Abstract
Beta‐trace protein (BTP) has emerged as a novel biomarker of cardiovascular risk. However, the level of circulating BTP in pregnancy‐induced hypertension (PIH) is still unknown. The aim of this study was to determine the concentration of serum BTP in healthy pregnant women and patients with PIH. No significant difference was found in the serum concentration of BTP in patients with a normal pregnancy. In contrast, serum BTP levels in women with PIH (n=46) were significantly higher than those in women with normal pregnancy (n=57). Receiver operating characteristic analysis revealed that using a serum BTP value of 321.3 ng/mL as a cutoff produced a sensitivity of 91.3% and a specificity of 89.5%. Taken together, these findings suggest that a higher serum BTP concentration in PIH patients compared with those with normal pregnancy and serum BTP might be a novel biomarker in the diagnosis of PIH.
Pregnancy‐induced hypertension (PIH) is one of the most common medical problems encountered during pregnancy. PIH and preeclampsia have been found to be characterized by insulin resistance,1, 2 sympathetic overactivity, and exaggerated systemic inflammation,3, 4 which are all features characteristic of the metabolic syndrome.5 Hypertensive pregnant women are at increased risk for later cardiovascular disease and mortality regardless of their proteinuric status.6, 7 Early diagnosis, close antenatal surveillance, and timely intervention are key to the management of PIH.8 Currently, there are no reliable, economic, and reproducible screening tests available.
Beta‐trace protein (BTP), also known as prostaglandin D2 synthase, is a protein with low molecular mass (23 kDa to 29 kDa) that belongs to the lipocalin protein family.9 BTP has been described as a more sensitive marker than serum creatinine in detecting impaired renal function, with performance comparable to that of cystatin C.10, 11 In addition, BTP has emerged as a novel biomarker of cardiovascular risk. Different studies have explored the role of BTP in hypertension.12, 13 Increase in BTP levels has been proposed to reflect injuries in the renal tubules and arterioles induced by hypertension.12 Moreover, earlier studies have shown that elevated serum BTP levels are associated with the presence of atrial fibrillation in hypertension patients.14 However, the role of BTP in the pathogenesis of PIH is still incompletely understood.
In this study, we tested the hypothesis that there is a relationship between BTP levels and PIH. The purpose of this was to investigate maternal serum BTP levels under physiologic and pathologic conditions in pregnant women. Further, we aimed to assess whether serum BTP levels in pregnancy are associated with development of hypertensive pregnancy.
Ethics Statement
Written informed consent was obtained from all patients, and our study was reviewed and approved by an independent ethical committee of the institution (institutional and regional research ethics committee of QiLu Hospital of Shandong University, China). Laboratory studies and interpretations were performed on coded samples lacking personal and diagnostic identifiers.
Definitions
According to a report of the National High Blood Pressure Education Program Working Group,15 PIH was defined as a systolic BP elevation to ≥140 mm Hg or a diastolic BP (DBP) elevation to ≥90 mm Hg after 20 weeks of gestation with (preeclampsia) or without proteinuria, on two occasions, at least 4 hours apart during pregnancy, with no hypertension before 20 gestational weeks. Normotensive women had normal BP (<140/90 mm Hg) and no proteinuria during pregnancy.
Study Design and Participants
A total of 1206 Asian pregnant women attended the outpatient facilities at QiLu Hospital of Shandong University between April 2013 and October 2014. Among them, 46 pregnant women who developed PIH (PIH group) and 57 normotensive pregnant women (control group) were enrolled in this study. Blood samples were collected during the first (1 to 12 gestational weeks), second (13 to 27 gestational weeks), and third (28 to 40 gestational weeks) trimester with informed consent from each woman. All cases were uncomplicated singleton pregnancies. The PIH and control groups were matched for gestational age and maternal age. Pregnant women who had secondary forms of hypertension, coronary heart disease, kidney disease, or diabetes mellitus were excluded from the study.16 Clinical risk factor data for the 103 pregnant women enrolled in the study were retrospectively obtained and analyzed.
Data Collection
A double‐blind procedure was used for this study. Information on all patients from early pregnancy to delivery, which included information such as patient age, prepregnant state (body mass index [BMI]), history of pregnancy and parity, and medical records regarding blood pressure, weight, and results of biochemical examinations of the blood and urine during the whole period of pregnancy, were extracted from the database.
