Abstract
How to identify the early signs of hypertensive heart disease is the key to block or reverse the process of heart failure. The aim of this study was to evaluate the predictive value of left atrial (LA) enlargement in the early stage of hypertensive heart disease and to explore the correlations between LA enlargement and heart failure with normal ejection fraction (HFnEF), as well as the metabolic syndrome (MetS). Baseline clinical characteristics, biochemical indices, electrocardiographic and echocardiographic data were collected from 341 consecutive patients with essential hypertension. Among those patients, LA enlargement was more frequently presented than LV enlargement (57.2% vs 17.9%). Compared with patients without HFnEF, the prevalence of LA enlargement was higher in patients with HFnEF (82.9% vs 49.0%, P<.0001). From grade 2 to grade 3 hypertension, LA size was significantly larger in patients with MetS (P<.01) than those without. Multivariate linear regression analyses showed that age, body mass index, waist circumference, triglyceride level, and left ventricular diameter were independent predictors of LA enlargement. The simple measurement for identification of LA enlargement potentially allows early recognition of those patients at risk for heart failure, particularly among patients with MetS.
Recent epidemiological data show that more than 200 million adults present with hypertension in China, which was found as the second leading cause of heart failure. From hypertension to hypertensive heart disease is a slow and progressive process, and persistent high pressure load may lead to compensatory left ventricular (LV) hypertrophy. LV hypertrophy or enlargement confirmed by electrocardiography (ECG) or echocardiography is about 10% to 30% in unselected hypertensive patients. Previous studies1 have shown that echocardiographic left atrial (LA) enlargement occurring before LV hypertrophy is an early sign of hypertensive heart disease. LA volume provides a sensitive morphophysiologic expression of the severity of LV diastolic dysfunction, and appears to be a useful index of cardiovascular risk and disease burden, and LA volume indexed to body surface is independently associated with outcome of cardiovascular diseases.2 A report by Nicolaou and colleagues3 has recently revealed that the metabolic syndrome (MetS) increases LA diameter in paroxysmal atrial fibrillation patients and obesity was an important covariate of LA size in hypertensive patients. However, the prevalence of LA enlargement and its correlation to heart failure with normal ejection fraction (HFnEF) and MetS have not been assessed in Chinese hypertensive patients.
The aims of the present study were 3‐fold: (1) to investigate the prevalence of LA enlargement in patients with essential hypertension; (2) to assess the relationship between LA enlargement and HFnEF, as well as MetS; and (3) to explore independent predictors of LA enlargement.
Methods
Patients and Baseline Procedures
A total of 341 consecutive hypertensive patients hospitalized in Union Hospital, which is affiliated with Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China), were enrolled in our study from August 2007 to March 2008. Patients were included if they were older than 18 years and fulfilled the diagnostic criteria of hypertension:4 systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg on ≥2 occasions or on treatment with antihypertensive drugs. Grade 1 hypertension was defined as SBP of 140 mm Hg to 159 mm Hg and/or DBP of 90 mm Hg to 99 mm Hg, grade 2 as SBP of 160 mm Hg to 179 mm Hg and/or DBP of 100 mm Hg to 109 mm Hg, and grade 3 as SBP of ≥180 mm Hg and/or DBP of ≥110 mm Hg.4, 5 Hospitalization was defined as care at a hospital lasting for at least 24 hours. Exclusion criteria included coronary heart disease, congenital heart disease, valvular heart disease, cardiomyopathy, severe anemia, hyperthyroidism, or permanent pacemaker implantation. Informed consent was obtained from all participants, and the study protocol was approved by the ethics committee on human research of Tongji Medical College of Huazhong University of Science and Technology.
