To the Editor:
Atrial fibrillation (AF) and peripheral artery disease (PAD) are prevalent in the aging population and share some common risk factors. The coexistence of both represents a population at very high risk for vascular complications.1 The prevalence of left ventricular systolic dysfunction is significantly greater in patients with PAD compared with the general population and is associated with worse outcome.2 Since this fact was not included in previous outcome studies3, 4, 5, 6 we investigated the prognostic impact of AF on major adverse cardiovascular events (MACEs; composite endpoint of acute myocardial infarction, urgent coronary revascularization, stroke, and death) in a cohort of consecutive mostly hypertensive patients with symptomatic PAD and preserved left ventricular ejection fraction (LVEF >50%).
Between January 2010 and January 2014 we prospectively studied 319 patients (66% men, 87% hypertensive, mean age 70±10 years, ankle brachial index 0.59±0.14) with symptomatic PAD in Rutherford stages 3 (58%), 4 (24%), and 5 (18%). The diagnosis of PAD was established by clinical examination, ankle brachial index measurement, duplex sonography, and/or computed tomography or magnetic resonance angiography and confirmed with peripheral angiography using the criteria of the European Society of Cardiology.7 The diagnosis of hypertension was in accordance with the European Society of Cardiology/European Society of Hypertension 2013 guidelines.8 The diagnosis of AF was based on history and electrocardiographic evidence of arrhythmia. Baseline anemia was defined as hemoglobin level <13 g/dL for men and <12 g/dL for women.9 LVEF was assessed using transthoracic echocardiography (Simpson method). Cardiovascular disease (CVD), in addition to confirmed PAD, was defined as history of angina, myocardial infarction, coronary revascularization, history of stroke, transient ischemic attack, or carotid revascularization.
Differences between the groups were analyzed with t test and Mann‐Whitney test for continuous variables and with chi‐square test for categorical variables. Cox proportional hazards regression analysis was performed to determine the independent predictors of MACE and results were expressed as hazard ratios and 95% confidence intervals (CIs). Covariate selection included known correlates of poor cardiovascular outcome and those that were found to be significant in the univariate analysis, namely age, sex, traditional cardiovascular risk factors, anemia, impaired renal function (estimated glomerular filtration rate [eGFR] <60 mL/min), AF, history of CVD, critical limb ischemia (CLI), and statin treatment. Statistical analysis was performed using MedCalc version 11.3.1.0 (Ostend, Belgium).
The prevalence of AF was 17.9% among PAD patients and was associated with unfavorable outcome (Figure). When compared with patients without AF, these patients were older (76 vs 69 years; P<.001), with higher CHADS2 (congestive heart failure, hypertension, age 75 years, diabetes mellitus, stroke) scores (2.64 vs 1.99; P<.001) and more likely to have CLI (72% vs 35%; P<.001), impaired renal function (61% vs 40%; P=.006), and anemia (35% vs 18%; P=.007). No significant difference between groups was found with regards to antiplatelet therapy or use of statins and angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers. All patients with AF were on warfarin therapy. In logistic regression analysis, age (odds ratio, 1.09; 95% CI, 1.05–1.14; P<.001), and CLI (odds ratio, 3.62; 95% CI, 1.88–6.96; P<.001) were independently associated with AF. During the median follow‐up period of 24 months (interquartile range, 16–34 months), 77 patients (24%) had a MACE, with 20 myocardial infarctions, eight percutaneous coronary interventions/coronary artery bypass graft procedures, 11 strokes, and 38 deaths. In the univariable analysis, AF, age, hypertension, CLI, anemia, history of cardiovascular disease, and decreased eGFR were significantly associated with MACEs. Multivariable Cox regression analysis revealed that only AF, renal impairment, and polyvascular disease independently predicted MACEs (Table). Patients with AF, polyvascular disease, and impaired renal function at baseline were 11.23 times (95% CI, 3.15–40.03) more likely to experience MACEs than patients in sinus rhythm with single vascular disease and preserved renal function.
Figure 1.
