Abstract
Twenty‐seven patients with resistant hypertension and chronic kidney disease were treated by renal sympathetic denervation (RSD) and followed for 12 months. Patients were retrospectively divided into controlled and uncontrolled blood pressure (BP) groups. Increases in mean estimated glomerular filtration rate (eGFR) were found at months 1, 3, 6, and 12 in the controlled group (P<.0001, for every time point). The mean change in eGFR after 12 months was 18.54±8.15 mL/min/1.73m2 higher in the controlled group (P=.0318). In patients in the controlled group with baseline eGFR <45 mL/min/1.73 m2, responders (with an increase in eGFR >6.2%) corresponded to 50% at 6 months and 83% at 12 months. In the patients with baseline eGFR ≥45 mL/min/1.73 m2, all patients were labeled as responders at months 6 and 12. Median albumin:creatinine ratio after 12 months was lower than baseline only in the controlled group (P=.0003). Our results suggest that patients with this profile who reached BP control by RSD also experienced a significant improvement in renal function.
Essential hypertension represents a significant and growing global health issue. It is also recognized as one of the most important risk factors for the development and progression of chronic kidney disease (CKD).1 Treatment strategies for hypertension are mainly based on lifestyle intervention and pharmacotherapy.2
Based on a number of recently published international data, catheter‐based transluminal renal artery sympathetic denervation (RSD) has been suggested as a promising treatment option for patients with resistant hypertension.3, 4, 5, 6 Accordingly, a previous report from our group showed that RSD was associated with substantially improved blood pressure (BP) control among hypertensive patients with mild to moderate CKD.7 However, it is still unknown whether the magnitude of BP reduction following RSD is related to the reported improvement in renal function in patients with resistant hypertension and CKD. In this study, we aim to evaluate whether the control of BP at the 12 months following RSD in patients with resistant hypertension and mild to moderate CKD confers benefit on estimated glomerular filtration rate (eGFR) after a follow‐up period of 1 year.
Methods
Study Patients
We conducted a prospective, longitudinal study in 27 patients with refractory hypertension and CKD stages 2, 3, and 4 who underwent RSD. The Committee of Ethics in Research of the Medical School of Universidade Federal Fluminense approved the study and informed consent was signed by all patients. Furthermore, this study investigated whether systolic office BP reduction following RSD can be useful in predicting changes in eGFR in the long term.
The study was conducted in the state of Rio de Janeiro, Brazil, as a partnership of the Universidade Federal Fluminense and the Hospital Regional Darcy Vargas. Patients were recruited from June 2011 to December 2012 and were derived from the university hospital and the public health network of the county. Patients who had the combination of the following criteria were consecutively enrolled: (1) office systolic blood pressure ≥160 mm Hg (or ≥150 mm Hg for patients with type 2 diabetes mellitus), confirmed by multiple measurements,7 despite treatment with nonpharmacologic measures and use of at least three antihypertensive drugs (including a diuretic) on maximally tolerated doses or confirmed intolerance to medications; (2) GFR estimated by the Chronic Kidney Disease Epidemiology Collaboration equation8 between 15 and 89 mL/min/1.73 m2 (patients with eGFR >60 mL/min/1.73 m2 were required to have microalbuminuria); and (3) age 18 to 70 years.
Exclusion criteria were pregnancy; valvular heart disease with significant hemodynamic consequences; stenotic valvular heart disease for which the reduction in BP could be dangerous; acute myocardial infarction, unstable angina, stroke, or transitory ischemic attack within the previous 6 months; renovascular anomalies (including renal artery stenosis, angioplasty with or without stenting, or double or multiple main arteries in the same kidney); and diabetes mellitus type 1 or other secondary cause for hypertension.
All patients involved in this study were already treated for hypertension for at least a year. Baseline medication was unchanged for at least 3 months before RSD.