Venous blood samples were drawn into heparinized (serum) tubes on ice, centrifuged (4°C and 600 × g) for 10 minutes and the samples stored at −80°C until analysis. At baseline, a venous sample was drawn from an antecubital vein after at least 12 hours of fasting for assay of serum high‐sensitivity C‐reactive protein (hs‐CRP), total testosterone, sex hormone–binding globulin (SHBG), and BTP.
Measurement of BTP Levels
The serum BTP concentration was quantitatively determined using a Human Prostaglandin D Synthase, Lipocalin‐type enzyme‐linked immunosorbent assay (ELISA) kit (BioVendor, Candler, NC). Samples were treated in strict compliance with the manufacturer's instructions, and the BTP concentration present in samples was determined directly from a dose‐response curve (based on a four‐parameter algorithm). The measurement of the concentrations in this study was performed in duplicate. The inter‐assay coefficient of variability (CV) was <6% and reliability was confirmed by control samples.
Statistical Analysis
The differences between PIH patients and normal pregnancy controls were examined using a post hoc test followed by Student t test for continuous variables with a normal or nonnormal distribution and the chi‐square test for categorical variables. The nonparametric test was used for ELISA validation. Potential risk factors identified above were used to draw receiver operating characteristic (ROC) curves (MedCalc version 9.2.0.1; Ostend, Belgium), and the specificity, sensitivity, and area under the ROC curve (AUC) were determined. The AUCs were used to estimate model performance. Unless otherwise noted, all statistical analyses and tests were two‐sided and performed at the 5% significance level using the SPSS 13.0 software package (SPSS Inc, Chicago, IL).
Results
Clinical Characteristics of the Study Participants
The clinical characteristics of the study groups are summarized in Table 1 according to pregnancy outcome. There were no significant differences between the groups in maternal age, childbearing history, or ethnicity. Compared with gestational age– and maternal age–matched controls, women in the PIH group had a higher prepregnancy BMI (P<.05), greater incidence of anemia (P<.001), and higher incidence of a family history of PIH (P<.001). In addition, measurements of hs‐CRP, testosterone, and SHBG showed that there were no significant differences at second and/or at third trimester in women with PIH compared with normal controls.
Table 1.
Clinical Characteristics of the Study Participants
| Characteristics | Normal Pregnancy (n=57) | PIH (n=46) | P Value |
|---|---|---|---|
| Maternal average age, y | 27.7±2.8 | 28.4±3.4 | .2 |
| Prepregnancy BMI, kg/m2 | 19.7±1.7 | 24.6±2.5 | .026 |
| Anemia | 3 (5.3) | 17 (37) | <.001 |
| Asian ethnicity | 57 (100.0) | 46 (100.0) | 1 |
| Family history of PIH | 4 (7.0) | 18 (39.1) | <.001 |
| Childbearing history | |||
| History of spontaneous abortion (>3) | 7 (12.3) | 4 (8.7) | .75 |
| Gravidity (>3) | 14 (24.6) | 13 (28.3) | .82 |
| hs‐CRP at second trimester, mg/L | 3.8±1.9 | 4.4±2.8 | .31 |
| hs‐CRP at third trimester, mg/L | 3.6±1.8 | 4.3±2.5 | .26 |
| Testosterone at second trimester, nmol/L | 3.2±1.6 | 2.8±1.4 | .38 |
| Testosterone at third trimester, nmol/L | 2.7±1.3 | 2.5±1.6 | .87 |
| SHBG at second trimester, nmol/L | 397.6±106.2 | 362.4±89.1 | .44 |
| SHBG at third trimester, nmol/L | 448.2±124.7 | 407.5±96.8 | .41 |
Abbreviations: BMI, body mass index; hs‐CRP, high‐sensitivity C‐reactive protein; PIH, pregnancy‐induced hypertension; SHBG, sex hormone–binding globulin. Data are expressed as mean±standard deviation or number (percentage). Student t test (for continuous data) and chi‐square or Fisher exact test (for categorical data) were used to compare PIH patients with normotensive controls.
