Abstract
It has long been thought that there is a close association between hypertension and atrial fibrillation (AF). However, the efficacy of an angiotensin II receptor blocker for the prevention of organ damage in hypertensive individuals with AF is still controversial. The present study was a multicentered, prospective, randomized, open‐label clinical trial investigating the differences in the effect of treatment with telmisartan/amlodipine combination tablets on blood pressure (BP) levels and BP variability between morning and bedtime administration in hypertensive patients with paroxysmal AF, using ambulatory BP monitoring (ABPM) and home BP. With this treatment, the patients' 24‐hour BP, nighttime BP, preawake BP, and morning BP shown by ABPM were significantly reduced, and the antihypertensive effects were similar regardless of the timing of the drug administration. The standard deviation of day‐by‐day home systolic BP and the maximum home systolic BP were also significantly reduced, and these effects were similar regardless of the treatment timing. The N‐terminal pro‐brain natriuretic peptide level was significantly decreased only in the bedtime administration group. A larger study will demonstrate whether the bedtime administration of telmisartan/amlodipine combination tablets maximizes the risk‐lowering effect against AF recurrence in paroxysmal AF hypertensive patients.
A close association between hypertension and atrial fibrillation (AF) has long been suspected. The Framingham Heart Study1 showed that hypertension was an independent risk factor for incident AF in a general population, and uncontrolled elevated blood pressure (BP) was associated with an increased risk of incident AF in patients treated for hypertension.2 Based on overseas clinical trials, the main etiology of AF is hypertension in approximately 60% of AF patients.3 In addition, the Suita Study,4 a cohort study in Japan, showed that hypertension was associated with an increased risk of incident AF.
It is also suspected that hypertension not only facilitates the onset and persistence of AF, but also raises the risk of a thromboembolism. Antihypertensive treatment is therefore considered essential for hypertensive individuals with AF to prevent recurrent AF, cerebral infarction, or heart failure.5 In several countries, angiotensin II receptor blockers (ARBs) have attracted attention as a therapeutic drug for hypertensive individuals with AF.6, 7, 8, 9 However, the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE I)10 comparing the effect of irbesartan on patients with AF (mainly persistent and permanent types) with that of placebo showed no differences between irbesartan and placebo for the recurrence of AF, indicating that ARBs did not suppress the recurrence of AF. Valsartan also did not reduce the recurrent AF rate in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico‐Atrial Fibrillation (GISSI‐AF) trial.11 The J‐RHYTHM II Study12 in Japan also demonstrated that candesartan did not have a significant advantage over amlodipine in reducing recurrent AF.
Patton and colleagues13 reported that N‐terminal pro‐brain natriuretic peptide (NT‐ProBNP) serves as a biomarker for AF recurrence regardless of the patients' race/ethnicity. High BNP and relatively low atrial natriuretic peptide compared with BNP were also demonstrated to be independent biomarkers for recurrent AF in patients with mild congestive heart failure.14
Hypertension is also one of the independent predictors of progression from paroxysmal to persistent AF.15 It would thus be essential to strictly control BP over the entire 24‐hour day in patients with paroxysmal AF. However, few studies of hypertension and coexisting AF using ambulatory BP monitoring (ABPM) have been reported.16, 17 In many patients with AF, it is difficult to obtain accurate BP measurements.18 In the present study, therefore, the BP measurements were performed by ABPM and home BP in hypertensive patients with paroxysmal AF who were in normal sinus rhythm during the run‐in period, and we compared the BP‐lowering effects of a long‐acting ARB/calcium channel blocker (CCB) between the patients who received morning administration of the medication and those who received bedtime administration.
One report indicated that the bedtime administration of an ARB was superior to the morning administration in reducing nighttime BP.19 On the other hand, in the Japan Morning Surge‐Target Organ Protection (J‐TOP) study,20 there was almost no difference in the effect of candesartan on home BP between morning and bedtime administration, and the urinary albumin/creatinine ratio (UACR) was significantly reduced with the bedtime administration. Thus, we designed an ARB and CCB longest combination treatment on ambulatory and home BP in hypertension with atrial fibrillation Multicenter study on time of dosing (ACROBAT) to address the issues. In addition, the potential differences in the changes in biomarkers for AF recurrence between a morning administration and a bedtime administration of an ARB/CCB had not been investigated prior to the present study. Here, we also examined the influence of the timing of drug administration on the levels of biomarkers such as NT‐ProBNP.
Methods
Study Design
The present study was a multicentered, prospective, randomized, open‐label clinical trial investigating the effect of treatment with a telmisartan/amlodipine combination tablet (40 mg/5 mg per tablet) on 24‐hour BP when administered in the morning vs at bedtime in hypertensive patients with paroxysmal AF. This study was conducted in accord with the principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the institutional review boards of the participating study sites. All patients provided written informed consent to participate in the study.
The study design is illustrated in Figure 1. During the run‐in period, all patients underwent 4‐week monotherapy with either 40 mg/d of telmisartan or 5 mg/d of amlodipine. Those who already had more than 4 weeks of telmisartan 40 mg/d or amlodipine 5 mg/d continued the existing treatment.
Figure 1.
