Abstract
In a community survey, 4432 persons aged 15 years and older in two districts in Uganda were studied. Blood pressure was measured and predictors for subtypes of uncontrolled hypertension (HTN) were assessed using bivariate and multivariate logistic regression modeling. Prevalence of uncontrolled HTN was 20.2% and the subgroups of isolated systolic HTN (ISH), isolated diastolic HTN (IDH), and systolic‐diastolic HTN (SDH) were 7.2%, 4.2%, and 8.8%, respectively. No difference was observed between the sexes. For all HTN subtypes, middle (35–49 years) and older age (50+) groups had a higher prevalence compared with younger subjects (15–34 years) (all P<.001). IDH prevalence in older age was not higher compared with younger age (P=.417). After multivariate analysis, middle age predicted all subtypes of HTN and old age predicted ISH and SDH. Alcohol consumption predicted IDH and SDH. Uncontrolled HTN in this population increases in the order IDH, ISH, and SDH, with more than 1 in 5 having uncontrolled HTN.
Uncontrolled hypertension (HTN) is one of the most important preventable causes of cardiovascular diseases.1, 2 Approximately 54% cases of stroke and 47% cases of coronary heart disease worldwide are attributable to HTN.3 Moreover, uncontrolled HTN is associated with increased mortality and morbidity.4, 5 HTN is rapidly becoming a major public health burden in sub‐Saharan Africa.6, 7 Unlike in higher‐income countries, HTN‐related mortality in developing countries is occurring in relatively younger age groups.6, 8, 9, 10
Uncontrolled HTN is defined as systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg.11 Isolated systolic HTN (ISH) is defined as SBP ≥140 mm Hg and DBP <90 mm Hg, isolated diastolic HTN (IDH) is defined as SBP <140 mm Hg and DBP ≥90 mm Hg, and systolic‐diastolic HTN (SDH) is defined as SBP ≥140 mm Hg and DBP ≥90 mm Hg.12
Adequate treatment and control of HTN can save many lives from cardiovascular‐related mortality and morbidity.13, 14 However, pharmaceutical control of HTN on a population scale is not feasible, especially in low‐income settings, where there is already a high burden of communicable diseases.15 Recent studies show that detection rates of HTN are very low. Among patients who have detected HTN, few are taking treatment and, among those on treatment, very few are controlled.9, 16 Against this background, understanding the burden of uncontrolled HTN and its subtypes would provide critical information for public health policy and practice. In this study, we estimate the prevalence of uncontrolled HTN and subtypes and assess the associations between the risk factors and subtypes of HTN.
Methods
Setting and Study Population
This was a community cross‐sectional study that was conducted in the districts of the Mukono and Buikwe in Uganda using the World Health Organization (WHO) STEPS instrument. The detailed methodological and sampling process has been reported elsewhere.9 The study population was composed of persons aged 15 years and older in urban and rural communities of the Buikwe and Mukono districts in Uganda. For this analysis, 4432 surveys with complete datasets were analyzed. The dataset excluded study subjects who had achieved adequate control9 and those with missing data. Study subjects were classified as having uncontrolled HTN if their SBP was ≥140 mm Hg or DBP was ≥90 mm Hg or both.12
Measurements
Data were collected by trained research assistants using a standardized questionnaire at the respondents’ homes. The questionnaire was used to enlist sociodemographic, behavior, anthropometric measurements and blood pressure (BP). BP was measured on a single occasion using an independently validated HONSUN automated digital BP monitor (model LD; HONSUN Group, Shanghai, China)17 with appropriate cuff sizes. Three BP measurements (at least 1 minute apart) were taken after 5 minutes of rest with the participant in a seated position. For each participant, the mean of the 3 values was calculated to estimate their BP. Body weight was measured using calibrated Seca scales (Hamburg, Germany) with the patients lightly clothed, and height was measured using standard height meters. In both cases, participants removed their shoes. Body mass index (BMI) was calculated as weight measured in kilograms (kg) divided by the square of height in meters (m2). Waist circumference was measured around a horizontal plane through the mid‐point between the lower costal margin and the iliac crest waistline while clothed lightly or unclothed.
Study Variables
Outcome Variables
Participants’ BPs were categorized based on the WHO cutoff points for classification of BP.
