Abstract
Orthostatic hypotension (OH) is a relatively common heterogeneous and multifactorial disorder often caused by autonomic dysfunction. This condition has a deleterious impact on quality of life and contributes to higher mortality rates. Supine hypertension is very common in patients with autonomic failure, limits the use of pressor agents, and can result in end‐organ damage. Current recommendations on the optimal management of these patients are based on expert opinion and poor‐quality small cross‐sectional studies including patients with primary autonomic failure and severe orthostatic hypotension. The authors present their treatment approach in 12 patients with disabling orthostatic hypotension and supine hypertension not related to primary autonomic failure, presenting to a referral center over a 4‐year period. The first step is to educate the patient about the pathophysiology and course of their disorder. Nonpharmacologic therapies and maneuvers are usually effective in relieving symptoms and preventing syncope. If needed, pharmacologic options such as fludrocortisones and midodrine are also available in patients with severe symptoms. Supine hypertension represents a challenge in the treatment of this condition. Therefore, elevation of the bed of the head and dosing of short‐acting antihypertensive agents at bedtime is often indicated.
Etiologies of orthostatic hypotension are classified, generally, as mechanisms of either autonomic failure, volume depletion, or neurally mediated (vasovagal) reflex syncope. Among these pathophysiologic categories, autonomic failure represents the most complex and poorly understood array of disorders causing orthostatic hypotension because they vary greatly in both presentation and management.1
Causes of autonomic failure contributing to orthostatic hypotension can be divided into neurodegenerative diseases, ie, multiple system atrophy, and neuropathies, ie, diabetic neuropathy. Another less common etiology is associated with direct injury to the carotid sinus either secondary to stenting or angioplasty or neck irradiation for thyroid or other malignancies.
Nestled in the adventitia of the carotid body and aortic arch are mechano‐sensitive terminals of the vagus and glossopharyngeal nerves that project to the nucleus tractus solitarius (NTS) in the caudal medulla. In response to postural changes, efferent projections from the NTS to sympathetic and parasympathetic preganglionic neurons in the brain and spinal cord govern acute fluctuations in heart rate (HR) and blood pressure (BP). Impaired afferent signaling of this arterial baroreceptor reflex arc in humans causes a sustained increase in mean arterial pressure lability, often leading to both paroxysms of hypercatecholaminergic tone that resemble pheochromocytoma and contrasting episodes of profound orthostatic hypotension. In 1996, a small study by Shapiro and colleagues2 found that lightheadedness and defective cardiovascular reflexes after neck radiotherapy in patients with tongue and neck cancers may have been attributable to baroreceptor damage but was unlikely to be apparent on routine cardiovascular testing.
Since 1996, a myriad of case reports of cardiovascular dysautonomia have been linked to carotid sinus dysfunction occurring from both neck irradiation therapy and advanced bilateral carotid artery stenosis. Importantly, these particular etiologies do not respond to the traditional management that many physicians are accustomed to rely on when caring for patients whose autonomic failure is caused by centrally mediated dysfunction. Therefore, orthostatic hypotension that arises secondary to advanced diabetic peripheral neuropathy or trauma‐induced baroreceptor failure is commonly refractory to traditional therapies and leads to severely diminished quality of life with greater risk for cardiovascular morbidity and mortality.
Despite a rising prevalence of this relatively uncommon subclass of patients with dysautonomia, no uniform guidelines exist to manage patients.
Table 1 summarizes the characteristics of 12 patients from the American Society of Hypertension (ASH) Comprehensive Hypertension Center at the University of Chicago who were referred for management of orthostatic hypotension and supine hypertension related to carotid sinus dysfunction. The paper presents two detailed representative cases to illustrate separate etiologies and differences in therapeutic approach to reduce BP variability. Unlike previous reports, we studied patients without evidence of primary autonomic failure but with common comorbidities such as advanced age, hypertension, and diabetes.
Table 1.