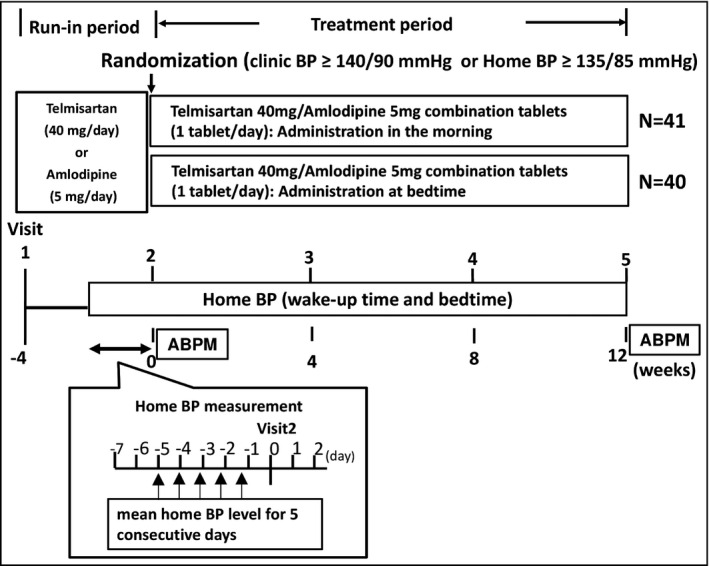
Study design. ABPM indicates ambulatory blood pressure monitoring; BP, blood pressure.
After the run‐in period, eligible patients were enrolled in the study and randomly allocated to either the morning or bedtime administration of telmisartan/amlodipine combination tablets. The allocated patients underwent 12 weeks of this treatment. The investigators instructed the patients regarding whether to take the prescribed telmisartan/amlodipine combination tablet in the morning or at bedtime.
Study Patients
Hypertensive patients with paroxysmal AF were screened, and the participants who met all of the following inclusion criteria were eligible: (1) hypertensive patients with systolic BP (SBP) ≥140 mm Hg (clinic) or 135 mm Hg (home) and/or diastolic BP (DBP) ≥90 mm Hg (clinic) or 85 mm Hg (home), (2) electrocardiography‐confirmed paroxysmal AF within 2 years prior to the date of informed consent and normal sinus rhythm during the run‐in period, and (3) age 20 years and older.
The exclusion criteria included: (1) severe liver or kidney disease, (2) prescription of antihypertensive drugs other than telmisartan or amlodipine during the run‐in period, (3) persistent or permanent AF, (4) secondary AF, (5) average SBP ≥180 mm Hg at a visit during the run‐in period, (6) class III or IV heart failure by the New York Heart Association classification or decreased left ventricular function, (7) history of stroke or myocardial infarction within 6 months prior to consent, and (8) planned pulmonary vein ablation or surgery including percutaneous coronary intervention.
BP Measurements
Noninvasive ABPM was performed with a well‐validated automatic ABPM device (TM‐2431; A&D Company, Limited, Tokyo, Japan), and the BP of the device's wearer was automatically measured every 30 minutes. The device was attached to the patients at the clinic during the treatment period (weeks 0 and 12) to measure BP continuously, starting between 8 am and 10 am for at least 25 hours. Nighttime BP was defined as the average BP from when the patient went to bed until he or she got out of bed in the morning. Daytime BP was defined as the average BP for the rest of the day. Morning BP was defined as the average BP during the first 2 hours after the patient woke up. Evening BP was defined as the average BP during the 2 hours before the patient went to bed. Preawake BP was defined as the average BP during the 2 hours before the patient woke up. Lowest BP was defined as the average BP of the three readings around the lowest nighttime reading. The sleep‐trough morning surge was defined as the morning BP minus the lowest BP. The prewaking morning surge was defined as the morning BP minus the preawake BP.21
Home BP was measured using HEM‐7251G (Omron Healthcare, Kyoto, Japan) consecutively for 5 days during the last week of the run‐in period prior to the study allocation, and weekly during the treatment period, four times daily (twice each at wake‐up time and bedtime). Home BP was calculated as the average of morning or evening BP values for five consecutive days.22 The home BP variability included the standard deviation (SD), the coefficient variation (CV), the maximum BP, and the morning minus evening BP. The SD and CV values were calculated based on the average daily BP (four readings daily) of five consecutive days,22 the maximum BP defined as the highest average among the average duplicate morning BP and average duplicate evening BP values,23 and the morning minus evening BP as the difference between the daily morning BP and the evening BP. Clinic BP was determined at the participating study sites to measure BP three times at each visit.
Primary and Secondary Endpoints
The primary endpoint was to investigate the efficacy of the treatment with telmisartan/amlodipine combination tablets by evaluating the changes in average 24‐hour BP from baseline to 12 weeks of the study drug administration.
The secondary endpoints were as follows: changes in nighttime BP, preawake BP, morning BP, daytime BP, home BP, clinic BP, ambulatory BP variability, home BP variability, the levels of high‐sensitivity troponin T (hsTnT), plasminogen activator inhibitor‐1 (PAI‐1), and NT‐ProBNP, and the urinary albumin/creatinine ratio (UACR) from baseline to 12 weeks of treatment.
Statistical Analysis
Assuming a change in SBP in the morning administration and bedtime administration groups of 8.2 mm Hg and the SD of 12.0 mm Hg, we calculated that the number of patients required to detect a difference with a statistical power of 80% and a two‐sided significance level of 0.05 was 35 patients, and the target number of patients was set at 40 patients per group in consideration of dropouts.
Fisher exact test was performed on all allocated patients to determine background differences, and the two‐sided significance level was 0.15. Student t test was performed on the primary and secondary endpoints including the average BP, average BP variability index, and changes in laboratory values from baseline to 12 weeks of treatment to determine any difference between the morning and bedtime administration groups, and the paired t test was used to determine intragroup differences. The two‐sided significance level was 5% for both tests.