Clinical Outcome of Normal Pregnancy and PIH
The clinical features of the women with a normal pregnancy and PIH are shown in Table 2. In the PIH group, the highest blood pressure during pregnancy was significantly greater than that in patients with normal pregnancy (P<.05), but the gestational age at birth, mode of delivery, and placental and birth weight were not significantly different from those in patients with normal pregnancy.
Table 2.
The Clinical Outcome of Patients With Normal Pregnancy vs Patients With PIH
| Characteristics | Normal Pregnancy (n=57) | PIH (n=46) | P Value |
|---|---|---|---|
| Systolic BP, mm Hg | 113.7±10.4 | 157.7±11.0 | .01 |
| Diastolic BP, mm Hg | 72.5±8.9 | 104.9±9.2 | .02 |
| Gestational age at birth, d | 274.3±13.8 | 277.9±11.7 | .77 |
| Mode of delivery | |||
| Normal vaginal delivery | 49 (86.0) | 37 (80.4) | |
| Scheduled C‐section | 8 (14.0) | 9 (19.6) | .59 |
| Placental weight, g | 557.0±68.0 | 546.7±80.7 | .31 |
| Newborn sex | |||
| Female | 25 (43.9) | 26 (56.5) | |
| Male | 32 (56.1) | 20 (43.5) | .23 |
| Birth weight, g | 3252.7±415.1 | 3016.0±294.6 | .5 |
Abbreviations: BP, blood pressure; PIH, pregnancy‐induced hypertension. Data are expressed as mean±standard deviation or number (percentage). Student t test (for continuous data) and chi‐square or Fisher exact test (for categorical data) were used to compare results.
Serum BTP Levels at the First, Second, and Third Trimesters
Serum BTP levels in normal pregnancy did not differ significantly between the first, second, and third trimester (247.3±38.6 ng/mL, 252.9±59.4 ng/mL, and 265.8±45.1 ng/mL, respectively). In addition, no significant difference was detected in the concentration of BTP in normal pregnancy based on maternal age and BMI. However, serum BTP levels at the second and third trimesters in the PIH patients were significantly higher than those in normal controls (405.1±79.2 ng/mL vs 252.9±59.4 ng/mL [P<.05]; 442.1±83.4 ng/mL vs 265.8±45.1 ng/mL [P<.05], respectively) (Figure 1), and no significant difference was observed in PIH patients between the second trimester and third trimester. Moreover, comparing PIH patients with women with a normal pregnancy, no significant difference was found in BTP levels at first trimester (268.7±41.2 vs 247.3±38.6).
Figure 1.
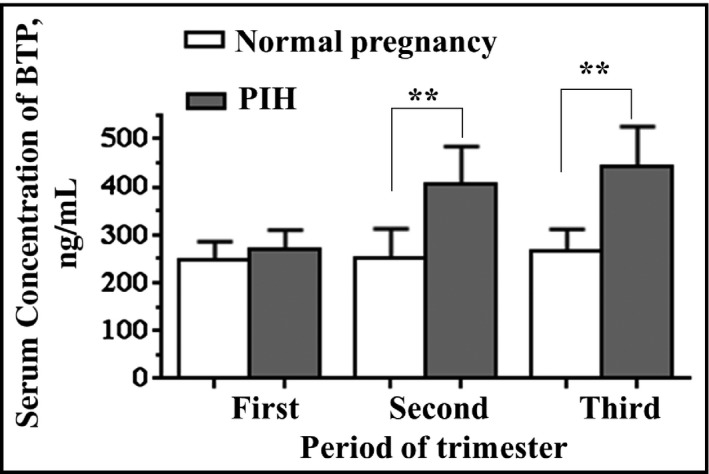
Serum beta‐trace protein (BTP) concentration was measured in serum from women with a normal pregnancy (n=57) and those with pregnancy‐induced hypertension (PIH) (n=46) during the first, second, and third trimester. Data are presented as mean±standard deviation. Significant differences were seen between patients with a normal pregnancy and those with PIH. **P<.01.
ROC Analysis of BTP Values in Normal Pregnancy and PIH
The potential value of BTP in the determination of PIH was analyzed using ROC analysis. The ROC curve of BTP was shown in Figure 2. BTP had an AUC of 0.968 (95% confidence interval [CI], 0.94–0.99) for the prediction of PIH. The cutoff value of BTP to discriminate between normal pregnancy and PIH was 321.3 ng/mL (sensitivity, 91.3% [95% CI, 79.21–97.58]; specificity, 89.47% [95% CI, 78.48–6.04]). Based on these data, BTP appears to be a potential tool for diagnosing PIH.