Normotension: Subjects with SBP <140 mm Hg and DBP <90 mm Hg were classified as normotensive.
ISH: Subjects with SBP ≥140 mm Hg and DBP <90 mm Hg were classified as having ISH.
IDH: Subjects with DBP ≥90 mm Hg and SBP <140 mm Hg were classified as having IDH.
SDH: Subjects with SBP ≥140 mm Hg and DBP ≥90 mm Hg had combined elevated BP and were classified as having SDH.
Covariates/Predictor Variables
Rural/urban residence: People were classified as urban dwellers if they resided in a town with a population of more than 10,000 persons as defined by the Uganda national bureau of statistics. All others who did not meet this criterion were classified as rural dwellers.18
BMI: Using the WHO classification of body weight (underweight, normal weight, overweight, and obese), a cutoff of 25 was utilized to classify participants as overweight/obese if their BMI was ≥25.
Age: Age was classified into 3 groups: young (<35), middle age (35–49), and older (50+) based on the following reasons. Adults older than 35 years have ≥1 vascular risk factor.19 Before reaching 50 years of age, most people with HTN have elevated diastolic pressure.20 After the age of 50 years, systolic pressure rises as diastolic pressure tends to fall.20 Moreover, after age 50 or 60, SBP increases and the risk of cardiovascular events are highly attributed to systolic HTN.21 The WHO guidelines for the prevention of CVDs indicate that people 50 years and older are at highest risk for CVDs and therefore are candidates for intervention with a preventive polypill.22 Because the onset of HTN and CVDs occur at a relatively lower age group in low‐income settings compared with higher‐income countries,21, 23 and the fact that data are scant in this setting, we opted for the above age stratification.
Education status: Level of education was classified into two groups. All those who had attained a level of training at a secondary or higher level were classified as post‐primary, whereas those who did not receive any formal education or attained only some level of education at any primary level were classified as pre‐secondary.
Alcohol consumption: Participants were asked whether they had ever consumed alcoholic beverages and whether they were currently drinking alcohol.
Waist circumference: Participants were classified as having an elevated waist circumference if it was >94 cm for men or >80 cm for women.
Statistical Analysis
Data extraction and computations to generate outcome variables (ISH, IDH, and SDH) was conducted using SPSS version 17 (IBM, Armonk, NY). Using STAT transfer, data were transferred to STATA 10.0 software (StataCorp, College Station, TX) for further analysis. To control for clusters and standard errors, computations for robust standard errors and cluster options in STATA 10 were used. To estimate the overall and type‐specific (ISH, IDH, and SDH), prevalence of uncontrolled HTN proportions were used. To determine whether subtypes of uncontrolled HTN differed with risk factors (sex, age, level of education, residential status, alcohol consumption, and BMI), crude odds ratios (ORs) were performed using binary logistic regression. To compare and identify independent predictors of ISH, IDH, and SDH, adjusted ORs (aOR) with their 95% CIs were computed using multivariate logistic regression analysis. All predictor variables (sex, age, residence status, education, alcohol consumption, and BMI) were entered and controlled for each other in the model. For all tests, a P value of <.05 was taken as statistically significant.
Ethics Statement
This study received approval from the institutional review board of Makerere University School of Public Health and the Uganda National Council of Science and Technology. Written informed consent and ascent were obtained from the adults aged 18 years and older and minors younger than 18 years, respectively. People diagnosed with HTN were referred to health facilities.
Results
Demographic Characteristics
Table 1 shows the basic characteristics of the studied population. The sample was composed of 36.3% (1911) men and 63.7% (2821) women. Most participants were rural residents (66.9% [2963]), and 44.3% (1965) had attained post‐primary education, most of whom were men (P=.0001). No difference was observed across the sexes with regard to residential status (P=.321). The mean age of the studied population was 34.5 years (standard deviation [SD]±15.5). Mean age was higher among men (mean, 36.12; SD±16.6) compared with women (mean, 33.69; SD±14.8). About 1 in 5 was overweight (19.2% [852]). Distribution of study subjects according to the WHO/ISH stages of HTN reveal that 13.6% (601) were in stage 1 and 6.6% (293) were in stage 2 and 3. More men were in the category of stage 1 compared with women (men=15.1 vs women=12.7). Equal proportions were observed for stage 2 and 3 combined across the sexes (men=6.8 vs women=6.5).