The study was registered with ClinicalTrials.gov number NCT01748253.
Results
Patient Disposition and Baseline Characteristics
A total of 81 patients were randomly allocated to the morning (41 patients) or bedtime (40 patients) administration groups. In each group, 37 patients completed the treatment. The reasons for withdrawal during the treatment period included adverse events (AEs), voluntary withdrawal, and lack of drug efficacy only in the morning administration group (Figure 2). There were no significant differences in baseline characteristics between the morning and bedtime groups (Table S1). However, a mildly increased NT‐ProBNP level at baseline was observed in both groups (approximately 190 pg/mL [normal: ≤125 pg/mL]), which might have indicated a slightly high cardiac load as a result of paroxysmal AF.
Figure 2.
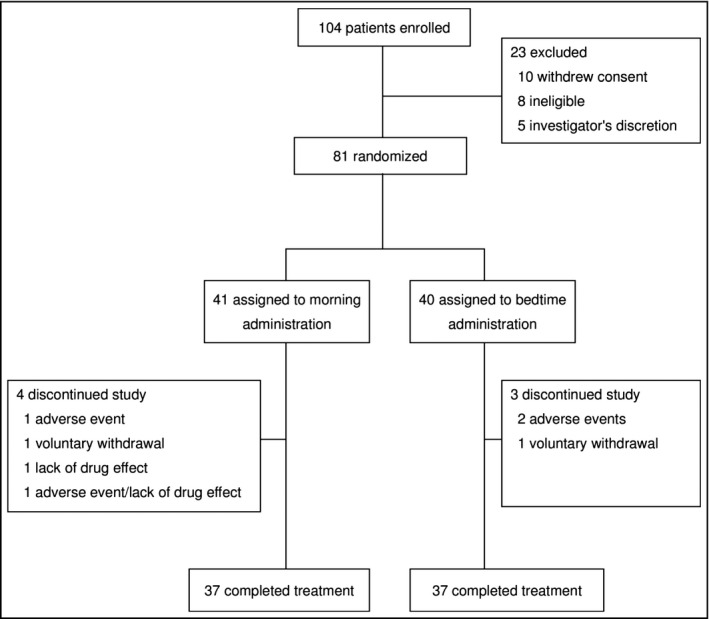
Patient disposition. One patient randomized to the morning group actually took the tablet at bedtime; two patients randomized to the bedtime group took the tablet in the morning. Therefore, the full analysis set consisted of 42 patients in the morning group and 39 patients in the bedtime group.
Ambulatory BP Monitoring
Concerning the primary endpoint, in the morning group, the changes in 24‐hour SBP and DBP from baseline to the end of the 12‐week treatment were −11.6 mm Hg and −5.0 mm Hg, respectively (both P<.001). In the bedtime group, the corresponding changes in 24‐hour SBP and DBP were −12.8 mm Hg and −6.4 mm Hg, respectively (both P<.001). There were no significant differences in 24‐hour SBP or DBP between the two groups (Table 1).
Table 1.
ABPM Parameters
| ABPM | Overall (N=68) | Morning Administration (n=32) | Bedtime Administration (n=36) | Comparison Between Groups | |||
|---|---|---|---|---|---|---|---|
| Change, mm Hg, Mean (SD) | P Value | Change, mm Hg, Mean (SD) | P Value | Change, mm Hg, Mean (SD) | P Value | P Value | |
| 24‐hour | |||||||
| Mean SBP | −12.3 (13.7) | <.001 | −11.6 (14.6) | <.001 | −12.8 (13.1) | <.001 | .725 |
| SD of SBP | −1.1 (5.2) | .075 | −1.5 (5.5) | .126 | −0.8 (4.9) | .343 | .560 |
| Mean DBP | −5.7 (6.7) | <.001 | −5.0 (7.2) | <.001 | −6.4 (6.2) | <.001 | .420 |
| SD of DBP | −0.7 (3.1) | .066 | −1.1 (2.6) | .028 | −0.4 (3.5) | .508 | .378 |
| Nighttimea | |||||||
| Mean SBP | −12.8 (17.4) | <.001 | −12.4 (20.6) | .002 | −13.1 (14.3) | <.001 | .867 |
| SD of SBP | −1.5 (5.0) | .014 | −1.6 (5.5) | .115 | −1.5 (4.6) | .060 | .948 |
| Mean DBP | −5.7 (8.8) | <.001 | −5.5 (10.1) | .004 | −5.9 (7.5) | <.001 | .842 |
| SD of DBP | −1.3 (3.4) | .003 | −1.3 (3.3) | .031 | −1.3 (3.6) | .038 | .972 |
| Daytimeb | |||||||
| Mean SBP | −12.0 (13.5) | <.001 | −11.5 (12.8) | <.001 | −12.5 (14.2) | <.001 | .765 |
| SD of SBP | −1.0 (6.4) | .218 | −1.5 (6.7) | .222 | −0.5 (6.2) | .619 | .545 |
| Mean DBP | −5.8 (7.3) | <.001 | −4.8 (7.2) | .001 | −6.6 (7.4) | <.001 | .328 |
| SD of DBP | −0.3 (3.8) | .501 | −0.9 (3.2) | .133 | 0.2 (4.1) | .771 | .237 |
| Eveningc | |||||||
| Mean SBP | −13.5 (24.1) | <.001 | −13.0 (24.9) | .006 | −13.9 (23.8) | .001 | .886 |
| Mean DBP | −5.9 (13.2) | .001 | −5.3 (14.6) | .048 | −6.4 (12.1) | .003 | .747 |
| Preawaked | |||||||
| Mean SBP | −12.5 (19.2) | <.001 | −11.4 (21.1) | .004 | −13.4 (17.7) | <.001 | .683 |
| Mean DBP | −6.3 (11.1) | <.001 | −5.3 (11.6) | .015 | −7.2 (10.7) | <.001 | .485 |
| Morninge | |||||||
| Mean SBP | −13.9 (19.1) | <.001 | −12.3 (21.1) | .002 | −15.4 (17.3) | <.001 | .514 |