Blood samples were collected after an overnight fast in the clinical biochemical laboratory. Baseline data including age, sex, blood pressure (BP), waist circumference, body mass index (BMI), fasting plasma glucose (FPG), serum total cholesterol (TC), triglycerides (TGs), high‐density lipoprotein cholesterol (HDL‐C), and low‐density lipoprotein cholesterol (LDL‐C) were recorded. BMI was calculated as weight in kilograms divided by the square of height in meters. The final diagnosis of MetS was according to the 2005 International Diabetes Federation (IDF) definition,6 which defines MetS as having central obesity plus ≥2 of the following abnormalities: TGs ≥150 mg/dL, HDL‐C <40 mg/dL in men and <50 mg/dL in women, SBP ≥130 mm Hg or DBP ≥85 mm Hg, FPG >100 mg/dL, or previously diagnosed type 2 diabetes.
Electrocardiography
ECG was performed using a 3‐channel standard 12‐lead synchronous ECG apparatus (Cardiofax GEM‐9020 K, Nihon Kohden, Japan) with a rate of 25 mm/s and amplitude of 10 mV including at least 3 QRS complexes for each derivation. ECG criteria for LA enlargement included P‐wave duration in lead I, II, or III >110 ms; or P‐wave notching in lead I, II, or III with interpeak duration >40 ms (P mitrale); or area subtended by the terminal negative component of a biphasic P wave in precordial lead V1 >40 ms·mm (Morris index).7, 8, 9 A total of 22 patients with atrial fibrillation were excluded for LA enlargement analysis by ECG because there would not be a P wave present in these patients. ECG criteria for determining LV hypertrophy were defined by Sokolow‐Lyon index:10 sum of the largest R wave of the V5 or V6 derivation with wave S of the V1 ≥3.5 mV (35 mm) and/or R wave in aVL ≥1.1 mV (11 mm).
Echocardiographic Measurements
Echocardiography was performed using Acuson Sequoia C256 Echocardiography System (Philips Ultrasound, Bothell, WA) for the patients at steady state in the supine or left lateral decubitus position. Two‐dimensional–guided M‐mode measurement of LA posteroanterior dimension was measured from the parasternal long‐axis view according to the American Society of Echocardiography standards.11 Two‐dimensional–guided M‐mode measurement of LV end‐diastolic dimension (LVDd), interventricular septum (IVS) thickness, and posterior wall (PW) thickness were gained in the LV minor axis at end‐diastole. Early (E) and late (A) transmitral flow velocity, deceleration time (DT), and LV diastolic filling were assessed by pulse‐wave Doppler. Measurements from at least 3 different cardiac cycles were averaged and used in the analyses. Echocardiographic parameters were measured by consensus of the two experienced observers, blinded to the clinical data. LA enlargement was defined as a posteroanterior dimension >35 mm, while LV enlargement was defined as end‐diastolic dimension >55 mm according to Chinese criteria. The diagnosis of HFnEF was confirmed by satisfying the following obligatory conditions: (1) presence of signs or symptoms of congestive heart failure; (2) presence of normal or mildly abnormal LV systolic function; and (3) evidence of diastolic LV dysfunction, according to the consensus statement by the Heart Failure and Echocardiography Associations of the European Society of Cardiology.12
Statistical Analysis
Statistics were analyzed using SPSS 12.0 software (SPSS, Inc, Chicago, IL). Data were presented as mean±standard deviation or median (interquartile range) for continuous variables and as percentages for categorical variables. Comparisons between groups were analyzed by 2‐tailed unpaired Student t test for continuous variables and chi‐square test for categorical variables. The interaction of level of hypertension and MetS on LA size were determined by 2‐way factorial analysis of variance. Pearson correlation coefficients were used to assess the relationship between LA size and the following variables: LV diameter, MetS, and other echocardiographic parameters, as well as age, BMI, and BP. Multivariate logistic regression analysis was then performed to determine the independent predictors of LA enlargement. A 2‐tailed value of P<.05 was considered to be statistically significant.