Table 1.
Demographic and Basic Descriptive Characteristics of the Studied Variables
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Male | 1611 (36.3) |
| Female | 2821 (63.7) |
| Age, y | |
| Mean (SD) | 34.5 (±15.5) |
| >35 | 2589 (58.4) |
| 35–49 | 1016 (22.9) |
| 50+ | 827 (18.7) |
| Residence status | |
| Rural | 2963 (66.9) |
| Urban | 1469 (33.1) |
| Educational level | |
| Pre‐secondary | 2467 (55.7) |
| Post‐primary | 1965 (44.3) |
| Body mass indexa | |
| <25 | 3574 (80.8) |
| ≥25 | 852 (19.2) |
| Hypertension classification according to WHO's ISH stages (SBP/DBP) – (Total) | |
| Optimal | 1912 (43.1) |
| Normal | 991 (22.4) |
| Hi normal | 635 (14.3) |
| Stage 1 | 601 (13.6) |
| Stage 2 | 189 (4.3) |
| Stage 3 | 104 (2.3) |
Abbreviations: DBP, diastolic blood pressure; ISH, isolated systolic hypertension; SBP, systolic blood pressure; SD, standard deviation; WHO, World Health Organization. aBMI ≥25 classified as overweight/obese.
Prevalence of Uncontrolled HTN and Subtypes of HTN
The prevalence of uncontrolled HTN in our sample was 20.2% (Table 2). The prevalence of ISH, IDH, and SDH was 7.2%, 4.2%, and 8.8%, respectively, suggesting that uncontrolled HTN in this population increases in the order of IDH, ISH, and SDH. The prevalence of ISH (P=.205) and SDH (P=.435) did not differ by sex. Subjects with ISH were significantly older within and across sexes (P<.0001).
Table 2.
Prevalence of Subtypes of Uncontrolled HTN and Age Distribution by Sex and Subtypes of HTN
| Variable | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|
| NT | ISH | IDH | SDH | NT | ISH | IDH | SDH | |
| No. (%) | 2280 (80.8) | 193 (6.8) | 114 (4.1) | 234 (8.3) | 1258 (78.1) | 124 (7.7) | 71 (4.4) | 158 (9.8) |
| Age, mean (SD), y | 30.6 (12.5) | 53 (17.5) | 34.1 (11.4) | 47.3 (14.6) | 33.7 (15.4) | 46.3 (21.5) | 36.6 (12.5) | 46.9 (16.1) |
Abbreviations: HTN, hypertension; IDH, isolated diastolic hypertension; ISH, isolated systolic hypertension; NT, normotension; SD, standard deviation; SDH, systolic‐diastolic hypertension.
Distribution of Studied Characteristics by Subtypes of HTN
The Figure illustrates the distribution of studied characteristics by subtypes of uncontrolled HTN. Urban residents were more predominate in the IDH category and the proportions were higher among women (women 43% vs men 40.8%). In the SDH category, urban men were more prominent compared with urban women (men 46% vs women 34%). Pre‐secondary education was highest in the ISH group and across sexes. Women in the ISH group had the highest proportion (79.3%) of pre‐secondary education. The Figure also shows that a higher proportion of women with pre‐secondary education (72.3%) were in the SDH category compared with their male counterparts (50%). Participants who consumed alcohol were higher in the SDH male category (72.8%). Overweight/obese subjects (BMI ≥25) were significantly higher among women compared with men across all subtypes of HTN. Within the female category, overweight/obese subjects were highest among the SDH (47.4%) followed by IDH (31.6%) categories. In the male category, obese or overweight subjects (BMI ≥25) were very few (<10%). The proportion of study subjects with elevated waist circumference was highest among women in the SDH group (48.3%) and lowest among men in the IDH group (1.4%).
Figure 1.