| Mean DBP | −6.6 (11.4) | <.001 | −4.9 (11.9) | .027 | −8.1 (10.9) | <.001 | .253 |
| Sleep‐trough morning surgef | |||||||
| Mean SBP | −4.9 (20.5) | .054 | −3.7 (20.1) | .303 | −6.0 (21.1) | .102 | .653 |
| Mean DBP | −1.8 (16.3) | .361 | −0.8 (15.6) | .788 | −2.8 (17.0) | .334 | .607 |
| Prewaking morning surgeg | |||||||
| Mean SBP | −1.5 (10.4) | 0.431 | −0.9 (17.0) | .768 | −2.1 (14.8) | .405 | .755 |
| Mean DBP | −0.07 (15.5) | 0.971 | 0.4 (17.2) | .888 | −0.5 (14.1) | .827 | .804 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation. aAverage blood pressure (BP) from when the patient went to bed until he or she got out of bed in the morning. bAverage BP for the rest of the day. cAverage BP during the 2 hours before the patient went to bed. dAverage BP during the 2 hours before the patient woke up. eBP during the first 2 hours after the patient woke up. fThe morning BP minus the lowest BP (average BP of three readings around the lowest nighttime reading). gThe morning BP minus the preawake BP.
In the morning group, the changes in nighttime SBP and DBP from baseline to the end of the 12‐week treatment were −12.4 mm Hg (P=.002) and −5.5 mm Hg (P=.004), respectively, and in the bedtime group, the corresponding changes were −13.1 mm Hg and −5.9 mm Hg, respectively (both P<.001). There were no significant differences in nighttime SBP or DBP values between the two groups (Table 1).
In the morning group, the changes in morning SBP and DBP from baseline to the end of the 12‐week treatment were −12.3 mm Hg (P=.002) and −4.9 mm Hg, respectively (P=.027). In the bedtime group, the corresponding changes were −15.4 mm Hg and −8.1 mm Hg, respectively (both P<.001). There were no significant differences in the morning SBP or DBP between the two groups. The preawake SBP and DBP showed results similar to those of morning SBP and DBP.
The changes in the prewaking morning surge from baseline to the end of the 12‐week treatment were −0.9 mm Hg (SBP) and +0.4 mm Hg (DBP) in the morning group, which were not significant reductions. In the bedtime group, the changes in the prewaking morning surge were −2.1 mm Hg (SBP) and −0.5 mm Hg (DBP), which were also not significant reductions. In addition, the sleep‐trough morning surge did not show a significant reduction in SBP or DBP. The changes in the prewaking morning surge and sleep‐trough morning surge SBP/DBP were not significantly different between the two groups (Table 1).
In the overall group administered the telmisartan/amlodipine combination tablets, the change in nighttime SBP‐SD from baseline to the end of the 12‐week treatment was −1.5 mm Hg (P=.014), and that in the nighttime DBP‐SD was −1.3 mm Hg (P=.003), showing a significant reduction. As for the change in nighttime SBP‐SD after the 12‐week treatment, the reduction was not significantly different in the morning group or bedtime group. On the other hand, the nighttime DBP‐SD after the 12‐week treatment showed a significant reduction in both the morning (P=.031) and bedtime (P=.038) groups. There were no significant differences in the change in nighttime SBP‐SD and DBP‐SD between the two groups (Table 1).
Home BP
Regarding the change in morning home SBP/DBP after the 12‐week treatment, a significant reduction was observed in both the morning and bedtime groups (each P<.001). There was no significant difference between the groups in this change (Table 2). The evening home SBP/DBP showed results similar to those of morning SBP/DBP (Table II).
Table 2.