Study Procedures and Assessment
In this study, we treated 27 patients (11 men and 16 women) with CKD (stages 2, 3, and 4)9 and grade 2 and 3 systemic arterial hypertension.10 Patients underwent a complete medical history and physical examination. Hypertension was diagnosed on the basis of the current Brazilian Society of Cardiology guidelines and of the current European Society of Cardiology guidelines for the management of arterial hypertension.2, 11 Patients had previously been screened for secondary forms of hypertension according to current guidelines.2, 11 All patients underwent history and physical examination and antihypertensive medication was reviewed. BP measurements were performed in the standing, sitting, and supine positions on at least two subsequent visits in both arms. Patients also underwent blood sampling for whole blood cell count and biochemistry (including serum creatinine to estimate GFR). Urine samples were obtained for determination of albuminuria, protein, and creatinine. Echo Doppler to evaluate the anatomy of the renal arteries of patients was also performed.
To evaluate the true effects of RSD on BP and additional measures, baseline medication was unchanged for at least 3 months before RSD. The patients and physicians were instructed not to change the medications and dosages after the procedure unless clinically indicated. Drug records and adherence of each patient were comprehensively reviewed and documented at each visit. All patients received intravenous sodium bicarbonate (3 mL/kg) and 0.9% saline for 1 hour, as prophylaxis for attenuation of iodinated contrast media‐associated nephrotoxicity.12, 13
RSD Procedure
After completion of bilateral renal arteriography to confirm that the patient had no anatomic contraindication, the RSD was performed using a 7F irrigated ablation catheter as previously described.7
After the procedure, patients remained hospitalized for a period of 24 hours. The follow‐up was performed weekly for the first month, monthly from the second to the sixth month, and bimonthly from the seventh through the 12th month. In every visit to the office, BP was measured after standing for 10 minutes in both upper limbs in the sitting and supine positions, with the mean of four measures. For every change in patient position (standing, sitting, and supine), there was a pause of 5 minutes. Samples were collected for blood and urine tests to monitor the variables at month 1, 3, 6, and 12. Echo Doppler was also performed at month 1 and month 6 after the RSD to evaluate the anatomy of the renal arteries of patients. The following variables were monitored during the follow‐up period: mean systolic and diastolic office BP, number and doses of antihypertensive medications, creatinine, eGFR, and albuminuria.
Recently, the Eighth Joint National Committee (JNC 8)14 reported a new goal: systolic BP <140 mm Hg and diastolic BP <90 mm Hg for the population aged 18 years or older with CKD. Based on the inclusion criteria used in the randomized controlled trials reviewed by experts from the panel of JNC 8, this recommendation should apply to individuals younger than 70 years with an eGFR or measured GFR <60 mL/min/1.73 m2 and in people of any age with albuminuria defined as >30 mg of albumin/g of creatinine at any level of GFR.
According to this new recommendation, the patients in this study were divided into two groups: patients with controlled and noncontrolled BP (office systolic BP after 12 months of follow‐up <140 mm Hg and ≥140 mm Hg, respectively). Their longitudinal course was retrospectively analyzed to check whether BP reduction was predictive of changes in eGFR.
The determination of the serum creatinine was performed using an autoanalyzer (Selectra; Vital Scientific NE, Dieren, The Netherlands) whose results are provided as standardized serum creatinine values. To account for the intra‐assay variation coefficient of creatinine determination of the kit used (Vitros; Johnson & Johnson Medical, São Paulo, Brazil), only changes in eGFR with order of magnitude higher than 6.2% were taken into consideration. Accordingly, only patients with at least this improvement were labeled as responders regarding GFR.
Statistical Analysis
The results were expressed as mean and standard deviation (mean±SD) of the mean in case of normal distribution and as the median with interquartile range otherwise. Statistical tests were all two‐sided. Comparisons between two‐paired values were performed by paired t test in case of Gaussian distribution or, alternatively, by Wilcoxon test. Comparisons between more than two paired values were performed by analysis of variance for repeated measures or with Kruskal‐Wallis analysis of variance as appropriate, complemented by a post hoc test. Frequencies were compared with chi‐square test with Yates’ correction or with Fisher test. P values <.05 were considered significant. Correlations between two variables were performed by Pearson in case of Gaussian distribution or, alternatively, with the Spearman correlation test. All statistical analyses were performed using the program GraphPad Prism v 6.0 (GraphPad software, La Jolla, CA).