Results
Clinical Characteristics of the Study Population
Three hundred forty‐one patients comprised our study population, of which the mean age was 60±14.4 years (range, 58 to 75 years). In the patients (n=341), mean baseline LA end‐systolic diameter (LAD) and left ventricular end‐diastolic diameter (LVDd) were 37.1±6.2 and 47.8±5.9 mm, respectively. LA enlargement was more frequently presented than LV enlargement (57.2% vs 17.9%) in the hypertensive patients. The baseline clinical characteristics of patients are demonstrated in Table 1.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | |
|---|---|
| Age, y | 60±14.4 |
| Male sex, % | 62.5 |
| BMI, kg/m2 | 25.2±3.8 |
| Metabolic syndrome, % | 42.2 |
| SBP, mm Hg | 175±23.0 |
| DBP, mm Hg | 105±14.5 |
| Current smoker, % | 35.5 |
| Alcohol drinking history, % | 28.5 |
| Family history of hypertension, % | 26.4 |
| Median duration of hypertension, y | 6.0 |
| Diabetes, % | 15.5 |
| Atrial fibrillation, % | 6.4 |
| LAD, mm | 37.1±6.2 |
| LVDd, mm | 47.8±5.9 |
| LV enlargement determined by ECG, % | 10.3 |
| LA enlargement determined by echocardiography, % | 57.2 |
| LV enlargement determined by echocardiography, % | 17.9 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; ECG, electrocardiography; LA, left atrial; LAD, left atrial diameter; LV, left ventricular; LVDd, left ventricular end‐diastolic dimension; SBP, systolic blood pressure. Categorical variables are presented as absolute (relative) frequencies and continuous variables as mean±standard deviation or median (interquartile range).
Relationship Between LA Enlargement and HFnEF
A total of 82 patients (25.2%) were diagnosed as having HFnEF in the study population. Patients with HFnEF had larger diameter of LA and significantly higher prevalence of LA enlargement than those without HFnEF (82.9% vs 49.0%, P<.0001), as shown in Table 2.
Table 2.
Relationship Between LA Enlargement and HFnEF
| Patients | With HFnEF (n=82) | Without HFnEF (n=259) | P Value |
|---|---|---|---|
| Early peak velocity, E, cm/s | 89.7±23.4 | 72.3±16.5 | <.001 |
| Late peak velocity, A, cm/s | 86.2±21.5 | 82.5±17.6 | .015 |
| E/A ratio | 1.14±0.57 | 1.05±0.30 | .041 |
| Deceleration time, ms | 201±49 | 239±42 | <.001 |
| LAD, mm | 38.0±5.6 | 34.5±4.3 | <.001 |
| LA enlargement, No. (%) | 68 (82.9) | 127 (49.0) | <.001 |
Abbreviations: A, late peak velocity; E, early peak velocity; HFnEF, heart failure with normal ejection fraction; LA, left atrial; LAD, left atrial diameter. Data are expressed as mean±standard deviation.
LA Dimension and Enlargement
LA enlargement was noted in 195 hypertensive patients (57.2%), who were older and had longer duration of hypertension compared with those without LA enlargement (9.0 years vs 4.5 years, P<.01). There was also significant difference in BMI, waist circumference, TG, presence of MetS, and LVDd between the patients with and without LA enlargement (P<.05), as shown in Table 3.
Table 3.
Comparison of Clinical Characteristics Between Patients With and Without LA Enlargement
| Without LA Enlargement (n=146) | With LA Enlargement (n=195) | P Value | |
|---|---|---|---|
| Age, y | 56±15.0 | 63±13.2 | .001 |
| Male sex, % | 63.0 | 62.1 | .78 |
| Median duration of hypertension, y | 4.5 | 9.0 | .006 |
| BMI, kg/m2 | 24.23±3.57 | 25.85±3.75 | .004 |
| Waist circumference, cm | 86.4±7.71 | 91.2±8.81 | .003 |
| Total cholesterol, mmol/L | 5.22±1.15 | 5.31±1.21 | .14 |
| HDL‐C, mmol/L | 1.22±0.30 | 1.17±0.30 | .13 |
| Triglycerides, mmol/L | 1.64±0.75 | 1.76±0.67 | .02 |
| FPG, mg/dL | 93.6±19.98 | 97.2±24.84 | .15 |
| SBP, mm Hg | 172±19.9 | 177±25.1 | .050 |
| DBP, mm Hg | 105±15.4 | 104±13.9 | .53 |
| Metabolic syndrome, % | 32.19 | 49.74 | .003 |
| LVDd, cm | 4.5±0.41 | 5.0±0.64 | .005 |
| LVEF, % | 66.7±6.52 | 63.4±11.2 | .15 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; LA, left atrial; LVDd, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure.