Home and Clinic BP
| Home BP | Overall (N=67) | Morning Administration (n=32) | Bedtime Administration (n=35) | Comparison Between Groups | |||
|---|---|---|---|---|---|---|---|
| Change, mm Hg, Mean (SD) | P Value | Change, mm Hg, Mean (SD) | P Value | Change, mm Hg, Mean (SD) | P Value | P Value | |
| Morning time | |||||||
| SBP | −16.7 (18.5) | <.001 | −17.9 (20.4) | <.001 | −15.6 (16.7) | <.001 | .844 |
| DBP | −9.2 (10.8) | <.001 | −10.2 (12.7) | <.001 | −8.3 (8.9) | <.001 | .470 |
| Evening time | |||||||
| SBP | −14.4 (26.5) | <.001 | −18.1 (28.4) | <.001 | −10.8 (24.2) | <.001 | .249 |
| DBP | −8.1 (14.8) | <.001 | −10.5 (17.4) | <.001 | −5.8 (11.5) | <.001 | .181 |
| SD of day‐by‐day BPa | |||||||
| SBP | −2.2 (3.3) | <.001 | −2.2 (2.5) | <.001 | −2.2 (4.0) | .003 | .911 |
| DBP | −1.3 (2.4) | <.001 | −1.7 (2.5) | <.001 | −0.9 (2.3) | .024 | .191 |
| CV of day‐by‐day BPa | |||||||
| SBP | −0.9 (2.2) | <.001 | −0.8 (2.0) | .023 | −0.9 (3.1) | .049 | .862 |
| DBP | −0.9 (2.9) | .018 | −1.2 (2.5) | .031 | −0.5 (2.7) | .269 | .342 |
| Maximumb | |||||||
| SBP | −22.2 (32.7) | <.001 | −26.3 (29.0) | <.001 | −20.7 (33.6) | <.001 | .490 |
| DBP | −13.1 (19.4) | <.001 | −16.6 (22.6) | <.001 | −9.9 (15.6) | <.001 | .160 |
| Morning‐eveningc | |||||||
| SBP | 1.0 (20.7) | .890 | 1.0 (19.2) | .980 | 1.1 (22.8) | .630 | .994 |
| DBP | −3.2 (17.1) | <.001 | −1.0 (20.0) | .356 | −5.6 (13.3) | .140 | .395 |
| Clinic BP | |||||||
| SBP | −21.7 (15.7) | <.001 | −19.6 (15.9) | <.001 | −23.7 (15.4) | <.001 | .269 |
| DBP | −10.4 (9.7) | <.001 | −10.1 (8.1) | <.001 | −10.7 (11.0) | <.001 | .790 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure. aStandard deviation (SD) and coefficient variation (CV) were calculated based on the average daily blood pressure (BP) of five consecutive days. bThe highest BP among morning BP and evening BP. cDifference between the morning BP and evening BP.
The changes in the SD of day‐by‐day home SBP and the CV of day‐by‐day home SBP after 12 weeks were −2.2 mm Hg (P<.001) and −0.8 mm Hg (P=.023) in the morning group and −2.2 mm Hg (P=.003) and −0.9 mm Hg (P=.049) in the bedtime group, respectively, with a significant reduction in both groups. The change in the maximum home SBP after 12 weeks showed a significant reduction in both groups, ie, −26.3 mm Hg in the morning group and −20.7 mm Hg in the bedtime group (both P<.001). Neither group showed a significant decrease in morning − evening home BP after the 12‐week treatment. No tested parameters showed a significant difference between the two groups (Table 2).
Biomarkers
The biomarker data are provided in Tables 3 and S2. The morning group did not show a significant decrease in hsTnT, NT‐ProBNP, or UACR levels after the 12‐week treatment, but there were significant decreases in these biomarkers in the bedtime group (hsTnT: P=.010, NT‐ProBNP: P=.033, UACR: P=.001). There were no significant differences in any biomarkers between the morning and bedtime groups (Table 3).
Table 3.
Investigation of Biomarkers
| Biomarker | Overall (N=72) | Morning Administration (n=36) | Bedtime Administration (n=36) | Comparison Between Groups | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | P Value | Mean (SD) | P Value | Mean (SD) | P Value | P Value | |
| hsTnT | |||||||
| Change, ng/mL | −0.04 (0.14) | .017 | −0.01 (0.11) | .479 | −0.07 (0.16) | .010 | .067 |
| PAI‐1 | |||||||
| Change, ng/mL | −0.01 (0.24) | .728 | −0.02 (0.24) | .712 | 0.00 (0.23) | .914 | .846 |
| NT‐ProBNP | |||||||
| Change, pg/mL | −0.07 (0.36) | .127 | 0.00 (0.35) | .985 | −0.13 (0.36) | .033 | .116 |
| UACR | |||||||
| Change, mg/g∙Cr | −0.16 (0.36) | .001 | −0.13 (0.41) | .069 | −0.19 (0.31) | .001 | .446 |
Abbreviations: Cr, creatinine; hsTnT, high‐sensitivity troponin T; NT‐ProBNP, N‐terminal pro‐brain natriuretic peptide; PAI‐1, plasminogen activator inhibitor‐1; SD, standard deviation; UACR, urinary albumin/creatinine ratio. Data are shown as logarithmically transformed values.
Adverse Events
Drug‐related AEs were observed in four patients. Persistent AF occurred in one patient, but it was not serious. Although AF also occurred in one other patient, a causal relationship was ruled out (data not shown).
Discussion
When telmisartan/amlodipine combination tablets were administered to hypertensive patients with paroxysmal AF in the morning or at bedtime, the patients' 24‐hour BP, nighttime BP, preawake BP, and morning BP were significantly reduced, and the antihypertensive effect was similar regardless of the timing of drug administration. The SD of nighttime BP by ABPM was also significantly reduced. The maximum home SBP and the SD of day‐by‐day home SBP were also significantly reduced regardless of the timing of the tablets' administration. Among the biomarkers, the NT‐ProBNP level was significantly decreased in the bedtime administration group, but not in the morning administration group. However, the difference between the two groups was not significant.
These findings indicate that treatment with telmisartan/amlodipine combination tablets could control morning BP and nighttime BP regardless of the timing of the tablets' administration. Several reports indicated that morning BP surge and morning hypertension increase the risk of organ damage and other conditions such as cardiovascular events, left ventricular hypertrophy, carotid intima‐media thickness, and asymptomatic cerebral infarction, independent of 24‐hour BP.21, 24, 25 Increased nighttime BP is also associated with a higher cerebrovascular and cardiovascular event risk26, 27, 28 and decreased cognitive and physical functions,29, 30 and vascular tissue damage has been shown to progress even if only nighttime BP is high, increasing the risk of cardiovascular events.31 The importance of managing morning BP and nighttime BP is therefore well recognized.
