metabolic risk factors and health‐related quality of life than nonresponders. Participants (N=126; age, 57.4 [9.1] years) had waist circumference (WC), resting BP, glycated hemoglobin, lipids, and fitness assessed at baseline and at 12, 24, and 52 months. The 36‐item short‐form survey was administered to assess HRQOL. At baseline, responders had higher mental health scores (P=.04) and systolic and diastolic BPs (P<.001) than nonresponders. Across 52 weeks, responders also had greater improvements in diastolic BP (P<.001), WC (P=.01), and maximal oxygen uptake (P=.04) compared with nonresponders. Participants with clinically important changes in systolic BP at 24 weeks had greater metabolic improvements across 52 weeks, compared with those without clinically important systolic BP changes.
Hypertension is one of the leading risk factors for cardiovascular diseases and was the leading risk factor for overall global disease burden in 2010.1, 2 In fact, hypertension is considered the major driver of cardiovascular risk.3 Systolic blood pressure (SBP) specifically is an important variable in cardiovascular risk calculators, including the Framingham4 and Systematic Coronary Risk Evaluation (SCORE)5 models. Importantly, SBP can often be treated, controlled, or prevented to some extent with optimal lifestyle habits.6, 7
Hypertension is often present with other cardiometabolic risk factors, including abdominal obesity, dysglycemia, and dyslipidemia. The metabolic syndrome is a clustering of three or more of these risk factors, which doubles the 5‐year risk of developing cardiovascular diseases and increases the lifetime risk of developing type 2 diabetes five‐fold.8 Management of risk factors for metabolic syndrome prior to the development of overt disease could have a positive impact on public health.
Lifestyle interventions have proved to effectively manage blood pressure (BP)9, 10, 11, 12 and metabolic syndrome risk factors.13 In addition, participation in regular physical activity has been shown to reduce cardiovascular and all‐cause mortality.14 Despite positive results from a number of studies, there is often considerable individual variability, with some participants having great success in improving health status and others showing no change or even decline in overall health during the course of an intervention period. It has been established that patients with greater dysfunction (ie, higher BP) at baseline have better response to treatment than those who are healthier;11, 15 however, other clinical characteristics or health‐related quality of life (HRQOL) may also play a role. Understanding patient characteristics that may increase or decrease the chance of response to an intervention may aid in proper management of risk factors and disease.
The purpose of this exploratory paper was to examine whether responders to a lifestyle intervention (defined as having reduced clinic SBP of at least 4 mm Hg16 after 24 weeks) had greater changes in metabolic risk factors, self‐efficacy, and HRQOL characteristics than nonresponders. It was hypothesized that those with clinically important changes in SBP would have greater improvements in other metabolic and HRQOL variables.
Methods
This paper presents a secondary analysis from our randomized controlled trial17, 18 (ClinicalTrials.gov registration NCT01944124). Community‐dwelling adults (n=149; aged 18–70 years) presenting with at least two metabolic syndrome risk factors according to Third Report of the Adult Treatment Panel National Cholesterol Education Program criteria19 were eligible for participation in this study. Participants with systolic BP >180 mm Hg and/or diastolic BP >110 mm Hg; type 1 diabetes; history of myocardial infarction, angioplasty, coronary artery bypass, or cerebrovascular ischemia/stroke; symptomatic congestive heart failure; atrial flutter; unstable angina; unstable pulmonary disease; use of medications known to affect heart rate; second‐ or third‐degree heart block; history of alcoholism, drug abuse, or other emotional cognitive or psychiatric problems; pacemaker; unstable metabolic disease; or orthopedic or rheumatologic problems that could impair the ability to exercise were excluded. Voluntary informed consent was obtained from each participant. This study was approved by the University of Western Ontario Health Sciences research ethics board.