Characteristics of Patient Cases With Orthostatic Hypotension and Supine Hypertension Related to Baroreceptor Dysfunction
| Case | Age, y | Sex | Follow‐Up, mo | Cause of Baroreceptor Dysfunction | Pharmacologic Interventions | Nonpharmacologic Interventionsa | α‐Agonists | Fludrocortisone | Mean Home BPs/HR at Baseline (Supine/Standing) | Mean Home BPs/HR at Last Visit (Supine/Standing) |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 84 | M | 1 | Neck radiation therapy for laryngeal cancer and advanced bilateral carotid stenosis | Atenolol 25 mg BID and isradipine 5 mg at bedtime | + | – | − | 167/67/65 | 145/65/60 |
| 94/62/61 | 117/55/58 | |||||||||
| Case 2 | 40 | F | 12 | Uncontrolled diabetes mellitus | Verapamil 240 mg at bedtime | + | Midodrine 5 mg in am | − | 165/80/89 | 120/70/77 |
| 90/65/92 | 105/65/74 | |||||||||
| Case 3 | 63 | M | 14 | Neck radiation therapy for tongue cancer | Atenolol 25 mg BID and isradipine 5 mg at bedtime | + | – | − | 146/91//57 | 136/86/58 |
| 97/63/60 | 104/84/62 | |||||||||
| Case 4 | 74 | F | 12 | Advanced bilateral carotid stenosis | Atenolol 100 mg at night and nicardipine 40 mg at bedtime and 20 mg in the am | + | – | − | 162/90/77 | 155/85/70 |
| 110/76/75 | 147/70/70 | |||||||||
| Case 5 | 84 | M | 1 | Advanced bilateral carotid stenosis | Atenolol 100 mg at night and nicardipine 40 mg at bedtime | − | – | − | 180/80/75 | 160/92/68 |
| 80/50/80 | 120/70/67 | |||||||||
| Case 6 | 82 | M | 12 | Neck radiation therapy for head and neck cancer | None | + | Midodrine 5 mg in am as needed | − | 130/68/62 | 122/66/60 |
| 86/58/64 | 104/60/61 | |||||||||
| Case 7 | 63 | M | 12 | Neck radiation therapy for tongue cancer | Atenolol 50 mg at bedtime | + | – | + | 185/105/62 | 152/82/58 |
| 155/76/68 | 146/80/60 | |||||||||
| Case 8 | 83 | M | 12 | Diabetes | Clonidine 0.1 mg at bedtime | + | Midodrine 5 mg in am | − | 140/85/62 | 105/65/58 |
| 110/70//62 | 100/62/57 | |||||||||
| Case 9 | 65 | F | 8 | Diabetes | Verapamil 240 mg and guanfacine 1 mg daily | + | – | − | 154/70/86 | 142/60/80 |
| 100/48/89 | 118/54/82 | |||||||||
| Case 10 | 65 | M | 36 | Neck radiation therapy for head and neck cancer | Atenolol 50 mg at bedtime and guanfacine 2 mg at bedtime | + | – | − | 156/80/84 | 145/77/56 |
| 128/60/82 | 123/67/60 | |||||||||
| Case 11 | 50 | M | 1 | Neck radiation therapy for head and neck cancer | Nebivolol 10 mg qam and 5 mg qpm with ramipril 5 mg BID | + | – | + | 136/86/85 | 128/80/80 |
| 110/75/95 | 118/75/87 | |||||||||
| Case 12 | 58 | F | 24 | Multiple sclerosis | Atenolol 50 mg and nicardipine 20 mg at night | + | – | − | 185/95/58 | 143/75/53 |
| 85/55/65 | 110/65/56 |
Abbreviations: BID, twice a day; BP, blood pressure; F, female; HR, heart rate; M, male.
Nonpharmacological interventions include: moderate sodium intake (2500‐5000 mg/d), predominantly before noon on a given day, use of support stocking to thighs (25‐30 mmHg), and when needed sleeping with head/neck elevated to 15‐20 degree angle.
Case Reviews
We present a review of 12 orthostatic hypotension cases with supine hypertension not associated with neurodegenerative diseases or synucleinopathies managed at the University of Chicago Medicine (UCMC) between 2009 and 2013. Table 1 briefly summarizes their presentation and management. A standardized treatment approach including nonpharmacologic and pharmacologic interventions was followed (Figure 1). The goals of care are the following: (1) symptomatic relief with posture changes, (2) decrease of BP drop with standing position, (3) maintenance of intravascular volume, (4) treatment of supine hypertension and prevention of end‐organ damage, and (5) patient education. As shown in Table 2 and Figure 2 and Figure 3, treatment measures were effective in significantly decreasing supine and increasing orthostatic BP for all patients independent of the underlying condition. Two representative cases are presented below.
Figure 1.