Results
Baseline Features of Patients
General features of the 27 patients enrolled in the study are shown in the Table. Twelve months after RSD, patients were divided in two groups according to the final office systolic BP at 12 months in the controlled (n=22) and noncontrolled (n=5) groups. Eighteen of the 27 patients had stage 2 CKD, 4 had stage 3, and 5 had stage 4. At baseline, the mean office systolic/diastolic arterial BP was 184.4±18.7/106.0±13.3 mm Hg for all patients, 183.1±17.3/107.2±13.3 mm Hg for patients who had final office systolic BP <140 mm Hg, and 190.4±25.1/100.5±13.5 mm Hg for patients who had final office systolic BP ≥140 mm Hg. There was no difference in the mean eGFR between groups at baseline. Noncontrolled patients had a higher proportion of type 2 diabetes and sympatholytic use.
Table 1.
General Features of Patients at Study Entrance
| Parameters | All Patients (n=27) | Final Office Systolic BP <140 mm Hg (n=22) | Final Office Systolic BP ≥140 mm Hg (n=5) | P Value |
|---|---|---|---|---|
| Age, y | 54.8±10.8a | 52.5±10.5a | 64.8±4.8a | 0.0567 |
| Women, % | 16 (59.3) | 12 (54.5) | 4 (80) | 0.6185 |
| Ethnicity (white), % | 19 (70.4) | 16 (72.7) | 3 (60) | 0.6159 |
| Body mass index, kg/m² | 31.4±4.7a | 30.7±4.9a | 34.4±2.3a | 0.2590 |
| Coronary artery disease, % | 4 (14.8) | 4 (18.2) | 0 (0) | 0.5613 |
| Atrial fibrillation, % | 2 (7.4) | 2 (9.1) | 0 (0) | 1.0000 |
| Stroke, % | 4 (14.8) | 4 (18.2) | 0 (0) | 0.5613 |
| Type 2 diabetes, % | 10 (37) | 6 (27.3) | 4 (80) | 0.0473 |
| LDL cholesterol >130 mg/dL, % | 17 (63) | 14 (63.6) | 3 (60) | 1.0000 |
| Tobacco smoking, % | 3 (11.1) | 2 (9.1) | 1 (20) | 0.4735 |
| Creatinine, mg/dL | 1.49 ±1.00 | 1.50±0.98 | 1.42±1.17 | 0.9859 |
| eGFR, mL/min/1.73 m² | 62.2±25.1a | 62.2±25.3a | 62.2±25.4a | >0.9999 |
| ACR, mg/g | 84.3 (35.9–169.0)b | 47.7 (35.6–168.3)b | 154.4 (75.5–323.2)b | 0.4417 |
| CKD stage, % | ||||
| 2 | 18 (66.7) | 15 (68.2) | 3 (60) | 1.0000 |
| 3 | 4 (14.8) | 3 (13.6) | 1 (20) | 1.0000 |
| 4 | 5 (18.5) | 4 (18.2) | 1 (20) | 1.0000 |
| Office BP, mm Hg | 184.4±18.7/106.0±13.3a | 183.1±17.3/107.2±13.3a | 190.4±25.1/100.5±13.5a | 0.7120/0.5731 |
| Classes of antihypertensive drugs | 4.6±1.3a | 4.4±1.4a | 5.4±0.5a | 0.1363 |
| Drug class, % | ||||
| ACE inhibitors/ARBs | 25 (92.6) | 20 (90.9) | 5 (100) | 1.0000 |
| Direct renin inhibitors | 2 (7.4) | 2 (9.1) | 0 (0) | 1.0000 |
| β‐Blockers | 22 (81.5) | 17 (77.3) | 5 (100) | 0.5469 |
| Calcium channel blockers | 22 (81.5) | 17 (77.3) | 5 (100) | 0.5469 |
| Diuretics | 27 (100) | 22 (100) | 5 (100) | 1.0000 |
| Oral sympatholytics | 10 (37) | 6 (27.3)s | 4 (80) | 0.0473 |
| Vasodilator | 4 (14.8) | 3 (13.6) | 1 (20) | 1.0000 |
| α1‐Adrenergic blocker | 1 (3.7) | 0 (0) | 1 (20) | 0.1852 |
Abbreviations: ACE‐I, angiotensin‐converting‐enzyme inhibitor; ACR, albumin:creatinine ratio; ARB, angiotensin II receptor antagonist; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure. aMean+SD; bMedian (IQR).