Full details of the study protocol have been reported elsewhere.17 Following an overnight fast, participants reported to the clinic at baseline and after 12, 24, and 52 weeks. At each visit, seated resting BP was calculated as the average of the last two of three readings measured with an automated cuff (BPTru VSM MedTech Ltd, Coquitlam, British Columbia, Canada) after a minimum 5‐minute rest. BP was measured from the same arm at each visit. Waist circumference was measured and blood drawn and sent to a central laboratory for analysis of glycated hemoglobin (HbA1c) and lipids. The Exercise Self‐Efficacy scale was used to assess confidence in participating in an exercise program.20 HRQOL was assessed with the 36‐item short‐form questionnaire (SF‐36), which evaluates self‐reported health status, function, and well‐being across eight dimensions, with lower scores indicating a higher degree of disability.21 Based on previous findings of change in HRQOL in response to a comprehensive lifestyle modification,22 an a priori decision was made to examine only four of the eight health status domains (physical functioning, general health, vitality, and mental health) in this analysis. Aerobic fitness (maximal oxygen uptake [VO2max]) was assessed and individualized exercise programs were prescribed according to the Step Test and Exercise Prescription (STEP) protocol.23, 24
SBP Response
An a priori SBP response of −4 mm Hg was chosen based on findings from a meta‐analysis.16 SBP response was chosen as the group determinant because of the importance of SBP to global cardiovascular risk status4, 5 and because it is a clinical variable that is easily measured and commonly used in practice.
Participants were randomized (1:1 allocation) to either a mobile Health (mHealth) intervention group or an active control group. Participants in both groups received an exercise prescription tailored to individual fitness levels and goals. All prescriptions aimed to increase activity according to global physical activity guidelines (150 minutes per week of moderate to vigorous intensity physical activity and resistance training a minimum of two times per week).25 In addition to the exercise prescription, the mHealth intervention group also received a technology kit, which included a smartphone (Blackberry Curve 8300 or 8530; Blackberry, Waterloo, Canada) equipped with Healthanywhere health monitoring application (Biosign Technologies Inc, Markham, Ontario, Canada), a Bluetooth‐enabled BP monitor (A & D Medical, UA‐767PBT, San Jose, CA), a glucometer (Lifescan One Touch Ultra2, Milpitas, CA) with Bluetooth adapter (Polymap Wireless, PWR‐08‐03, Tucson, AZ), and a pedometer (Omron, HJ‐150, Kyoto, Japan). Details of the monitoring protocols and database security are reported elsewhere.17
Statistical Analysis
All participants from our randomized controlled trial who completed baseline study visits with complete BP data (ie, three clinic SBP measurements) and at least one other study visit (12‐week, 24‐week, or 52‐week visit) were included in the analyses (n=126). A last‐observation‐carried‐forward design was used to impute missing data; baseline data were only carried forward in 2% of cases where participants had missed the 12‐week visit but completed a subsequent visit (ie, missed week 12 but attended week 24). Approximately 15% of participants had some data carried forward in time. Final sample sizes for each analysis varied slightly and depended on missing values in the outcome measure of interest at baseline (sample sizes ranged between n=123 and n=126).
At baseline, descriptive statistics were computed for demographic and clinical characteristics by responder status. Specifically, means and standard deviations were calculated for continuous variables and frequency counts and percentages were calculated for categoric variables. Baseline physiological variables were compared between responders using independent samples t test and scores for the SF‐36 were compared using the Wilcoxon rank‐sum test, since there was evidence to suggest that variables were not normally distributed. The Pearson chi‐square test was used to compare categoric variables. Mixed design analysis of variance with two between‐subject variables and one repeated (within‐subject) variable was used for the main analysis. Outcomes examined included diastolic BP, waist circumference, HbA1c, low‐density lipoprotein (LDL) cholesterol, VO2max, self‐efficacy, and four domain scores from the SF‐36 (Physical Functioning, General Health, Vitality, and Mental Health). For each model (outcome), the terms SBP response (response), study group (group), time, response × group, response × time, group × time, and response × group × time were included. Because of non‐normality, the square transformation was used for all of the SF‐36 scores. Analyses were performed using R 3.0.1.26 Data are presented as mean (standard deviation) unless otherwise specified. Two‐sided P values <.05 were considered statistically significant.
Results
At 24 weeks, 83 of the 126 study participants (65.9%) were classified as responders (ie, had a decrease in SBP of at least 4 mm Hg from 0 to 24 weeks). The proportion of responders was similar between the mHealth and active control groups (64.2% and 67.8%, respectively). Baseline characteristics of the subset of individuals with sufficient data for this secondary analysis (n=126; age, 57.4 [9.1] years; 74% women) are shown in Table 1. At baseline, systolic and diastolic BP were greater in responders compared with nonresponders (P<.001). In addition, the mental health score from the SF‐36 was higher in responders compared with nonresponders (P=.04). There were no differences in any other outcomes compared at baseline.