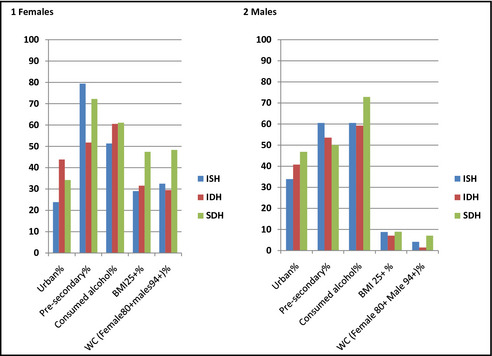
Percentage distribution of characteristics by subtypes of hypertension across sex. BMI indicates body mass index; IDH, isolated diastolic hypertension; ISH, isolated systolic hypertension; SDH, systolic‐diastolic hypertension; WC, waist circumference. .
Predictors of Subtypes of HTN
Table 3 represents crude ORs for the different covariates with respect to the various subtypes of HTN groups. Increasing age was associated with the risk of ISH and SDH. The risk appeared more marked among the ISH subtype. The 3 age groups had different odds of ISH (older vs young: OR, 12.4; 95% CI, 9.4–16.5; middle vs young: OR, 2.10; 95% CI, 1.47–3.01; and older vs middle: OR, 5.33; 95% CI, 4.44–6.64). In the SDH category, the risk was not as explicit (middle vs young: OR, 4.63; 95% CI, 3.48–6.15; older vs young: OR, 10.7; 95% CI, 8.1–14.1). Older age was not associated with the IDH subtype (middle vs young: OR, 2.11; 95% CI, 1.53–2.92; older vs young: OR, 1.32; 95% CI, 0.84–2.10). Persons in urban areas compared with rural areas differed in the IDH and SDH groups but did not differ among the ISH group (IDH: OR, 1.55; 95% CI, 1.15–2.09; SDH: OR, 1.34; 95% CI, 1.08–1.67; and ISH: OR, 0.80; 95% CI, 0.62–1.03). Level of education differed significantly only in the ISH and SDH subtypes (ISH: OR, 2.22; 95% CI, 1.72–2.86; SDH: OR, 1.49; 95% CI, 1.20–1.85). Alcohol consumption was significantly associated with all 3 categories of HTN (ISH: OR, 1.44; 95% CI, 1.14–1.81; IDH: OR, 1.77; 95% CI, 1.31–2.40; and SDH: OR, 2.28; 95% CI, 1.83–2.83). Being overweight or obese was only associated with the SDH subtype of HTN (OR, 2.83; 95% CI, 2.27–3.54).
Table 3.
Crude ORs and 95% CIs of Subtypes of HTN According to Sex, Age, Residential Status, Education, Alcohol, and BMI
| Variable | ISH OR (95% CI) | IDH OR (95% CI) | SDH OR (95% CI) |
|---|---|---|---|
| Sex | |||
| Female | 1 | 1 | 1 |
| Male | 1.16 (0.92–1.47) | 1.12 (0.83–1.53) | 1.24 (0.98–1.15) |
| Age, y | |||
| Young age (<35) | 1 | 1 | 1 |
| Middle age (35–49) | 2.10 (1.47–3.01)a | 2.11 (1.53–2.92)a | 4.63 (3.48–6.15)a |
| Older age (50+) | 12.4 (9.39–16.5)a | 1.32 (0.84–2.10) | 10.7 (8.11–14.1)a |
| Residential status | |||
| Rural | 1 | 1 | 1 |
| Urban | 0.80 (0.62–1.03) | 1.55 (1.15–2.09)b | 1.34 (1.08–1.67)b |
| Education | |||
| Post‐primary | 1 | 1 | 1 |
| Primary and below | 2.22 (1.72–2.86)a | 0.95 (0.71–1.28) | 1.49 (1.20–1.85)a |
| Consumed alcohol | |||
| No | 1 | 1 | 1 |
| Yes | 1.44 (1.14–1.81)c | 1.77 (1.31–2.40)a | 2.28 (1.83–2.83)a |
| BMI | |||
| <25 | 1 | 1 | 1 |
| 25+ | 1.31 (0.98–1.73) | 1.39 (0.97–1.99) | 2.83 (2.27–3.54)a |
Abbreviations: BMI, body mass index; CI, confidence interval; IDH, isolated diastolic hypertension; HTN, hypertension; ISH, isolated systolic hypertension; OR, odds ratio; SDH, systolic‐diastolic hypertension. a P<.001. b P<.05.c P<.005.