Categorical variables are presented as absolute (relative) frequencies and continuous variables as mean±standard deviation or median (interquartile range).
Effect of MetS on LA Size With Different Levels of Hypertension
A significant interaction was found between MetS and level of hypertension for LA size (P<.01, Figure). From grade 1 to grade 2 hypertension, the two lines were parallel, showing that there was no relation between levels of hypertension and LA size in patients with or without MetS. From grade 2 to grade 3 hypertension, the LA size was significantly larger in patients with MetS, which suggested that the MetS may evidently increase the dimension of LA during the higher level of hypertension.
Figure 1.
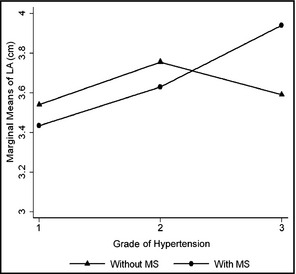
The effect of metabolic syndrome (MS) on the relationship between level of hypertension and left atrial (LA) size.
Logistic regression analyses demonstrated that age older than 60 years, BMI >25 kg/m2, waist circumference >90 cm in men and >80 cm in women, TG >1.7 mmol/L, and LVDd >4.5 cm were independent predictors of LA enlargement (P<.01). Among them, LVDd >4.5 cm was the most important predictor of LA enlargement (odds ratio, 4.43; 95% confidence interval, 2.61–7.50; P<.001), as shown in Table 4.
Table 4.
Independent Predictors of LA Enlargement
| Risk Factors | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age older than 60, y | 1.03 | 1.02–1.06 | .03 |
| BMI >25, kg/m2 | 2.64 | 1.60–4.38 | .004 |
| Waist circumference >90 in men, and >80 in women, cm | 2.07 | 1.23–3.48 | <.001 |
| TG >1.7, mmol/L | 1.82 | 1.10–3.00 | .002 |
| HDL‐C >1.0 in men, and >1.25 in women, mmol/L | 0.61 | 0.37–1.00 | .02 |
| LVDd >4.5, cm | 4.43 | 2.61–7.50 | <.001 |
Abbreviations: BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; LVDd, left ventricular end‐diastolic dimension; TG, triglyceride. Values are presented as hazard ratios (95% confidence intervals [CIs]).
Relationship of ECG Criteria for LA Enlargement to Echocardiographic LA Dimension Measurement
The 3 ECG criteria used to detect LA enlargement defined by dimension >35 mm performed poorly. Sensitivities and specificities of the ECG criteria are listed in Table 5. When used as individual tests, the highest sensitivity (69%) for LA dimension enlargement was with a P‐wave duration >110 ms in limb lead I, II, or III, but it had low specificity (47%). The highest specificity (73%) for LA dimension enlargement was for a bifid P wave separated by >40 ms (P mitrale) in lead I, II, or III, but it had low sensitivity (14%).
Table 5.
Relation of Electrocardiographic Criteria for LA Enlargement to Echocardiographic LA Dimension Measurement
| ECG Criteria | Detection of LA Enlargement (Dimension >35 mm) | |||
|---|---|---|---|---|
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
| P duration >110 ms in lead I, II, or III | 69 | 47 | 61 | 55 |
| Biphasic P wave >40 ms in lead I, II, or III | 14 | 73 | 39 | 41 |
| Negative terminal P force in lead V1 >40 ms·mm | 58 | 61 | 64 | 54 |
Abbreviations: ECG, electrocardiography; LA, left atrial; NPV, negative predictive value; PPV, positive predictive value.