In the present study, the telmisartan/amlodipine combination tablets reduced the SD of nighttime BP. Parati and colleagues32 reported that the telmisartan/amlodipine combination provided a better reduction of the SD of nighttime BP compared with monotherapy (ie, telmisartan, amlodipine, valsartan) and placebo. Palatini and colleagues33 reported that a nighttime SBP‐SD of ≥12.2 mm Hg was associated with a 41% greater risk of cardiovascular events compared with the SD of <12.2 mm Hg. The corresponding value for a DBP‐SD of ≥7.9 mm Hg was 48%. In the present study, the SD of nighttime SBP changed from 14.3 mm Hg at the baseline to 12.8 mm Hg at week 12, and the SD of nighttime DBP changed from 9.6 mm Hg to 8.3 mm Hg, which did not achieve 12.2 mm Hg in the nighttime SBP‐SD and 7.9 mm Hg in the DBP‐SD. Further investigations regarding how to reduce the SD of nighttime BP are needed.
Our results also revealed that the telmisartan/amlodipine combination tablets significantly reduced the maximum home SBP and the SD of day‐by‐day home SBP regardless of the timing of their administration. Matsui and colleagues23 showed that the maximum home SBP in untreated hypertensive patients was an independent determinant of the left ventricular mass index, carotid intima‐media thickness, and UACR, indicating a possible predictive value of organ damage in hypertensive patients. Matsui and colleagues22 also reported that the combination of olmesartan and azelnidipine reduced the SD of home SBP significantly more than the combination of olmesartan and a thiazide diuretic (−1.7 mm Hg vs −0.6 mm Hg; P=.01), and that the SD of home SBP was an independent determinant of aortic pulse wave velocity, indicative of arteriosclerosis. In the present study, the change in SD of home SBP was −2.2 mm Hg in both the morning and bedtime administration groups, which is consistent with the results reported by Matsui and colleagues. Managing the maximum home SBP and the SD of day‐by‐day home SBP is thought to be necessary for a reduction in the risk of organ damage.
In the present investigation, the levels of UACR, NT‐ProBNP, and hsTnT were significantly reduced only when the telmisartan/amlodipine combination was administered at bedtime, and not in the morning administration group. Since the geometric mean UACR and hsTnT levels at baseline were normal (approximately 3.0 ng/mL and 5.0 pg/mL, respectively), it appears that the reductions did not show clinical significance (Table S2). On the other hand, the bedtime administration of the telmisartan/amlodipine combination changed the geometric mean NT‐ProBNP level from 188.7 ng/mL at baseline to 140.2 ng/mL at week 12, which may be clinically significant (Table S2). Shimizu and colleagues34 reported that a decrease in the plasma BNP concentration was associated with a decrease in bedtime SBP and nighttime SBP. Hoshide and colleagues35 also reported that amlodipine monotherapy significantly reduced the hsTnT concentration more than the combination of losartan and a diuretic. In the J‐TOP study,20 there was almost no difference in the effect of candesartan on home BP between morning and bedtime administration, but the UACR was significantly reduced with bedtime administration. The reduction in the UACR was especially greater when administered at bedtime to patients with morning hypertension. A possible reason for this result is the activation of the renin‐angiotensin system at night and early morning.36, 37, 38, 39, 40 To confirm this, it will be necessary to measure the UACR in a future study using fractional urine by a method such as the collection of urine every 2 hours. Regarding our biomarker investigation, we propose that bedtime administration of a telmisartan/amlodipine combination may reduce the risk of AF recurrence in hypertensive patients with paroxysmal AF, and a larger‐scale randomized controlled study with the primary endpoint of AF recurrence is thus needed.
Unlike the report of Hermida and colleagues,19 the present study did not show a significant difference in the BP‐lowering effect of ARB/CCB treatment between morning and bedtime administration, but we observed that the bedtime administration of the ARB/CCB reduced organ damage–related biomarker levels more. These results are consistent with those of the J‐TOP study.20 In the present study, the addition of amlodipine (which has the longest half‐life among antihypertensive drugs) to telmisartan would have led to continuous BP‐lowering effects, unlike the study by Hermida and colleagues using telmisartan monotherapy. In the J‐TOP study, the patients were allowed to use diuretics, which preferentially reduce nighttime BP even by morning administration; this might have led to the different results reported between the study by Hermida and colleagues and the J‐TOP study.
This study has the following limitations. It included a small sample size, and it was not a randomized controlled trial with the endpoint of AF onset or cardiovascular events.
Conclusions
Telmisartan/amlodipine treatment maintained its effects by significantly reducing nighttime BP and morning BP as well as nighttime BP‐SD and home BP‐SD regardless of the timing of administration in high‐risk hypertensive patients with paroxysmal AF. The NT‐ProBNP level was significantly decreased only when telmisartan/amlodipine combination tablets were administered at bedtime. A larger‐scale randomized controlled study may determine whether the bedtime administration of telmisartan/amlodipine combination tablets maximizes the risk‐lowering effects for AF recurrence in paroxysmal AF hypertensive patients.
Disclosure
This study was funded by the Waksman Foundation of Japan Inc.
Supporting information
Table S1. Patient baseline characteristics.
Table S2. Investigation of biomarkers.