Efficacy in BP Reduction
Initially, there were 14 patients (52%) with stage 3 hypertension (systolic BP >180 mm Hg) and 13 patients (48%) with stage 2 (systolic BP between 160 mm Hg and 179 mm Hg). Twelve months after RSD, 22 patients (81%) were normotensive, 4 (15%) had stage 1 hypertension (systolic BP between 140 mm Hg and 159 mm Hg), and 1 (4%) had stage 2 hypertension. The office systolic BP values at baseline and months 1, 3, 6, and 12 of follow‐up after RSD for patients with controlled BP were 183.1±17.3 mm Hg, 133.8±8.5 mm Hg, 134.4±10.6 mm Hg, 131.8±9.6 mm Hg, and 124.9±8.7 mm Hg, respectively (P<.001 vs baseline, for all time points). For those with noncontrolled BP, the corresponding values were 190.4±25.1 mm Hg, 142.9±9.9 mm Hg, 148.5±10.1 mm Hg, 151.7±6.5 mm Hg and 151.3±8.7 mm Hg, respectively (P<.001 vs baseline, for all time points post‐procedure) (Figure 1A). At month 12 after RSD, the magnitude of office systolic BP fall between groups was more pronounced in patients with controlled BP (P=.0346) (Figure 2A).
Figure 1.
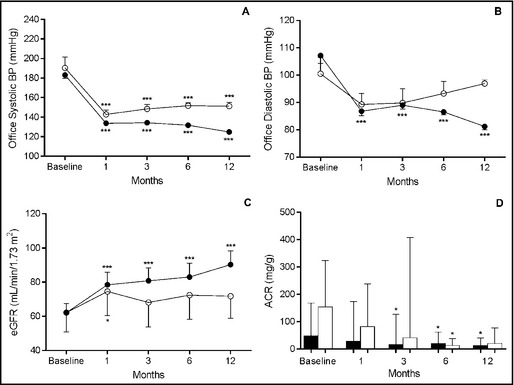
Office systolic (A) and diastolic (B) blood pressure (BP), estimated glomerular filtration rate (eGFR) (C), and albumin:creatinine ratio (ACR) (D) of patients with controlled BP (office systolic BP <140 mm Hg at month 12 [closed circles or bars, n=22] and those with noncontrolled BP (open circles or bars, n=5) during follow‐up. *P<.05 and ***P<.001 vs correspondent baseline values. Values are presented as mean±standard error in (A), (B), and (C) and as median (interquartile range) in (D).
Figure 2.
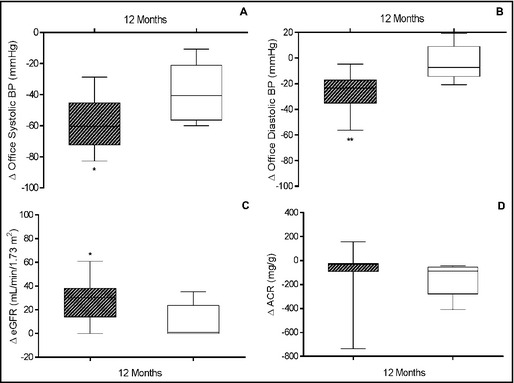
Variations (∆) in office systolic (A) and diastolic (B) blood pressure (BP), estimated glomerular filtration rate (eGFR) (C), and albumin:creatinine ratio (ACR) (D) of patients with controlled BP (office systolic BP <140 mm Hg at month 12 [hatched boxes, n=22]) and those with noncontrolled BP (open boxes, n=5) 12 months after renal sympathetic denervation. *P<.05 and **P<.01 vs correspondent difference between variations. Values are presented as minimum to maximum.
Diastolic BP fell from 107.2±13.3 mm Hg at baseline to 86.8±7.5 mm Hg, 89.0±6.7 mm Hg, 86.6±5.2 mm Hg, and 81.2±5.6 mm Hg at months 1, 3, 6, and 12 after the procedure, respectively, for patients with controlled BP, as shown in Figure 1B (P<.001 vs baseline, for all time points post‐procedure). In noncontrolled patients, changes during follow‐up exhibited the same trend but statistical significance was not found. The mean magnitude of the fall in mean diastolic BP at month 12 was 22.37±6.65 mm Hg higher in patients with controlled BP (P=.0025) (Figure 2B).