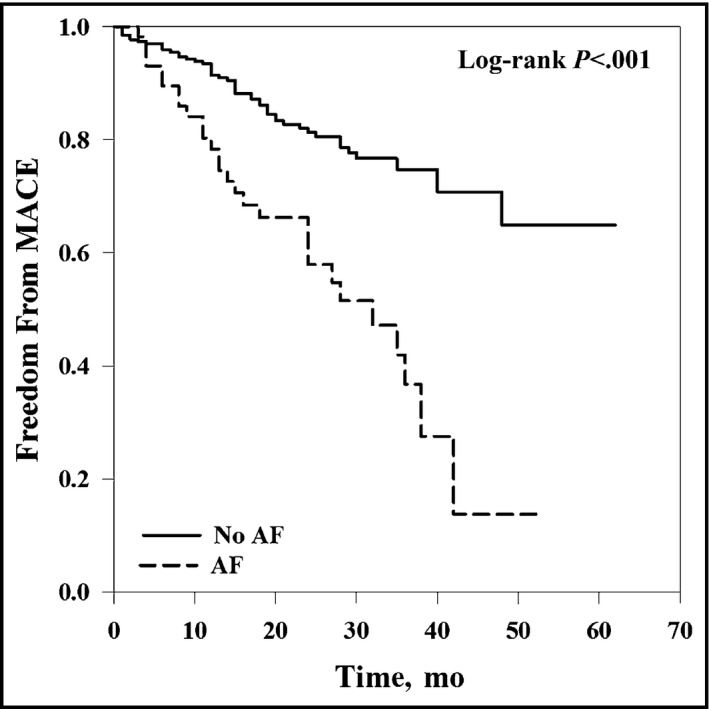
Cumulative major adverse cardiovascular event (MACE)–free survival according to presence/absence of atrial fibrillation (AF) in 319 patients with symptomatic peripheral artery disease.
Table 1.
Univariate and Multivariate Cox Proportional Hazards Regression Analysis for Major Adverse Cardiovascular Events (n=319)
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Age | 1.048 (1.022–1.075) | <.001 | – | |
| Female sex | 1.254 (0.795–1.979) | .333 | – | |
| Hypertension | 2.905 (1.067–7.910) | .038 | – | |
| Diabetes mellitus | 1.084 (0.690–1.703) | .727 | – | |
| Smoking | 1.124 (0.715–1.765) | .615 | – | |
| Dyslipidemia | 1.077 (0.633–1.831) | .786 | – | |
| Critical limb ischemia | 1.798 (1.138–2.840) | .012 | – | |
| Atrial fibrillation | 2.934 (1.847–4.662) | <.001 | 2.938 (1.840–4.692) | <.001 |
| Polyvascular disease | 2.018 (1.289–3.159) | .002 | 1.977 (1.258–3.108) | .003 |
| eGFR (<60 mL/min) | 2.222 (1.408–3.506) | <.001 | 1.944 (1.225–3.085) | .005 |
| Anemia | 1.775 (1.103–2.855) | .018 | – | |
| Statin therapy | 1.001 (0.635–1.578) | .996 | – | |
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Disclosures
The authors declare no conflicts of interest.
References
- 1. Violi F, Lip GY, Basili S. Peripheral artery disease and atrial fibrillation: a potentially dangerous combination. Intern Emerg Med. 2012;7:213–218. [DOI] [PubMed] [Google Scholar]
- 2. Hedberg P, Hammar C, Selmeryd J, et al. Left ventricular systolic dysfunction in outpatients with peripheral atherosclerotic vascular disease: prevalence and association with location of arterial disease. Eur J Heart Fail. 2014;16:625–632. [DOI] [PubMed] [Google Scholar]
- 3. Conway DS, Lip GY. Comparison of outcomes of patients with symptomatic peripheral artery disease with and without atrial fibrillation (the West Birmingham Atrial Fibrillation Project). Am J Cardiol. 2004;93:1422–1425. [DOI] [PubMed] [Google Scholar]
- 4. Winkel TA, Hoeks SE, Schouten O, et al. Prognosis of atrial fibrillation in patients with symptomatic peripheral arterial disease: data from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Eur J Vasc Endovasc Surg. 2010;40:9–16. [DOI] [PubMed] [Google Scholar]
- 5. Goto S, Bhatt DL, Röther J, et al; REACH Registry Investigators. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156:855–863. [DOI] [PubMed] [Google Scholar]
- 6. Wasmer K, Unrath M, Köbe J, et al. Atrial fibrillation is a risk marker for worse in‐hospital and long‐term outcome in patients with peripheral artery disease. Int J Cardiol. 2015;199:223–228. [DOI] [PubMed] [Google Scholar]
- 7. Organization European Stroke, Tendera M, Aboyans V, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2851–2906. [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, Fagard R, Narkiewicz K, et al. Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2014;23:3–16. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization . Nutritional anemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]