Acknowledgments
We thank all patients, physicians, and medical staff who supported this study, including Dr Hiroshi Otani (Iwase General Hospital), Dr Shuichi Maeda (Kurosawa Hospital), Dr Masahiro Ono (Southern Tohoku Research Institute for Neuroscience), Dr Tomoharu Arakawa (Daido Hospital), Dr Akihiko Takahashi (Takahashi Hospital), Dr Mamoru Manita (Naha City Hospital), Dr Eiichi Tokutake (Tokutake Clinic), Dr Masahiko Kuroda (Kuroda Clinic), Dr Daishiro Yamada (Jiyugaoka Yamada Internal Medicine Clinic), Dr Takeshi Okuda (Okuda Clinic), Dr Junya Kamata (Odori Kamata Medicine Clinic), Dr Mari Nishizaka (Kimura Hospital), Dr Itaru Maeda (Miyanomori Memorial Hospital), Dr Harukazu Iseki (Sagamihara Kyodo Hospital), Dr Masatoshi Yanagisawa (Yanagisawa Clinic), Dr Kazuo Maeda (Maeda Clinic), Dr Naoki Kawai (Kawai Clinic), Dr Masaaki Techigawara (Techigawara Clinic), Dr Toshiki Fukui (NTT West Takamatsu Hospital), Dr Hideki Kuribayashi (Kuribayashi Clinic), Dr Takayuki Owada (Japanese Red Cross Fukushima Hospital), Dr Ichiro Watanabe (Nihon University Itabashi Hospital), Dr Shitoshi Hiroi (Takasaki General Medical Center), Dr Kuniyuki Takai (Takai Clinic), Dr Yuichiro Nakamura (Nakamura Cardiovascular Clinic), Dr Masayuki Watanabe (Omori Medical and Cardiovascular Clinic), and Dr Taiji Miyake (Gifu Heart Center). We also thank Ryuji Takeda for the statistical analysis (Department of Applied Biological Chemistry, Faculty of Agriculture, Kinki University), Satt Co., Ltd. for study administration and data management, and Mitsuharu Tanaka (WysiWyg Co., Ltd.) for editorial assistance in the preparation of this manuscript. The authors retain editorial control over the content.
J Clin Hypertens (Greenwich). 2016;18:1036–1044. DOI: 10.1111/jch.12814. © 2016 Wiley Periodicals, Inc.
ClinicalTrials.gov number NCT01748253.
References
- 1. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 2. Thomas MC, Dublin S, Kaplan RC, et al. Blood pressure control and risk of incident atrial fibrillation. Am J Hypertens. 2008;21:1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camm AJ, Savelieva I, Bharati S, et al. Atrial tachycardia, flutter, and fibrillation. In: Saksena S, Camm AJ, eds. Electrophysiological Disorders of the Heart. London: Elsevier Churchill‐Livingstone; 2005:283–363. [Google Scholar]
- 4. Kokubo Y, Watanabe M, Higashiyama A, et al. Interaction of blood pressure and body mass index with risk of incident atrial fibrillation in a Japanese urban cohort: The Suita study. Am J Hypertens. 2015;28:1355–1361. [DOI] [PubMed] [Google Scholar]
- 5. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 6. Lévy S. Drug Insight: angiotensin‐converting‐enzyme inhibitors and atrial fibrillation‐indications and contraindications. Nat Clin Pract Cardiovasc Med. 2006;3:220–225. [DOI] [PubMed] [Google Scholar]
- 7. Patlolla V, Alsheikh‐Ali AA, Al‐Ahmad AM. The renin‐angiotensin system: a therapeutic target in atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:1006–1012. [DOI] [PubMed] [Google Scholar]
- 8. Makkar KM, Sanoski CA, Spinler SA. Role of angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, and aldosterone antagonists in the prevention of atrial and ventricular arrhythmias. Pharmacotherapy. 2009;29:31–48. [DOI] [PubMed] [Google Scholar]
- 9. Schneider MP, Hua TA, Böhm M, et al. Prevention of atrial fibrillation by renin‐angiotensin system inhibition a meta‐analysis. J Am Coll Cardiol. 2010;55:2299–2307. [DOI] [PubMed] [Google Scholar]
- 10. ACTIVE I Investigators , Yusuf S, Healey JS, et al. Irbesartan in patients with atrial fibrillation. N Engl J Med. 2011;364:928–938. [DOI] [PubMed] [Google Scholar]
- 11. GISSI‐AF Investigators , Disertori M, Latini R, et al. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009;360:1606–1617. [DOI] [PubMed] [Google Scholar]
- 12. Yamashita T, Inoue H, Okumura K, et al. Randomized trial of angiotensin II‐receptor blocker vs. dihydropiridine calcium channel blocker in the treatment of paroxysmal atrial fibrillation with hypertension (J‐RHYTHM II study). Europace. 2011;13:473–479. [DOI] [PubMed] [Google Scholar]
- 13. Patton KK, Heckbert SR, Alonso A, et al. N‐terminal pro‐B‐type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi‐Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99:1832–1836. [DOI] [PubMed] [Google Scholar]
- 14. Mabuchi N, Tsutamoto T, Maeda K, et al. Plasma cardiac natriuretic peptides as biochemical markers of recurrence of atrial fibrillation in patients with mild congestive heart failure. Jpn Circ J. 2000;64:765–771. [DOI] [PubMed] [Google Scholar]
- 15. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–731. [DOI] [PubMed] [Google Scholar]
- 16. Giantin V, Perissinotto E, Franchin A, et al. Ambulatory blood pressure monitoring in elderly patients with chronic atrial fibrillation: is it absolutely contraindicated or a useful tool in clinical practice and research? Hypertens Res. 2013;36:889–894. [DOI] [PubMed] [Google Scholar]