Renal Function
There was a significant reduction in serum creatinine at months 1, 3, 6, and 12 of follow‐up (1.26±0.99 mg/dL, 1.24±0.99 mg/dL, 1.21±0.91 mg/dL, and 1.10±0.99 mg/dL, respectively) compared with baseline (1.50±0.98 mg/dL) for the patients with controlled BP (P<.05, for all time points). The patients with noncontrolled BP did not show a difference in creatinine values at months 1, 3, 6, and 12 post‐procedure (1.24±1.04 mg/dL, 1.34±1.17 mg/dL, 1.30±1.18 mg/dL, and 1.30±1.18 mg/dL, respectively) compared with baseline (1.42±1.17 mg/dL). The mean reduction in serum creatinine at month 12 was 0.28±0.09 mg/dL higher in patients with controlled BP (P=.0132).
A significant increase in eGFR was observed in every point analyzed in patients with controlled BP (P<.001, for all time points). Meanwhile, in patients with noncontrolled BP, a discrete but significant increase was seen at the first month (P<.05), which was no longer present in the following measurements (Figure 1C). Again, the mean increase in eGFR at month 12 was 18.54±8.15 mL/min/1.73 m2 higher in controlled patients (P=.0318) (Figure 2C).
The median of albumin:creatinine ratio (ACR) values measured at months 3, 6, and 12 were lower than baseline for patients who achieved BP control (P<.05 for every time point). However, patients with noncontrolled BP showed a brief and unsustained reduction in ACR levels at month 6 (P<.05) compared with baseline levels (Figure 1D). Comparison between groups showed a fleeting significant difference in the magnitude of changes in ACR levels at month 6 (P=.0060) in patients with controlled BP (Figure 2D).
The percentage of patients who increased their eGFR in the group with controlled BP (panel A) and noncontrolled BP (panel B), taking into account their baseline eGFR dichotomized as <45 mL/min/1.73 m2 and ≥45 mL/min/1.73 m2, are shown in Figure 3.
Figure 3.
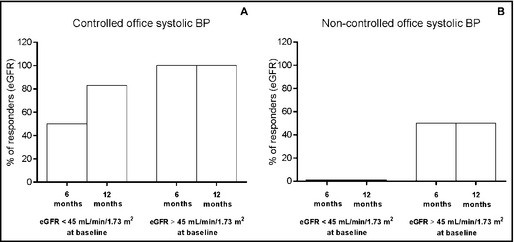
Percentage of cases who had increased estimated glomerular filtration rate (eGFR) (responders) at 6 and 12 months after renal sympathetic denervation stratified by baseline eGFR (< or ≥45 mL/min/1.73 m2) in patients with controlled blood pressure (BP; office systolic BP <140 mm Hg at month 12 [panel A, n=22]) and those with noncontrolled BP (panel B, n=5).
For patients in the lower baseline eGFR range (<45 mL/min/1.73 m2), 50% and 83% in the controlled BP group could be classified as responders at 6 and 12 months, respectively. In the noncontrolled BP group, correspondent values were 0% at both time points. Meanwhile, for patients in the higher eGFR range, 100% of patients in the controlled BP group were found to be responders either at month 6 or at month 12. In the noncontrolled BP group, correspondent figures were 50% for every time point.
Discussion
Hypertension affects the majority of patients with chronic renal disease and is one of the leading causes of end‐stage renal disease, besides being a major factor for progression of diabetic and nondiabetic renal disease.15 Recently, 68 of 88 (77%) patients were reported to have a reduction of at least 20 mm Hg in systolic BP at 36 months after RSD.6 In the present study, 96.3% of patients exhibited such a reduction after 12 months of RSD. Only one patient who underwent RSD had bleeding that required intervention at the puncture site of the femoral artery after the end of the procedure. The complication was resolved by mechanical compression, fluid infusion, and blood transfusion.