Discussion
The present study clearly demonstrated that LA enlargement, which reflected cardiac remodeling, presented earlier than LV enlargement in patients with essential hypertension and might be an early marker of hypertensive heart disease. The prevalence of LA enlargement in our study (54.9%) was higher than those in the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE)13 trial (46%) and the Evaluation of Target Organ Damage in Hypertension (ETODH) trial14 (23%) probably attributable to different definitions of LA enlargement. We defined LA enlargement as LAD >35 mm according to Chinese criteria, while in the aforementioned two clinical trials the definition of LAD exceeded 38 mm in women and 42 mm in men, respectively. In addition, the ETODH trial included only outpatients without atrial fibrillation, while we enrolled in‐patients in the present study who might have higher levels of BP. Furthermore, 6.4% of patients in our study presented with atrial fibrillation. Otherwise, prevalence of echocardiographic LA enlargement in hypertension consistently varied among studies, from 16.0% to 83.0%, with a prevalence in the pooled population of 32%.15
We also found a significant association between LA enlargement and HFnEF, which was consistent with previous studies.16, 17 The Irbesartan in HFPEF trial (I‐PRESERVE)10 indicated that LA enlargement was present in the majority of patients with HFnEF and might be the best diastolic function prognostic index, while Park and colleagues18 reported that LA volume was strongly correlated with the degree of diastolic dysfunction. Another study19 found that the higher LA volume index in HFnEF with the diastolic wall strain ≤0.03 might be a useful marker for assessing LV diastolic stiffness. Moreover, in the Strong Heart Study (SHS),20 LA diameter was proved to be an independent predictor of incident cardiovascular events. This relationship may be explained by several potential mechanisms. Firstly, function of the LA and ventricle are known to be interactional. During the LV systolic phase, the LA acts as a reservoir, while in early LV diastole, the LA works as a conduit for the influx of blood to the left ventricle. Consequently, LA contracts for LV filling in the late diastolic phase. Thus, it is credible that increased LA size plays an important role in the development of LV diastolic dysfunction.21, 22 By multiple regression analysis in the present study, we found that LV diameter was an independent predictive factor of LA enlargement. In accordance with our study, a recent meta‐analysis reported that the prevalence of LV hypertrophy was about 3‐fold greater in patients with LA enlargement than those without.9 Additionally, investigators23, 24 have shown that LA diameter is independently associated with arterial stiffness, which may affect the development of LV diastolic dysfunction. Furthermore, in patients with HFnEF, common comorbidities such as coronary artery disease, diabetes mellitus, obesity, and renal dysfunction may result in volume overload and myocardial injury of atrium.18, 25 Therefore, the European Association of Echocardiography had already included LA volume index into the diagnostic criteria of diastolic heart failure.26 Otherwise, we also found that commonly used ECG criteria for LA enlargement do not reliably reflect LA enlargement defined by a dimension of >35 mm and lack sufficient predictive value to be useful clinically.