Table 1.
Population Characteristics
| Responders | Nonresponders | P Value | |
|---|---|---|---|
| No. (%) | 83 (65.9) | 43 (34.1) | |
| Age, mean (SD), y | 56.8 (8.9) | 56.7 (11.2) | .73 |
| Female sex, No. (%) | 59 (71.1) | 34 (79.1) | .45 |
| Systolic BP, mm Hg | 146.9 (18.8) | 130.4 (13.7) | <.001 |
| Diastolic BP, mm Hg | 88.7 (9.4) | 81.3 (10.0) | <.001 |
| Waist circumference, cm | 104.2 (13.4) | 101.9 (13.1) | .35 |
| LDL, mmol/L | 3.23 (0.81) | 3.10 (0.82) | .40 |
| HbA1c, % | 5.8 (0.7) | 5.7 (0.3) | .08 |
| VO2max, mL/kg/min | 31.2 (6.4) | 30.5 (6.6) | .58 |
| SF‐36 Physical Function | 85 (20.0) | 85 (17.5) | .20 |
| SF‐36 General Health | 77 (25.0) | 72 (22.0) | .46 |
| SF‐36 Vitality | 60 (27.5) | 60 (20.0) | .65 |
| SF‐36 Mental Health | 84 (14.0) | 76 (24.0) | .04 |
Abbreviations: BP, blood pressure; HbA1c, glycated hemoglobin; LDL, low‐density lipoprotein cholesterol; SF‐36, 36‐item short‐form questionnaire; VO2max, maximal oxygen uptake (aerobic fitness).
Metabolic Risk Factors
Across 52 weeks, diastolic BP, waist circumference, HbA1c, and LDL cholesterol decreased and VO2max increased for the entire study population (all P<.001), with no differences in change between study groups (Table 2). Across both study groups, SBP responders had diastolic BP and waist circumference values that decreased and VO2max values that increased to a greater extent across 52 weeks, compared with nonresponders (Figure 1 and Table 2). For HbA1c and LDL cholesterol, profiles over time did not differ between responders and nonresponders. For each metabolic risk factor outcome, relationships between SBP response and metabolic risk factors did not vary by study group (ie, the three‐way interaction of response × study group × time).
Table 2.
Select Main Results From Repeated‐Measures ANOVA Models With Two Between‐Subject Factors
| Outcomes | No. | Time | Responder × Time | Responder × Group × Time | |||
|---|---|---|---|---|---|---|---|
| F Value | P Value | F Value | P Value | F Value | P Value | ||
| DBPrest, mm Hg | 126 | 38.92 | <.001 | 17.58 | <.001 | 0.26 | .86 |
| WC, cm | 126 | 23.66 | <.001 | 3.54 | .01 | 0.78 | .51 |
| HbA1c, % | 126 | 12.46 | <.001 | 0.45 | .72 | 1.21 | .31 |
| LDL, mmol/L | 124 | 10.21 | <.001 | 0.95 | .42 | 0.29 | .84 |
| VO2max | 126 | 19.61 | <.001 | 3.15 | .03 | 0.66 | .58 |
| SE, total score | 125 | 1.26 | .29 | 0.55 | .65 | 0.07 | .98 |
| SF‐36: PFa | 126 | 3.53 | .02 | 1.24 | .30 | 0.76 | .52 |
| SF‐36: GHa | 123 | 4.50 | .004 | 0.60 | .61 | 0.33 | .80 |
| SF‐36: Vitalitya | 125 | 13.35 | <.001 | 0.44 | .73 | 0.30 | .83 |
| SF‐36: MHa | 125 | 5.65 | <.001 | 1.02 | .38 | 1.09 | .35 |
Abbreviations: ANOVA, analysis of variance; DBPrest, resting diastolic blood pressure; GH, General Health; HbA1c, glycated hemoglobin; LDL, low‐density lipoprotein cholesterol; MH, Mental Health; PF, Physical Functioning; SE, Self‐Efficacy; SF‐36, 36‐item short‐form questionnaire; VO2max, maximal oxygen uptake (aerobic fitness); WC, waist circumference. aSquare transformation applied due to non‐normality on original scale.