- 17. Lip GY, Zarifis J, Beevers M, et al. Ambulatory blood pressure monitoring in atrial fibrillation. Am J Cardiol. 1996;78:350–353. [DOI] [PubMed] [Google Scholar]
- 18. The Japanese Society of Hypertension . Guidelines for the Management of Hypertension 2014. Tokyo: Life Science Publishing Co., Ltd.; 2014. [Google Scholar]
- 19. Hermida RC, Ayala DE, Fernández JR, et al. Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension. 2007;50:715–722. [DOI] [PubMed] [Google Scholar]
- 20. Kario K, Hoshide S, Shimizu M, et al. Effect of dosing time of angiotensin II receptor blockade titrated by self‐measured blood pressure recordings on cardiorenal protection in hypertensives: the Japan Morning Surge‐Target Organ Protection (J‐TOP) study. J Hypertens. 2010;28:1574–1583. [DOI] [PubMed] [Google Scholar]
- 21. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 22. Matsui Y, O'Rourke MF, Hoshide S, et al. Combined effect of angiotensin II receptor blocker and either a calcium channel blocker or diuretic on day‐by‐day variability of home blood pressure: the Japan Combined Treatment With Olmesartan and a Calcium‐Channel Blocker Versus Olmesartan and Diuretics Randomized Efficacy Study. Hypertension. 2012;59:1132–1138. [DOI] [PubMed] [Google Scholar]
- 23. Matsui Y, Ishikawa J, Eguchi K, et al. Maximum value of home blood pressure: a novel indicator of target organ damage in hypertension. Hypertension. 2011;57:1087–1093. [DOI] [PubMed] [Google Scholar]
- 24. Metoki H, Ohkubo T, Kikuya M, et al. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension. 2006;47:149–154. [DOI] [PubMed] [Google Scholar]
- 25. Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56:765–773. [DOI] [PubMed] [Google Scholar]
- 26. Kario K, Matsuo T, Kobayashi H, et al. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. [DOI] [PubMed] [Google Scholar]
- 27. Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. [DOI] [PubMed] [Google Scholar]
- 28. Hoshide S, Kario K, Hoshide Y, et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community‐dwelling normotensives. Am J Hypertens. 2003;16:434–438. [DOI] [PubMed] [Google Scholar]
- 29. Nagai M, Hoshide S, Ishikawa J, et al. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens. 2008;26:1636–1641. [DOI] [PubMed] [Google Scholar]
- 30. Yano Y, Inokuchi T, Hoshide S, et al. Association of poor physical function and cognitive dysfunction with high nocturnal blood pressure level in treated elderly hypertensive patients. Am J Hypertens. 2011;24:285–291. [DOI] [PubMed] [Google Scholar]
- 31. Hoshide S, Ishikawa J, Eguchi K, et al. Masked nocturnal hypertension and target organ damage in hypertensives with well‐controlled self‐measured home blood pressure. Hypertens Res. 2007;30:143–149. [DOI] [PubMed] [Google Scholar]
- 32. Parati G, Dolan E, Ley L, et al. Impact of antihypertensive combination and monotreatments on blood pressure variability: assessment by old and new indices. Data from a large ambulatory blood pressure monitoring database. J Hypertens. 2014;32:1326–1333. [DOI] [PubMed] [Google Scholar]
- 33. Palatini P, Reboldi G, Beilin LJ, et al. Added predictive value of night‐time blood pressure variability for cardiovascular events and mortality: the Ambulatory Blood Pressure‐International Study. Hypertension. 2014;64:487–493. [DOI] [PubMed] [Google Scholar]
- 34. Shimizu M, Ishikawa J, Yano Y, et al. Association between asleep blood pressure and brain natriuretic peptide during antihypertensive treatment: the Japan Morning Surge‐Target Organ Protection (J‐TOP) study. J Hypertens. 2012;30:1015–1021. [DOI] [PubMed] [Google Scholar]
- 35. Hoshide S, Fukutomi M, Eguchi K, et al. Change in high‐sensitive cardiac troponin T on hypertensive treatment. Clin Exp Hypertens. 2013;35:40–44. [DOI] [PubMed] [Google Scholar]
- 36. Kawasaki T, Cugini P, Uezono K, et al. Circadian variations of total renin, active renin, plasma renin activity and plasma aldosterone in clinically healthy young subjects. Horm Metab Res. 1990;22:636–639. [DOI] [PubMed] [Google Scholar]
- 37. Naito Y, Tsujino T, Fujioka Y, et al. Augmented diurnal variations of the cardiac renin‐angiotensin system in hypertensive rats. Hypertension. 2002;40:827–833. [DOI] [PubMed] [Google Scholar]
- 38. Kala R, Fyhrquist F, Eisalo A. Diurnal variation of plasma angiotensin II in man. Scand J Clin Lab Invest. 1973;31:363–365. [DOI] [PubMed] [Google Scholar]
- 39. Kawasaki T, Ueno M, Uezono K, et al. Differences and similarities among circadian characteristics of plasma renin activity in healthy young women in Japan and the United States. Am J Med. 1980;68:91–96. [DOI] [PubMed] [Google Scholar]
- 40. Brandenberger G, Follenius M, Goichot B, et al. Twenty‐four‐hour profiles of plasma renin activity in relation to the sleep‐wake cycle. J Hypertens. 1994;12:277–283. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient baseline characteristics.
Table S2. Investigation of biomarkers.