Figure 2.
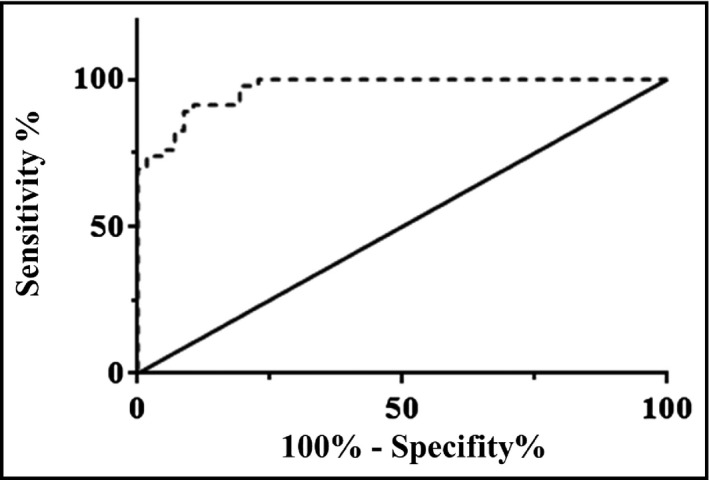
Serum beta‐trace protein (BTP) values in women with a normal pregnancy and those with pregnancy‐induced hypertension (PIH) were used to draw receiver operating characteristic (ROC) curves. Specificity, sensitivity, and area under the ROC curve were determined.
Discussion
PIH is a disease that is unique to pregnancy. Because there is no specific treatment, the predictive method would be of great importance. Therefore, the aim of this study was to assess serum BTP levels in normal pregnancy and PIH patients. To the best of our knowledge, this is the first study demonstrating the relationship between BTP levels and presence of PIH. We found that maternal serum BTP levels in the PIH group were obviously higher than those in the normal group. Findings from our study indicate that higher serum BTP levels are associated with presence of PIH.
It has been confirmed that BTP is linked to several processes, including inflammatory responses, atherogenesis, angina, vasomotor reactivity, and systemic arterial hypertension.17, 18 BTP is strongly accumulated within the fibrous plaque of atherosclerotic stenotic lesions of human coronary arteries, and plasma BTP levels are higher in patients with severe coronary heart disease.19, 20 A multicenter study reported that serum BTP level was elevated in patients with stable coronary heart disease and that the levels increased in association with the number of affected vessels, as well as with age and hypertension.21 BTP has also been found in vascular endothelial cells in the systemic atherosclerotic artery and is related to coronary spasm and atherosclerosis.9, 17 Interestingly, one study has demonstrated that higher BTP levels could predict adverse cardiovascular events, mortality, and major bleeding in patients with atrial fibrillation receiving anticoagulation treatment.22 These observations suggest that BTP may be implicated in the progression of cardiovascular diseases. In agreement with this, a number of studies have reported prognostic value for BTP in coronary heart disease23 and acute heart failure.24 Hirawa and colleagues12 compared BTP levels in normotensive and hypertensive patients and found higher levels of BTP in hypertension patients. In the current study, we found that serum BTP was significantly elevated in the second and third trimesters for women with PIH. In contrast, comparing PIH groups with normal controls, no significant difference was found in BTP levels in the first trimester. Indeed, one recent study demonstrated that BTP levels were elevated in patients with paroxysmal atrial fibrillation when compared with age‐ and sex‐matched control patients.14 The mean values in the present study were similar to those in previous studies. Taking into account the differences in the measurement techniques, this result suggests that BTP may be implicated in the progression of PIH.