In a multivariable logistic regression model, covariates (sex, age, residential status, education status, alcohol consumption, and BMI) were controlled for each other to determine the independent predictors of subtypes of uncontrolled HTN. Table 4 shows that middle age was a predictor for all subtypes (ISH: aOR, 1.95; 95% CI, 1.35–2.82; IDH: aOR, 2.04; 95% CI, 1.45–2.87; and SDH: aOR: 3.97, 95% CI, 2.95–5.34) and old age was a likely predictor for ISH and SDH (ISH: aOR, 11.9; 95% CI, 8.82–16.25; IDH: aOR, 1.32; 95% CI, 0.81–2.14; and SDH: aOR, 10.3; 95% CI, 7.65–13.9). Urban residents had a higher risk of IDH and SDH compared with their rural counterparts (IDH: aOR, 1.59; 95% CI, 1.16–2.19; SDH: OR, 1.66; 95% CI, 1.30–2.13). Low education predicted ISH (aOR: 1.39; 95% CI, 1.04–1.86). Alcohol consumption predicted IDH (aOR: 1.58; 95% CI, 1.16–2.16) and SDH (aOR: 1.48; 95% CI, 1.17–1.88). Overweight/obesity predicted ISH and SDH (aOR: 2.56; 95% CI, 1.97–3.32)
Table 4.
Adjusted ORs and 95% CIs of Different Types of HTN According to Sex, Age, Residential Status, Education, Alcohol Consumption, and BMI
| Variable | ISH OR (95% CI) | IDH OR (95% CI) | SDH OR (95% CI) |
|---|---|---|---|
| Sex | |||
| Female | 1 | 1 | 1 |
| Male | 1.02 (0.78–1.33) | 1.11 (0.81–1.54) | 1.20 (0.94–1.54) |
| Age, y | |||
| <35 | 1 | 1 | 1 |
| 35–49 | 1.95 (1.35–2.82)a | 2.04 (1.45–2.87)a | 3.97 (2.95–5.34)a |
| 50+ | 11.9 (8.82–16.25)a | 1.32 (0.81–2.14) | 10.3 (7.65–13.9)a |
| Residential status | |||
| Rural | 1 | 1 | 1 |
| Urban | 1.28 (0.95–1.71) | 1.59 (1.16–2.19)a | 1.66 (1.30–2.13)a |
| Education | |||
| Post‐primary | 1 | 1 | 1 |
| Primary and below | 1.39 (1.04–1.86)b | 0.92 (0.66–1.26) | 1.12 (0.87–1.43) |
| Consumed alcohol | |||
| No | 1 | 1 | 1 |
| Yes | 0.96 (0.74–1.24) | 1.58 (1.16–2.16)c | 1.48 (1.17–1.88)c |
| BMI | |||
| <25 | 1 | 1 | 1 |
| 25+ | 1.42 (1.03–1.95)b | 1.18 (0.80–1.72) | 2.56 (1.97–3.32)a |
Abbreviations: BMI, body mass index; CI, confidence interval; IDH, isolated diastolic hypertension; HTN, hypertension; ISH, isolated systolic hypertension; OR, odds ratio; SDH, systolic‐diastolic hypertension. a P<.001. b P<.05. c P<.005.
Discussion
Epidemiological studies addressing uncontrolled HTN and its subtypes are scarce in sub‐Saharan Africa and Uganda in particular. In this study, more than 1 in 5 of our study population had uncontrolled HTN. Stage 1 HTN was more common among men compared with women. ISH and SDH were more prevalent compared with IDH. The association between urban residents with ISH was not different from their rural counterparts, but the difference was observed with respect to IDH and SDH. Increasing age was associated with the risk of ISH and SDH, while older age was not significantly associated with the IDH subtype. About 1 in 5 were overweight or obese and were significantly higher among women compared with men across all subtypes of HTN. The proportion of elevated waist circumference was highest among women in the SDH group and lowest among men in the IDH group.