Figure 1.
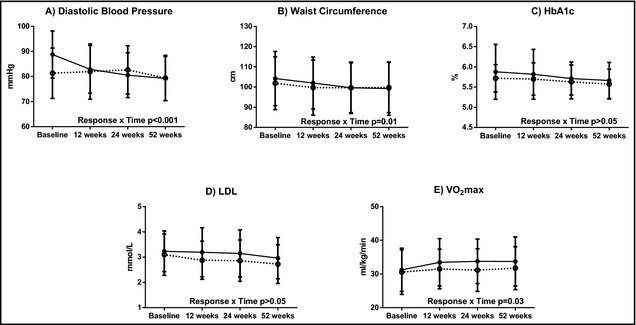
Cardiometabolic risk factors by response over time. Solid circles indicate responders; open circles, nonresponders; HbA1c, glycated hemoglobin; LDL, low‐density lipoprotein cholesterol; VO 2max, maximal oxygen uptake.
HRQOL and Self‐Efficacy Measures
Graphical representations of results are shown in Figure 2 and select numerical results from repeated‐measures analysis of variance models (with two between‐subject factors) are shown in Table 2. Across 52 weeks, physical function (P=.02), general health (P=.004), vitality (P<.001), and mental health (P<.001) scores improved across the entire study population (ie, across SBP response and study groups) (Table 2). Improvement over time was not seen for self‐efficacy (Table 2). For all HRQOL measures, there were no differences in change in profiles over time between responders and nonresponders (Figure 2 and Table 2). Three‐way interactions were nonsignificant for all models. Results for all of the HRQOL measures should be interpreted with caution since even after transformation, there was still a slight violation of normality.
Figure 2.
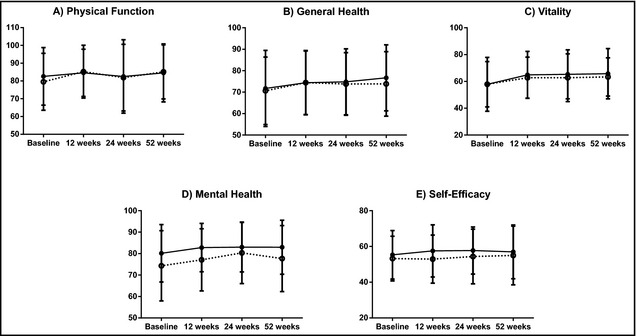
Health‐related quality of life and self‐efficacy by response over time. Solid circles indicate responders; open circles, nonresponders. Response × Time, all P>.05.
Discussion
The main findings of this study are that participants who showed a decrease in SBP of at least 4 mm Hg at 24 weeks also had greater improvements in diastolic BP, waist circumference, and aerobic fitness over 52 weeks compared with those with no clinically significant change in SBP. These findings are somewhat in line with the hypotheses, although changes in HbA1c, LDL cholesterol, and HRQOL were not greater in responders compared with nonresponders as hypothesized. Across the entire study population, all metabolic risk factors and HRQOL measures improved across the 52‐week follow‐up period; self‐efficacy was the only measure examined that did not show improvement over time. There were no differences in improvements in HbA1c, LDL cholesterol, or any of the HRQOL measures between responders and nonresponders. Relationships between SBP response and all outcomes (five metabolic risk factor measures and four HRQOL measures) also did not vary by study group. Overall, this study showed that lifestyle management of hypertension goes beyond BP control. Effects are also seen in other cardiometabolic risk factors, thus having greater impact on global cardiometabolic risk.