Recently, interest has grown in the study of BTP as an alternative to serum creatinine for the evaluation of renal function as well as being a biomarker for cardiovascular diseases. The expression of BTP is not influenced by race, body mass index, or sex of the patient, when standardized to creatinine, β2‐microglobulin, and cystatin C.25, 26 In newborns, BTP is promising as a marker of glomerular filtration rate because its level rises as the gestational period advances.27 These studies indicate that BTP can be considered as a promising biomarker of kidney dysfunction in diabetes and is associated with the presence of the metabolic syndrome and greater cardiovascular risk.28 It has been reported that elevated BTP levels in patients with paroxysmal atrial fibrillation may be explained by the fact that chronic kidney disease is associated with increased atrial fibrillation risk, as shown in a prospective population‐based study.29 Although contrary results have been reported that BTP is a poor marker of glomerular filtration rate during pregnancy,30 one recent study demonstrated an association between presence of atrial fibrillation and serum BTP levels in atrial fibrillation patients.14 In our study, ROC curve analysis showed that serum BTP had a certain capability in predicting PIH. Thus, a high serum BTP at late gestation might be associated with the development of PIH, and such patients should be closely watched.
Study Limitations
Our study has a number of limitations. First, other risk factors for PIH may exist that were not identified in this study. Second, all participants in this study were of 100% Asian ethnicity. Lastly, these findings are marker dependent, not assay dependent, and additional markers are needed to achieve clinical utility. Future studies are necessary and need to include more patients at different stages of disease to evaluate BTP level in response to different treatment and to predict PIH progression.
Conclusions
Our results demonstrate that serum BTP level was elevated in women with PIH compared with women with a normal pregnancy. Our study suggests that serum BTP may be a potential biomarker in Asian patients with PIH.
Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Acknowledgments
None.
J Clin Hypertens (Greenwich). 2016;18:1022–1026. Doi: 10.1111/jan.12801. © 2016 Wiley Periodicals, Inc.
References
- 1. Roberts JM, Bodnar LM, Patrick TE, Powers RW. The role of obesity in preeclampsia. Pregnancy Hypertens. 2011;1:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seely EW, Solomon CG. Insulin resistance and its potential role in pregnancy‐induced hypertension. J Clin Endocrinol Metab. 2003;88:2393–2398. [DOI] [PubMed] [Google Scholar]
- 3. Greenwood JP, Scott EM, Walker JJ, et al. The magnitude of sympathetic hyperactivity in pregnancy‐induced hypertension and preeclampsia. Am J Hypertens. 2003;16:194–199. [DOI] [PubMed] [Google Scholar]
- 4. Wolf M, Kettyle E, Sandler L, et al. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol. 2001;98:757–762. [DOI] [PubMed] [Google Scholar]
- 5. Lambert GW, Straznicky NE, Lambert EA, et al. Sympathetic nervous activation in obesity and the metabolic syndrome–causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126:159–172. [DOI] [PubMed] [Google Scholar]
- 6. Mannisto T, Mendola P, Vaarasmaki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valdiviezo C, Garovic VD, Ouyang P. Preeclampsia and hypertensive disease in pregnancy: their contributions to cardiovascular risk. Clin Cardiol. 2012;35:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yadav K, Aggarwal S, Verma K. Serum βhCG and lipid profile in early second trimester as predictors of pregnancy‐induced hypertension. J Obstet Gynaecol India. 2014;64:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orenes‐Pinero E, Manzano‐Fernandez S, Lopez‐Cuenca A, et al. β‐Trace protein: from GFR marker to cardiovascular risk predictor. Clin J Am Soc Nephrol. 2013;8:873–881. [DOI] [PubMed] [Google Scholar]
- 10. Priem F, Althaus H, Birnbaum M, et al. Beta‐trace protein in serum: a new marker of glomerular filtration rate in the creatinine‐blind range. Clin Chem. 1999;45:567–568. [PubMed] [Google Scholar]
- 11. Priem F, Althaus H, Jung K, Sinha P. Beta‐trace protein is not better than cystatin C as an indicator of reduced glomerular filtration rate. Clin Chem. 2001;47:2181. [PubMed] [Google Scholar]