The prevalence of uncontrolled HTN in this population is comparable to other estimates within sub‐Saharan Africa.7, 24 In agreement with studies conducted in high‐ and low‐income countries, age was strongly associated with uncontrolled HTN.4, 7, 25, 26, 27, 28 Middle age was associated with all subtypes of HTN; older age was associated with only ISH and SDH, but the association was stronger in the ISH group, which is considered the main cause of uncontrolled HTN in the older population.29 It was interesting to observe that study subjects of older age had about a 6‐fold risk of ISH compared with those of middle age and more than 12‐fold risk of ISH compared with subjects in the young age group. Physiological descriptions explain this association. Older age is associated with the rise in SBP and the decline in SBP that occurs as a result of arterial stiffening.30 Besides in the older population, middle age was also associated with ISH in this study, although the association was not as strong. This finding appears to suggest that onset of ISH in this population could be occurring at a younger age. Unlike ISH, IDH was only associated with middle age even after controlling for possible confounders. This finding is in agreement with evidence from higher‐income countries.31, 32 Other key predictors were alcohol consumption, which was associated with IDH and SDH, and BMI, which was associated with ISH and SDH after multivariate analysis. BMI is used as a proxy measure for overweight and obesity.33 In addition, BMI and waist circumference are regarded as simple measures of adiposity associated with metabolic abnormalities, especially in the elderly.34 Study subjects with a BMI ≥25 were more likely to have ISH and SDH.
Clinical, Research, and Policy Implications
ISH presents the greatest risk for CVD among the elderly, and control is reported to be difficult in this group.35, 36 Given that lifestyle modifications might be difficult to change in older patients, it is important for the healthcare system to indentify these population at all possible opportunities and initiate them on pharmaceutical control to reduce the risks of CVD morbidity and mortality. ISH in young people has been described as paradoxical with regard to treatment decision‐making.37 O'Rourk and Adji argue that it is not appropriate to apply the same treatment guidelines for ISH across all age groups since the causes of ISH vary among these populations. Whereas in the elderly population ISH is caused by aortic stiffening, ISH in younger populations is caused by higher amplification of the central pressure wave.37
Uncontrolled ISH in the populations suggests that CVD can be prevented by instituting behavior change modification measures to control high BP. Such measures should particularly target the young and middle‐aged populations given that their behaviors are more likely to be influenced compared with the older population. In the event that there is no effort to curb ISH in the face of increasing life expectancy and rapid demographic transition, greater challenges with ISH will be inevitable in the future.38
The clinical significance of IDH is still largely described as unclear due to mixed findings from various population studies.36, 39, 40, 41, 42 Whatever the case, however, the Framingham study found that individuals with IDH had a 23‐fold risk of developing SDH compared with their counterparts with normotension in a follow‐up study of up to 10 years.43 In this low‐income context where the population is largely young, with majority of the adult population in their 40s or younger, the findings reinforce the need for public health intervention to mitigate or postpone progression of IDH into SDH.
Measurement of BMI and waist circumference require anthropometric tools that are inexpensive, effective, and do not require highly skilled personnel. Increased access to these tools, such as weighing scales and height meters, provide opportunities for first‐level screening for HTN.33
Limitations
This was a cross‐sectional study, meaning that cause and effect could not be established. Although 3 BP measurements were taken to estimate the average, all of them were taken on a single occasion, which could result in overestimation of uncontrolled HTN. However, the effect should be minimal for the within‐sample comparison.9, 44 Generalizability of the study findings should also be applied with caution as most men were not found at home during the survey. In spite of these limitations, we had a large sample size, and all BP measurements were conducted at the study subjects’ homes, which is associated with minimal white‐coat HTN effect.
Conclusions
More than 1 in 5 adults in this population have uncontrolled HTN. Uncontrolled HTN in this population increases in the order IDH, ISH, and SDH. There is an urgent need for further research and resources to understand and mitigate the growing burden of uncontrolled HTN in Uganda. Greater partnerships with key players (WHO, Centers for Disease Control and Prevention, the Pan American Health Organization, and the World Hypertension League) should be harnessed to establish essential resources for screening, education, and improved lifestyle as well as understanding other risk factors such as dietary salt intake, which is still understudied in the country.
Disclosure
All authors declare that they have no competing interests.
J Clin Hypertens (Greenwich). 2015;17:63–69. DOI: 10.1111/jch.12371 © 2014 Wiley Periodicals, Inc.
