To the Editor:
A 45‐year‐old Caucasian man in hypertensive crisis was referred to our hospital with general malaise, cephalalgia, and chest pain (blood pressure [BP], 210/100 mm Hg). The patient's medical history included never‐treated hypertension, dyslipidemia, and smoking. At admission, the patient was oriented and alert. Results from physical examination were normal and the patient was in hemodynamic balance. The abdomen auscultation revealed a periumbilical bruit. Findings from resting electrocardiography showed signs of left ventricular hypertrophy and negative T‐wave changes in V4–V6. Laboratory findings showed a modest increase in troponin Ths (56 ng/L) and no other significant alterations. Findings from 2‐dimensional echocardiography showed concentric hypertrophy of the left ventricle, with no decrease of left ventricular contraction and with a conserved ejection fraction (0.50). Color Doppler analysis showed normal function of all heart valves. To correctly study the renin‐angiotensin‐aldosterone axis and noradrenergic system and to exclude secondary hypertension, the patient was initially treated with α‐blockers only.
All bioassay results were in the high‐normal range according to the presence of high BP.
Therapy was later modified and implemented with calcium antagonists, angiotensin‐converting enzyme (ACE) inhibitors, furosemide, and α‐ and β‐blockers without reaching adequate BP control (160/90 mm Hg).
Subsequent findings from computed tomography angiography of the abdomen showed a significant hemodynamic stenosis at the origin of the left kidney artery of 10 mm, normal contralateral renal artery, and no evidence of adrenal or renal parenchyma tumefactions (Figure 1).
Figure 1.
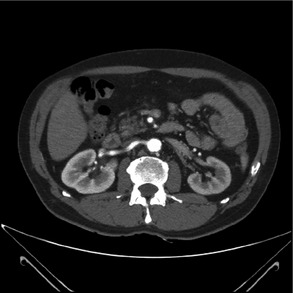
Computed tomographic angiography revealing a significant hemodynamic stenosis at the origin of the left kidney artery.
After case assessment by the radiologists and collegial discussion, it was decided to treat the renal artery stenosis (RAS).
Findings from angiography of the renal arteries confirmed the stenosis very tightened to the origin of the left renal artery. The attempt of crossing the stenosis via intraluminal procedure using a 0.014‐inch guidewire and a microcatheter failed. Following multiple subintimal passages, the renal artery dissection at the end of the stenotic occlusion was reported, and the procedure was stopped.
The patient was discharged, and, after 2 months, a new right transfemoral arteriography and an aortoiliac angiography were performed. The evidence was a revascularizated left kidney from the left spermatic artery, which was hypertrophic and with inverted flow. After several failed attempts of crossing the obstruction, it was decided to perform the procedure with retrograde access: the occlusion was crossed using a 0.035‐inch guidewire that was rescued in the aorta and via anterograde procedure and, after predilation, the premounted stent on a 6×18 mm balloon was positioned (Figure 2).
Figure 2.
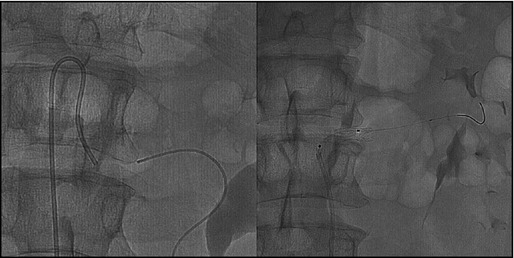
Renal artery angiography showing catheter position during interventional percutaneous transluminal coronary angioplasty of renal artery.
Following the procedure, the patient underwent treatment with 5 antihypertensive agents, ie, ACE inhibitors, diuretics, calcium antagonists, and α‐ and β‐blockers, and aspirin 100 mg/d was added to obtain good BP control (130/80 mm Hg).
Hypertension induced by RAS is a form of secondary hypertension caused by renin overproduction,1 and atherosclerotic disease is the most common cause.2 It is known that RAS affects 1% to 5% of hypertensive patients.3 Furthermore, many studies have shown an elevated prevalence of RAS in patients with coronary artery disease diagnosed by cardiac catheterization.4, 5, 6
Angiography is the gold standard for the diagnosis of RAS,7 and the most common technique uses a reverse‐curve catheter that advances cephalic from below the renal artery until it engages the renal artery ostium.
The specificity of this case was the unusual angiographic approach, ie, a retrograde access from the spermatic artery, due to the unavailability of the standard access. According to our knowledge, this approach is rarely used; therefore, information about the results is limited. In our patient, the results produced a complete resolution of RAS, as well as optimal BP control, with no procedural complications.
References
- 1. Piecha G, Wiecek A, Januszewicz A. Epidemiology and optimal management in patients with renal artery stenosis. J Nephrol. 2012;25:872–878. [DOI] [PubMed] [Google Scholar]
- 2. Carr TM 3rd, Sabri SS, Turba UC, et al. Stenting for atherosclerotic renal artery stenosis. Tech Vasc Interv Radiol. 2010;13:134–145. [DOI] [PubMed] [Google Scholar]
- 3. White CJ. Catheter‐based therapy for atherosclerotic renal artery stenosis. Prog Cardiovasc Dis. 2007;50:136–50. [DOI] [PubMed] [Google Scholar]
- 4. Dzielinska Z, Januszewicz A, Demkow M, et al. Cardiovascular risk factors in hypertensive patients with coronary artery disease and coexisting renal artery stenosis. J Hypertens. 2007;25:663–670. [DOI] [PubMed] [Google Scholar]
- 5. Liang F, Hu DY, Wu MY, et al. The incidence of renal artery stenosis in the patients referred for coronary artery bypass grafting. Indian J Nephrol. 2012;22:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mancia G, Fagard R, Narkiewicz K, et al. The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2013 ESH/ESC Guidelines for the management of arterial hypertension. J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 7. Boateng FK, Greco AB. Renal artery stenosis: prevalence of, risk factors for, and management of in‐stent stenosis. Am J Kidney Dis. 2011;61:147–160. [DOI] [PubMed] [Google Scholar]