- 12. Hirawa N, Uehara Y, Yamakado M, et al. Lipocalin‐type prostaglandin d synthase in essential hypertension. Hypertension. 2002;39:449–454. [DOI] [PubMed] [Google Scholar]
- 13. Huang M, Matsushita K, Sang Y, et al. Association of kidney function and albuminuria with prevalent and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2015;65:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yalcin MU, Gurses KM, Kocyigit D, et al. Elevated serum beta‐trace protein levels are associated with the presence of atrial fibrillation in hypertension patients. J Clin Hypertens (Greenwich). 2016;18:439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts JM, Pearson G, Cutler J, Lindheimer M, Pregnancy NWGoRoHD . Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–445. [DOI] [PubMed] [Google Scholar]
- 16. Mahmud A, Jatoi M, Chee YR, Feely J. History of gestational hypertension is associated with the metabolic syndrome and masked hypertension but not arterial stiffness in women with essential hypertension. J Clin Hypertens (Greenwich). 2008;10:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsumoto T, Eguchi Y, Oda H, et al. Lipocalin‐type prostaglandin D synthase is associated with coronary vasospasm and vasomotor reactivity in response to acetylcholine. Circ J. 2011;75:897–904. [DOI] [PubMed] [Google Scholar]
- 18. Taba Y, Sasaguri T, Miyagi M, et al. Fluid shear stress induces lipocalin‐type prostaglandin D(2) synthase expression in vascular endothelial cells. Circ Res. 2000;86:967–973. [DOI] [PubMed] [Google Scholar]
- 19. Eguchi Y, Eguchi N, Oda H, et al. Expression of lipocalin‐type prostaglandin D synthase (beta‐trace) in human heart and its accumulation in the coronary circulation of angina patients. Proc Natl Acad Sci USA. 1997;94:14689–14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inoue T, Eguchi Y, Matsumoto T, et al. Lipocalin‐type prostaglandin D synthase is a powerful biomarker for severity of stable coronary artery disease. Atherosclerosis. 2008;201:385–391. [DOI] [PubMed] [Google Scholar]
- 21. Miwa Y, Oda H, Shiina Y, et al. Association of serum lipocalin‐type prostaglandin D synthase levels with subclinical atherosclerosis in untreated asymptomatic subjects. Hypertens Res. 2008;31:1931–1939. [DOI] [PubMed] [Google Scholar]
- 22. Vilchez JA, Roldan V, Manzano‐Fernandez S, et al. β‐Trace protein and prognosis in patients with atrial fibrillation receiving anticoagulation treatment. Chest. 2013;144:1564–1570. [DOI] [PubMed] [Google Scholar]
- 23. Cipollone F, Fazia M, Iezzi A, et al. Balance between PGD synthase and PGE synthase is a major determinant of atherosclerotic plaque instability in humans. Arterioscler Thromb Vasc Biol. 2004;24:1259–1265. [DOI] [PubMed] [Google Scholar]
- 24. Manzano‐Fernandez S, Januzzi JL Jr, Boronat‐Garcia M, et al. β‐trace protein and cystatin C as predictors of long‐term outcomes in patients with acute heart failure. J Am Coll Cardiol. 2011;57:849–858. [DOI] [PubMed] [Google Scholar]
- 25. Juraschek SP, Coresh J, Inker LA, et al. Comparison of serum concentrations of β‐trace protein, β2‐microglobulin, cystatin C, and creatinine in the US population. Clin J Am Soc Nephrol. 2013;8:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshikawa R, Wada J, Seiki K, et al. Urinary PGDS levels are associated with vascular injury in type 2 diabetes patients. Diabetes Res Clin Pract. 2007;76:358–367. [DOI] [PubMed] [Google Scholar]
- 27. Filler G, Lopes L, Harrold J, Bariciak E. β‐trace protein may be a more suitable marker of neonatal renal function. Clin Nephrol. 2014;81:269–276. [DOI] [PubMed] [Google Scholar]
- 28. Bacci MR, Cavallari MR, de Rozier‐Alves RM, et al. The impact of lipocalin‐type‐prostaglandin‐D‐synthase as a predictor of kidney disease in patients with type 2 diabetes. Drug Des Devel Ther. 2015;9:3179–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sciacqua A, Perticone M, Tripepi G, et al. Renal disease and left atrial remodeling predict atrial fibrillation in patients with cardiovascular risk factors. Int J Cardiol. 2014;175:90–95. [DOI] [PubMed] [Google Scholar]
- 30. Akbari A, Lepage N, Keely E, et al. Cystatin‐C and beta trace protein as markers of renal function in pregnancy. BJOG. 2005;112:575–578. [DOI] [PubMed] [Google Scholar]