References
- 1. Arima H, Murakami Y, Lam TH, et al. Effects of prehypertension and hypertension subtype on cardiovascular disease in the Asia‐Pacific Region. Hypertension. 2012;59:1118–1123. [DOI] [PubMed] [Google Scholar]
- 2. Plange‐Rhule J, Phillips R, Acheampong JW, et al. Hypertension and renal failure in Kumasi, Ghana. J Hum Hypertens. 1999;13:37–40. [DOI] [PubMed] [Google Scholar]
- 3. Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood‐pressure‐related disease, 2001. Lancet. 2008;371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 4. Wang TJ, Vasan RS. Epidemiology of uncontrolled hypertension in the United States. Circulation. 2005;112:1651–1662. [DOI] [PubMed] [Google Scholar]
- 5. Inoue R, Ohkubo T, Kikuya M, et al. Stroke risk in systolic and combined systolic and diastolic hypertension determined using ambulatory blood pressure. The Ohasama study. Am J Hypertens. 2007;20:1125–1131. [DOI] [PubMed] [Google Scholar]
- 6. Williams EA, Keenan KE, Ansong D, et al. The burden and correlates of hypertension in rural Ghana: a cross‐sectional study. Diabetes Metab Syndr. 2013;7:123–128. [DOI] [PubMed] [Google Scholar]
- 7. Kayima J, Wanyenze RK, Katamba A, et al. Hypertension awareness, treatment and control in Africa: a systematic review. BMC Cardiovasc Disord. 2013;13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walker R, Whiting D, Unwin N, et al. Stroke incidence in rural and urban Tanzania: a prospective, community‐based study. Lancet Neurol. 2010;9:786–792. [DOI] [PubMed] [Google Scholar]
- 9. Musinguzi G, Nuwaha F. Prevalence, awareness and control of hypertension in Uganda. PLoS One. 2013;8:e62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. [DOI] [PubMed] [Google Scholar]
- 11. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2571. [DOI] [PubMed] [Google Scholar]
- 12. Fang XH, Zhang XH, Yang QD, et al. Subtype hypertension and risk of stroke in middle‐aged and older Chinese: a 10‐year follow‐up study. Stroke. 2006;37:38–43. [DOI] [PubMed] [Google Scholar]
- 13. Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med. 2010;38:600–609. [DOI] [PubMed] [Google Scholar]
- 14. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nuwaha F, Musinguzi G. Pre‐hypertension in Uganda: a cross‐sectional study. BMC Cardiovasc Disord. 2013;13:1471–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damasceno A, Azevedo A, Silva‐Matos C, et al. Hypertension prevalence, awareness, treatment, and control in mozambique: urban/rural gap during epidemiological transition. Hypertension. 2009;54:77–83. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y, Wang J, Huang QF, et al. Validation of the HONSUN LD‐578 blood pressure monitor for home blood pressure monitoring according to the European Society of Hypertension International Protocol. Blood Press Monit. 2009;14:128–131. [DOI] [PubMed] [Google Scholar]
- 18. Nuwaha F, Musinguzi G. Pre‐hypertension in Uganda: a cross‐sectional study. BMC Cardiovasc Disord. 2013;13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mulrow C, Kussmaul W. The middle‐aged and older American: wrong prototype for a preventive polypill? Ann Intern Med. 2005;142:467–468. [DOI] [PubMed] [Google Scholar]
- 20. Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. [DOI] [PubMed] [Google Scholar]
- 21. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the american society of hypertension and the international society of hypertension. J Hypertens. 2014;32:3–15. [DOI] [PubMed] [Google Scholar]
- 22. WHO . Prevention of Cardiovascular Disease. Guidelines for Assessment and Management of Cardiovascular Risk. Geneva: World Health Organisation; 2007. [Google Scholar]
- 23. WHO . Global Health Risks: Mortality and Burden of Disease Attributed to Selected Major Risks. Geneva: World Health Organisation; 2009. [Google Scholar]