Based on the JNC report,14 patients were divided into controlled BP (81.5% of cases) and noncontrolled BP (18.5% of cases) groups using the systolic BP values at month 12. Interestingly, only patients in the controlled BP group showed a marked decrease in diastolic BP and ACR. Accordingly, only this group, which achieved normalization of BP, had substantially improved in eGFR after 12 months of follow‐up. Of note, 80% of the patients in the noncontrolled BP group were diabetic or took sympatholytic agents, potentially implicating these conditions in the reduced response to RSD.
Sympathetic activation is a hallmark of the essential hypertensive state occurring early in the clinical course of the disease.16, 17, 18 In CKD, the sympathetic overactivity appears to be manifested at the earliest clinical stage of the disease, being directly related to the severity of the renal failure state.19, 20, 21, 22 In both conditions, hypertension and renal failure, the mechanisms of the hyperadrenergic state are manifold and include reflex and neurohumoral pathways.16, 17, 21 The adrenergic activation displays an adverse impact on cardiovascular morbidity and, in the case of renal failure, also on cardiovascular mortality.16, 17, 22, 23 The interruption of this sympathetic hyperactivity and of the feedback loop of the renin‐angiotensin‐aldosterone system may, at least in part, account for our findings. RSD, a safe therapeutic option for patients with hypertension and CKD,7, 24, 25, 26 can reduce the level of renin activity, angiotensin II, and aldosterone in humans.27 The drop in systolic BP of −55 mm Hg and in diastolic BP of −22 mm Hg is similar to that reported for a 6‐month follow‐up period after RSD7 and confirms the long‐term effect of the procedure.
In the group with controlled BP and eGFR <45 mL/min/1.73 m2 at baseline, the percentage of patients who had an augmentation in their eGFR increased from month 6 (50%) to month 12 (83%), demonstrating a late improvement in eGFR. This finding contrasts with results found in patients with controlled BP in the higher range of baseline eGFR in whom 100% of cases already exhibited an increase in eGFR by month 6 of follow‐up. The data suggest that recovery of eGFR in patients with baseline eGFR <45 mL/min/1.73 m2 can still take place but after a longer period. It is conceivable that the early improvement in eGFR found in patients in the higher range of baseline eGFR is dependent on hemodynamic changes, whereas the late improvement found in patients in the lower range of eGFR can involve tissue remodeling.
BP control seemed to have a pivotal role in eGFR improvement given the absence of responders in patients in the lower range of baseline eGFR in the noncontrolled BP group. This view is supported even in patients in the higher range of baseline eGFR in whom the percentage of responders in the noncontrolled BP group was lower than in those of the controlled BP.
As mentioned before, some patients in the higher range of eGFR had improved eGFR despite not meeting the criteria of BP control outlined by JNC 8. When comparing the two patients who responded with the patients who did not, we observed that the mean office systolic BP at baseline was higher in the nonresponders (208.0±22.5 mm Hg vs 168.3±9.5 mm Hg, P=.0026). This finding leads us to conclude that BP levels at baseline can in some way function as a predictor of an increase in eGFR, but this was not a main focus of the present study.
Irrespective of the benefits that RSD might confer on the treatment of resistant hypertension, our findings suggest that the procedure may be useful in the management of CKD, which, by itself, carries a substantial economic burden.
Conclusions
Our results suggest that the control of BP by RSD in patients with refractory hypertension and CKD provides a highly significant reduction in systolic and diastolic BP, as well as in albuminuria, and an improvement in eGFR in the long‐term. Although encouraging, our data are preliminary and need to be validated in more patients.
Funding
This study was supported by the Programme of Development of the Hospital Regional Darcy Vargas.
Conflict of interest
None declared.
J Clin Hypertens(Greenwich). 2014;16:794–800. © 2014 Wiley Periodicals, Inc.