As expected, responders had higher systolic and diastolic BP compared with nonresponders at baseline. Meta‐analyses have unequivocally shown that aerobic exercise training improves BP with greater effects in hypertensive compared with prehypertensive and healthy populations.11, 13, 15 Resistance exercise, on the other hand, was shown to affect only diastolic BP.11 Global physical activity recommendations include accumulation 150 minutes per week of moderate to vigorous activity for achieving and maintaining good health, including BP management.25 In this study, the STEP protocol23 was used to prescribe exercise according to best‐practice guidelines. The main focus was to increase aerobic activity to at least 150 minutes of moderate to vigorous intensity aerobic exercise per week, supplemented by full‐body resistance exercise 2 to 4 days per week. Despite the fact that exercise prescription was in line with global recommendations, not all participants realized clinically significant improvements in SBP. A review of interventions utilizing the STEP protocol showed that older adult and metabolic syndrome populations had systolic and diastolic BP reductions of 0% to 7.3% and 0% to 6.5%, respectively, with greater reductions in metabolic syndrome groups compared with healthy populations.24 Thus, baseline differences in systolic and diastolic BP or overall cardiovascular risk may be the fundamental difference between SBP responders and nonresponders.
Responders also had greater decreases in waist circumference and greater increases in estimated aerobic fitness compared with nonresponders. Obesity, especially central obesity, is also a major risk factor for cardiovascular diseases.8 Therefore, weight loss and reduced waist circumference may be important to reduce the overall incidence and burden of cardiovascular diseases. A systematic review concluded that for BP reduction, a weight‐reduction diet and regular exercise were indicated for BP reduction.10 Complex interactions may exist between BP, waist circumference, and aerobic fitness, and improvements in all outcomes may be related to each other and/or an underlying mechanism. It has been reported that increased aerobic fitness alters mortality risk in hypertensive men, such that during an average follow‐up of 7.2 years, increased aerobic fitness decreased mortality risk within every body mass index category, with greatest effects in the obese category.27 Thus, despite the fact that both responders and nonresponders in the present study remained obese on average based on guidelines for waist circumference, the greater increase in aerobic fitness in systolic BP responders may further improve overall risk status. It was recently reported that even “metabolically healthy” obese persons (ie, those who are obese but do not have other metabolic risk factors) have an increased risk of cardiovascular events and all‐cause mortality during a 10‐year follow‐up compared with metabolically healthy normal weight persons.28 Further research is needed to investigate the interactions between aerobic fitness, obesity, BP, and cardiovascular risk.
Similar to findings from another study, HRQOL increased over the course of the exercise intervention.22 Interestingly, while there were no differences in change in HRQOL between responders and nonresponders in this study, the mental health score was higher in responders compared with nonresponders at baseline. A very low SF‐36 mental health score indicates consistent feelings of nervousness and depression, while a very high score indicates that the respondent regularly feels peaceful, calm, and happy.29 The findings of the present study suggest that mental health characteristic may be important for optimal BP response to an exercise intervention. While more research is needed, patients with lower mental health scores may need components aimed to increase mental health added to the intervention to achieve optimal BP response.
Study Limitations
This study was limited to a group of volunteers from rural communities. Therefore, findings may not be generalizable to less motivated populations or to urban populations. In an attempt to increase adherence, exercise prescription was tailored to individual goals rather than assigning a predetermined exercise program. While this may have resulted in some variance in response to exercise, the exercise counsellor ensured that accumulation of a minimum of 150 minutes per week of moderate‐ to vigorous‐intensity physical activity as per global physical activity guidelines25 was included in each prescription. This secondary analysis of our randomized controlled trial is limited in that it was not powered for the present analysis. Therefore, there is a chance that results are caused by type 2 error. All of our findings are intended to be exploratory in nature and suggest themes for future research. Future studies should consider adjustments for other covariates within statistical models.
Conclusions
Participants randomized to either a standard or mHealth‐supported exercise intervention, with clinically important changes in SBP after 24 weeks, had greater improvements in diastolic BP, waist circumference, and aerobic fitness across 52 weeks compared with those without clinically important SBP changes. In addition, SBP responders had higher systolic and diastolic BPs and higher mental health scores at baseline compared with nonresponders. These findings may have implications for intervention design for optimal BP response.
Disclosures
MS received funding from Sykes Assistance Services Corporation as a part of the Mitacs Accelerate Research Internship Program during the final year of the study. No other relationships or activities that could appear to have influenced the submitted work were declared.
Acknowledgments
This study was funded by the Canadian Institute of Health Research Cardiovascular Complications of Diabetes Team grant CCT‐83029, Canadian Diabetes Association, the Heart and Stroke Foundation, and Sykes Assistance Services Corporation.