- 24. Commodore‐Mensah Y, Samuel LJ, Dennison‐Himmelfarb CR, Agyemang C. Hypertension and overweight/obesity in Ghanaians and Nigerians living in West Africa and industrialized countries: a systematic review. J Hypertens. 2014;32:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lloyd‐Jones DM, Evans JC, Larson MG, et al. Differential control of systolic and diastolic blood pressure: factors associated with lack of blood pressure control in the community. Hypertension. 2000;36:594–599. [DOI] [PubMed] [Google Scholar]
- 26. Alexander M, Tekawa I, Hunkeler E, et al. Evaluating hypertension control in a managed care setting. Arch Intern Med. 1999;159:2673–2677. [DOI] [PubMed] [Google Scholar]
- 27. Ornstein SM, Nietert PJ, Dickerson LM. Hypertension management and control in primary care: a study of 20 practices in 14 states. Pharmacotherapy. 2004;24:500–507. [DOI] [PubMed] [Google Scholar]
- 28. Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345:479–486. [DOI] [PubMed] [Google Scholar]
- 29. Franklin SS, Jacobs MJ, Wong ND, et al. Predominance of isolated systolic hypertension among middle‐aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. [DOI] [PubMed] [Google Scholar]
- 30. Safar ME, Levy BI, Struijker‐Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. [DOI] [PubMed] [Google Scholar]
- 31. Niiranen TJ, Rissanen H, Johansson JK, Jula AM. Overall cardiovascular prognosis of isolated systolic hypertension, isolated diastolic hypertension and pulse pressure defined with home measurements: the Finn‐home study. J Hypertens. 2014;32:518–524. [DOI] [PubMed] [Google Scholar]
- 32. Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med. 2007;357:789–796. [DOI] [PubMed] [Google Scholar]
- 33. Saeed AA, Al‐Hamdan NA. Anthropometric risk factors and predictors of hypertension among Saudi adult population – a national survey. J Epidemiol Glob Health. 2013;3:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wannamethee SG, Shaper AG, Morris RW, Whincup PH. Measures of adiposity in the identification of metabolic abnormalities in elderly men. Am J Clin Nutr. 2005;81:1313–1321. [DOI] [PubMed] [Google Scholar]
- 35. Antikainen R, Jousilahti P, Tuomilehto J. Systolic blood pressure, isolated systolic hypertension and risk of coronary heart disease, strokes, cardiovascular disease and all‐cause mortality in the middle‐aged population. J Hypertens. 1998;16:577–583. [DOI] [PubMed] [Google Scholar]
- 36. Hozawa A, Ohkubo T, Nagai K, et al. Prognosis of isolated systolic and isolated diastolic hypertension as assessed by self‐measurement of blood pressure at home: the Ohasama study. Arch Intern Med. 2000;160:3301–3306. [DOI] [PubMed] [Google Scholar]
- 37. O'Rourke MF, Adji A. Guidelines on guidelines: focus on isolated systolic hypertension in youth. J Hypertens. 2013;31:649–654. [DOI] [PubMed] [Google Scholar]
- 38. Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451:716–719. [DOI] [PubMed] [Google Scholar]
- 39. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. [DOI] [PubMed] [Google Scholar]
- 40. Arima H, Anderson C, Omae T, et al. Effects of blood pressure lowering on major vascular events among patients with isolated diastolic hypertension: the perindopril protection against recurrent stroke study (PROGRESS) trial. Stroke. 2011;42:2339–2341. [DOI] [PubMed] [Google Scholar]
- 41. Strandberg TE, Salomaa VV, Vanhanen HT, et al. Isolated diastolic hypertension, pulse pressure, and mean arterial pressure as predictors of mortality during a follow‐up of up to 32 years. J Hypertens. 2002;20:399–404. [DOI] [PubMed] [Google Scholar]
- 42. Broda G. Isolated systolic hypertension is a strong predictor of cardiovascular and all‐cause mortality in the middle‐aged population: warsaw Pol‐MONICA follow up project. J Clin Hypertens (Greenwich). 2000;2:305–311. [PubMed] [Google Scholar]
- 43. Franklin SS, Pio JR, Wong ND, et al. Predictors of new‐onset diastolic and systolic hypertension: the Framingham Heart Study. Circulation. 2005;111:1121–1127. [DOI] [PubMed] [Google Scholar]
- 44. Psaltopoulou T, Orfanos P, Naska A, et al. Prevalence, awareness, treatment and control of hypertension in a general population sample of 26,913 adults in the Greek EPIC study. Int J Epidemiol. 2004;33:1345–1352. [DOI] [PubMed] [Google Scholar]