Nonetheless, the MetS is a clustering of some cardiovascular risk factors in one patient. The core components of MetS include impaired glucose metabolism, obesity, dyslipidemia, and hypertension. To date, few studies have shed light on the explicit correlation of MetS to LA size in patients with essential hypertension. Previous studies suggest that patients with MetS are more susceptible to LA enlargement than those without MetS.27 An additional study presented by Ayer and colleagues28 showed that obesity was an independent predictor of LA size. In Japanese hypertensive patients, LA size was influenced by insulin resistance and obesity, integral components of the MetS, independently of LV hypertrophy, LV geometry, or LV diastolic function.1 Consistent with other authors, we found that MetS may accelerate the development of LA enlargement in patients with essential hypertension. Furthermore, we extend our knowledge by clearly demonstrating that LA size is strongly correlated to MetS in patients with grade 2 and 3 hypertension. Meanwhile, our study reveals that waist circumference, TGs, and HDL‐C, integral components of the MetS, are independent predictors of LA enlargement; however, the mechanism by which MetS contributes to LA enlargement is still inexplicit. It is suggested that MetS may evidently increase the dimension of LA during the higher levels of hypertension because of the hemodynamic overload or its direct effects on cardiovascular structure.25 Hypertension accompanied by MetS may exacerbate myocardial fibrosis, cadiomyocytes hypertrophy and myocardial micro‐vascular structure.29, 30 Other underlying mechanisms of MetS predisposed to LA enlargement may also include immunologic injury and oxidative stress. Atrial remodeling and MetS are both present with elevated circulating level of pro‐inflammatory cytokines and activated inflammatory signaling pathway.31, 32
Limitations
Our study has limitations. Firstly, recent studies have shown that LA volume may be more accurate for the definition of LA enlargement than LA diameter, therefore the lack of LA volume data is a limitation of the study. However, the simple linear measurement is more common and convenient in daily clinical practice and still helpful for calling attention to identify high‐risk individuals. Secondly, we included only in‐patients with essential hypertension and the results of the present study may not be generalized to all hypertensive patients. Finally, the sample size of the present study is relatively small.
Conclusions
Our findings reveal that LA enlargement is a common but easily ignored condition in Chinese hypertensive patients. We also found some independent predictors of LA enlargement and the close relationship between LA enlargement and HFnEF. Patients at heightened risk for developing heart failure warrant not only more aggressive antihypertensive therapy, but also reverse remodeling treatment and intensive risk factor modification. The simple measurement of LA diameter by echocardiography may be an effective method for identifying those high‐risk individuals. Further investigations are required in a larger population to support our preliminary results and elucidate the potential mechanisms.
Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
J Clin Hypertens (Greenwich).2014;16:192–197. ©2014 Wiley Periodicals, Inc.
References
- 1. Shigematsu Y, Norimatsu S, Ogimoto A, et al. The influence of insulin resistance and obesity on left atrial size in Japanese hypertensive patients. Hypertens Res. 2009;32:500–504. [DOI] [PubMed] [Google Scholar]
- 2. Tsang TS, Barnes ME, Gersh BJ, et al. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. [DOI] [PubMed] [Google Scholar]
- 3. Nicolaou VN, Papadakis JE, Karatzis EN, et al. Impact of the metabolic syndrome on atrial size in patients with new‐onset atrial fibrillation. Angiology. 2007;58:21–25. [DOI] [PubMed] [Google Scholar]
- 4. Mancia G, Fagard R, Narkiewicz K, et al. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–1938. [DOI] [PubMed] [Google Scholar]
- 5. Liu LS. [2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:579–615. [PubMed] [Google Scholar]
- 6. Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–2749. [DOI] [PubMed] [Google Scholar]
- 7. Hazen MS, Marwick TH, Underwood DA. Diagnostic accuracy of the resting electrocardiogram in detection and estimation of left atrial enlargement: an echocardiographic correlation in 551 patients. Am Heart J. 1991;122:823–828. [DOI] [PubMed] [Google Scholar]