References
- 1. DiBona GF. Neural control of the kidney: past, present, and future. Hypertension. 2003;41:621–624. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 3. Schlaich MP, Sobotka PA, Krum H, et al. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension. 2009;54:1195–1201. [DOI] [PubMed] [Google Scholar]
- 4. Mahfoud F, Lüscher TF, Andersson B, et al. Expert consensus document from the European Society of Cardiology on catheter‐based renal denervation. Eur Heart J. 2013;34:2149–2157. [DOI] [PubMed] [Google Scholar]
- 5. Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol. 2013;62:2031–2045. [DOI] [PubMed] [Google Scholar]
- 6. Krum H, Schlaich MP, Böhm M, et al. Percutaneous renal denervation in patients with treatment‐resistant hypertension: final 3‐year report of the Symplicity HTN‐1 study. Lancet. 2014;383:622–629. [DOI] [PubMed] [Google Scholar]
- 7. Kiuchi MG, Maia GL, de Queiroz Carreira MA, et al. Effects of renal denervation with a standard irrigated cardiac ablation catheter on blood pressure and renal function in patients with chronic kidney disease and resistant hypertension. Eur Heart J. 2013;34:2114–2121. [DOI] [PubMed] [Google Scholar]
- 8. Levey AS, Stevens LA, Schmid CH, et al. CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 10. National High Blood Pressure Education Program . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute (US); 2004. Report No.: 04‐5230. [PubMed] [Google Scholar]
- 11. Sociedade Brasileira de Cardiologia, Sociedade Brasileira de Hipertensão, Sociedade Brasileira de Nefrologia . VI Diretrizes Brasileiras de Hipertensão. Arq Bras Cardiol. 2010; 95: 1–51. [PubMed] [Google Scholar]
- 12. Merten GJ, Burgess WP, Rittase RA, et al. Prevention of contrast‐induced nephropathy with sodium bicarbonate: an evidence‐based protocol. Crit Path Cardiol. 2004;3:138–143. [DOI] [PubMed] [Google Scholar]
- 13. ten Dam MA, Wetzels JF. Toxicity of contrast media: an update. Neth J Med. 2008;66:416–422. [PubMed] [Google Scholar]
- 14. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 15. Hausberg M, Hillebrandt U, Lang D, et al. Sympathetic overactivity in patients with chronic renal failure – the culprit of increased cardiovascular mortality? Curr Hypertens Rev. 2006;2:297–300. [Google Scholar]
- 16. Grassi G. Sympathetic neural activity in hypertension and related diseases. Am J Hypertens. 2010;23:1052–1060. [DOI] [PubMed] [Google Scholar]
- 17. Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–697. [DOI] [PubMed] [Google Scholar]
- 18. Paton JF, Raizada MK. Neurogenic hypertension. Exp Physiol. 2010;95:569–571. [DOI] [PubMed] [Google Scholar]
- 19. Schlaich MP, Socratous F, Hennebry S, et al. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933–939. [DOI] [PubMed] [Google Scholar]
- 20. Neumann J, Ligtenberg G, Klein II, et al. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. 2004;65:1568–1576. [DOI] [PubMed] [Google Scholar]
- 21. McGrath BP, Ledingham JG, Benedict CR. Catecholamines in peripheral venous plasma in patients on chronic haemodialysis. Clin Sci Mol Med. 1978;55:89–96. [DOI] [PubMed] [Google Scholar]
- 22. Grassi G, Bertolli S, Seravalle G. Sympathetic nervous system: role in hypertension and in chronic kidney disease. Curr Opin Nephrol Hypertens. 2012;21:46–51. [DOI] [PubMed] [Google Scholar]
- 23. Zoccali C, Mallamaci F, Parlongo S, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with endstage renal disease. Circulation. 2002;105:1354–1359. [DOI] [PubMed] [Google Scholar]
- 24. Hering D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schlaich MP, Bart B, Hering D, et al. Feasibility of catheter‐based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end‐stage renal disease. Int J Cardiol. 2013;168:2214–2220. [DOI] [PubMed] [Google Scholar]
- 26. Luo D, Zhang X, Lu CZ. Renal sympathetic denervation for the treatment of resistant hypertension with chronic renal failure: first‐in‐man experience. Chin Med J. 2013;12:1392–1393. [PubMed] [Google Scholar]
- 27. Wang L, Lu CZ, Zhang X, et al. The effect of catheter based renal sympathetic denervation on renin‐angiotensin‐aldosterone system in patients with resistant hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2013;41:3–7. [PubMed] [Google Scholar]