J Clin Hypertens (Greenwich). 2015;17:375–380. DOI: 10.1111/jch.12531. © 2015 Wiley Periodicals, Inc.
References
- 1. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. JCvD. 2013;1:1–2. [Google Scholar]
- 3. Padwal RS, Hemmelgarn BR, Khan NA, et al. The 2009 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1–blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2009;25:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Agostino RBS, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 5. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 6. Geleijnse JM, Grobbee DE, Kok FJ. Impact of dietary and lifestyle factors on the prevalence of hypertension in western populations. J Hum Hypertens. 2005;19(Suppl 3):S1–S4. [DOI] [PubMed] [Google Scholar]
- 7. Beilin LJ. Lifestyle and hypertension–an overview. Clin Exp Hypertens. 1999;21:749–762. [DOI] [PubMed] [Google Scholar]
- 8. Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 9. Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure‐regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–675. [DOI] [PubMed] [Google Scholar]
- 10. Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–223. [DOI] [PubMed] [Google Scholar]
- 11. Fagard RH, Cornelissen VA. Effect of exercise on blood pressure control in hypertensive patients. Eur J Cardiovasc Prev Rehabil. 2007;14:12–17. [DOI] [PubMed] [Google Scholar]
- 12. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta‐analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. [DOI] [PubMed] [Google Scholar]
- 13. Pattyn N, Cornelissen VA, Eshghi SRT, Vanhees L. The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: a meta‐analysis of controlled trials. Sports Med. 2013;43:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nocon M, Hiemann T, Müller‐Riemenschneider F, et al. Association of physical activity with all‐cause and cardiovascular mortality: a systematic review and meta‐analysis. Eur J Cardiovasc Prev Rehabil. 2008;15:239–246. [DOI] [PubMed] [Google Scholar]
- 15. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelley GA. Effects of aerobic exercise in normotensive adults: a brief meta‐analytic review of controlled clinical trials. South Med J. 1995;88:42–46. [DOI] [PubMed] [Google Scholar]
- 17. Stuckey MI, Shapiro S, Gill DP, Petrella RJ. A lifestyle intervention supported by mobile health technologies to improve the cardiometabolic risk profile of individuals at risk for cardiovascular disease and type 2 diabetes: study rationale and protocol. BMC Public Health. 2013;13:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petrella RJ, Stuckey MI, Shapiro S, Gill DP. Mobile health, exercise and metabolic risk: a randomized controlled trial. BMC Public Health. 2014;14:1082,2458‐14‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 20. Atkin S, Manley J, Petrella RJ. Development and validation of a stage‐matched nutrition lifestyle intervention for primary care physicians. Med Sci Sports Exerc. 2005;37:S368. [Google Scholar]
- 21. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 22. Oh EG, Bang SY, Hyun SS, et al. Effects of a 6‐month lifestyle modification intervention on the cardiometabolic risk factors and health‐related qualities of life in women with metabolic syndrome. Metabolism. 2010;59:1035–1043. [DOI] [PubMed] [Google Scholar]
- 23. Petrella RJ, Koval JJ, Cunningham DA, Paterson DH. A self‐paced step test to predict aerobic fitness in older adults in the primary care clinic. J Am Geriatr Soc. 2001;49:632–638. [DOI] [PubMed] [Google Scholar]
- 24. Stuckey MI, Knight E, Petrella RJ. The step test and exercise prescription tool in primary care: a critical review. Crit Rev Phys Rehabil Med. 2012;24:109–123. [Google Scholar]
- 25. World Health Organization . Global recommendations on physical activity for health. 2010. [PubMed]
- 26. The R foundation for statistical computing [Internet]. www.R-project.org. Accessed November 14, 2014.
- 27. Faselis C, Doumas M, Panagiotakos D, et al. Body mass index, exercise capacity, and mortality risk in male veterans with hypertension. Am J Hypertens. 2012;25:444–450. [DOI] [PubMed] [Google Scholar]
- 28. Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: a systematic review and meta‐analysis. Ann Intern Med. 2013;159:758–769. [DOI] [PubMed] [Google Scholar]
- 29. SF‐36® health survey update [Internet]. http://www.sf-36.org/tools/SF36.shtml. Accessed November 14, 2014.