- 8. Munuswamy K, Alpert MA, Martin RH, et al. Sensitivity and specificity of commonly used electrocardiographic criteria for left atrial enlargement determined by M‐mode echocardiography. Am J Cardiol. 1984;53:829–832. [DOI] [PubMed] [Google Scholar]
- 9. Lee KS, Appleton CP, Lester SJ, et al. Relation of electrocardiographic criteria for left atrial enlargement to two‐dimensional echocardiographic left atrial volume measurements. Am J Cardiol. 2007;99:113–118. [DOI] [PubMed] [Google Scholar]
- 10. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. 1949. Ann Noninvasive Electrocardiol. 2001;6:343–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 12. Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 13. Gerdts E, Oikarinen L, Palmieri V, et al. Correlates of left atrial size in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Hypertension. 2002;39:739–743. [DOI] [PubMed] [Google Scholar]
- 14. Cuspidi C, Negri F, Lonati L, et al. Prevalence and correlates of echocardiographic left atrial enlargement in hypertensive outpatients in clinical practice. Clin Exp Hypertens. 2011;33:328–335. [DOI] [PubMed] [Google Scholar]
- 15. Cuspidi C, Rescaldani M, Sala C. Prevalence of echocardiographic left‐atrial enlargement in hypertension: a systematic review of recent clinical studies. Am J Hypertens. 2013;26:456–464. [DOI] [PubMed] [Google Scholar]
- 16. Zile MR, Gottdiener JS, Hetzel SJ, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. [DOI] [PubMed] [Google Scholar]
- 17. Carson P, Massie BM, McKelvie R, et al. The irbesartan in heart failure with preserved systolic function (I‐PRESERVE) trial: rationale and design. J Card Fail. 2005;11:576–585. [DOI] [PubMed] [Google Scholar]
- 18. Park SM, Park SW, Casaclang‐Verzosa G, et al. Diastolic dysfunction and left atrial enlargement as contributing factors to functional mitral regurgitation in dilated cardiomyopathy: data from the Acorn trial. Am Heart J. 2009;157:762–710. [DOI] [PubMed] [Google Scholar]
- 19. Ohtani T, Mohammed SF, Yamamoto K, et al. Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1742–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kizer JR, Bella JN, Palmieri V, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle‐aged and elderly adults: the Strong Heart Study (SHS). Am Heart J. 2006;151:412–418. [DOI] [PubMed] [Google Scholar]
- 21. Russo C, Jin Z, Homma S, et al. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JS, Shim CY, Wi J, et al. Left ventricular diastolic function is closely associated with mechanical function of the left atrium in patients with paroxysmal atrial fibrillation. Circ J. 2013;77:697–704. [DOI] [PubMed] [Google Scholar]
- 23. Myung Y, Seo HS, Jung IH, et al. The correlation of carotid artery stiffness with heart function in hypertensive patients. J Cardiovasc Ultrasound. 2012;20:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edelmann F, Stahrenberg R, Gelbrich G, et al. Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol. 2011;100:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chillo P, Rieck AE, Lwakatare J, et al. Left atrial volume index as a marker of left ventricular diastolic dysfunction in asymptomatic Tanzanian diabetic patients. Blood Press. 2013;22:86–93. [DOI] [PubMed] [Google Scholar]
- 26. Kindermann M. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2686; author reply 2686–2687. [DOI] [PubMed] [Google Scholar]
- 27. Uzun M, Koz C, Yildirim M, et al. [Does accompanying metabolic syndrome contribute to heart dimensions in hypertensive patients?]. Turk Kardiyol Dern Ars. 2008;36:446–450. [PubMed] [Google Scholar]
- 28. Ayer JG, Almafragy HS, Patel AA, et al. Body mass index is an independent determinant of left atrial size. Heart Lung Circ. 2008;17:19–24. [DOI] [PubMed] [Google Scholar]
- 29. Dzherieva IS, Volkova NI. [Arterial hypertension and metabolic disorders]. Klin Med (Mosk). 2010;88:4–8. [PubMed] [Google Scholar]
- 30. Campbell DJ, Somaratne JB, Jenkins AJ, et al. Impact of type 2 diabetes and the metabolic syndrome on myocardial structure and microvasculature of men with coronary artery disease. Cardiovasc Diabetol. 2011;10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanti JF, Ceppo F, Jager J, et al. Implication of inflammatory signaling pathways in obesity‐induced insulin resistance. Front Endocrinol (Lausanne). 2012;3:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pellegrino PL, Brunetti ND, De Gennaro L, et al. Inflammatory activation in an unselected population of subjects with atrial fibrillation: links with structural heart disease, atrial remodeling and recent onset. Intern Emerg Med. 2013;8:123–128. [DOI] [PubMed] [Google Scholar]