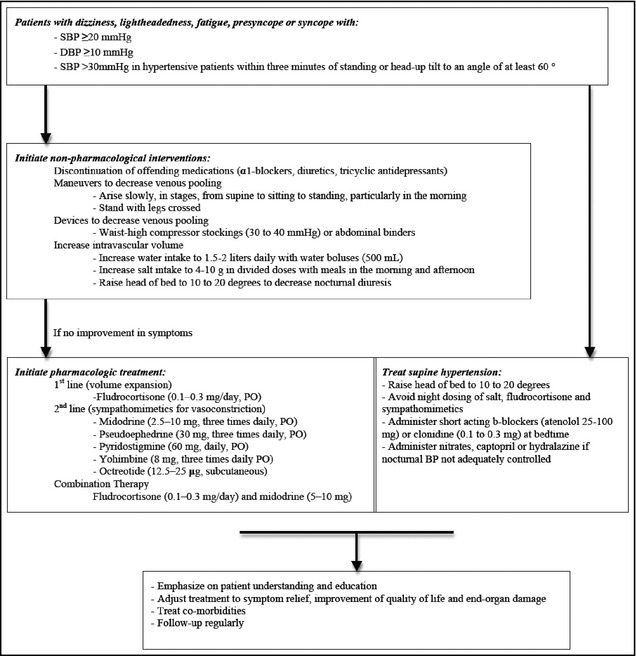
Treatment approach to patients with orthostatic hypotension with supine hypertension. The goal is to reduce delta blood pressure (BP) between supine and standing to <30 to 40 mm Hg and eliminate or reduce symptoms upon standing. Step 1: If symptomatic upon sitting or standing with significant BP drop >30 mm Hg (a) use moderate thigh‐high support stocking (about 30–35 mm Hg) or (b) use a teaspoon of salt with an intermediate β‐blocker, ie, atenolol or longer‐acting central α‐agonist, ie, such as guanfacine at bedtime if BP >150 mm Hg supine and heart rate >72 beats per minute. Consider nicardipine 20 mg if heart rate low. Step 2: If the above fail, add low‐dose fluoridated steroids daily. Step 3: Add midodrine 5 mg in the morning and perhaps another dose in early afternoon, but avoid titration too quickly as it can cause hypertension. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Table 2.
Effects of Selected Treatment Approach on Supine and Orthostatic BP and HR of Studied Patients
| Variables | Median (Minimum–Maximum) | Wilcoxon Signed‐Rank P Value |
|---|---|---|
| Age, y | 65 (40–84) | |
| Follow‐up, mo | 12 (1–36) | |
| Supine SBP baseline, mm Hg | 159 (130–185) | .003 |
| Supine SBP follow‐up, mm Hg | 143 (105–160) | |
| Supine DBP baseline, mm Hg | 82.5 (67–105) | .026 |
| Supine DBP follow‐up, mm Hg | 75 (60–92) | |
| Orthostatic SBP baseline, mm Hg | 98.5 (80–155) | .026 |
| Orthostatic SBP follow‐up, mm Hg | 117 (100–147) | |
| Orthostatic DBP baseline, mm Hg | 62.5 (48–76) | .241 |
| Orthostatic DBP follow‐up, mm Hg | 65 (54–84) | |
| Supine HR baseline, beats per min | 70 (57–89) | .05 |
| Supine HR follow‐up, beats per min | 71.5 (60–95) | |
| Orthostatic HR baseline, beats per min | 60 (53–80) | .259 |
| Orthostatic HR follow‐up, beats per min | 61 (56–82) |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
Figure 2.
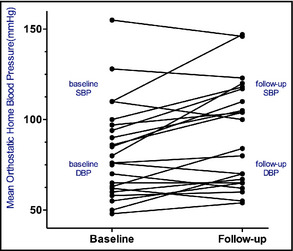
Mean standing home blood pressure.
Figure 3.

Mean supine home blood pressure.
Case 1: Orthostatic Hypotension in a Patient With Poor Glycemic Control
A 40‐year‐old black woman with a 20‐year history of uncontrolled type 1 diabetes and documented peripheral neuropathy presented to the UCMC emergency department (ED) complaining of a 1‐week history of worsening lightheadedness and near‐syncope upon standing. Associated symptoms included blurred vision, nausea, and headache, all of which resolved upon sitting or supine posture. Following a recent episode, the patient's home BP measurement was 86/53 mm Hg. This resulted in discontinuation of her antihypertensive regimen consisting of losartan‐hydrochlorothiazide 100/25 mg daily. Her diabetes was poorly controlled despite appropriate insulin dosing using a pump. BPs measured in the ED were 198/81 mm Hg supine, 169/92 mm Hg sitting, and 134/74 mm Hg standing. HR remained steady at 88 bpm on all measurements. The patient's orthostatic hypotension was primarily caused by autonomic dysfunction secondary to known diabetic peripheral neuropathy in the context of poorly controlled diabetes, and complicated by hypovolemia associated with decreased oral intake and recent emesis. The patient was volume‐repleted with 3L of normal saline in the ED and given clonidine 0.2 mg by mouth at night. The decision to use a β‐blocker was avoided because of known history of asthma. Instead, she was given 180 mg verapamil qHS with minimal concern for bradycardia. She was discharged on this regimen with instructions to take two salt tablets in the morning and wear abdominal binders and compression stockings while upright. However, she continued to have recurrent episodes, with multiple ED visits in the ensuing weeks for orthostatic hypotension and lower extremity edema. Her regimen was then changed to midodrine 5 mg in the morning and clonidine 0.2 mg and verapamil 120 mg at night. This regimen was further modified to midodrine 5 mg 3 times a day, verapamil 180 at night, and clonidine 0.2 mg at bedtime after another episode occurred 1 month later with associated sitting BP 160/80 mm Hg, HR 90 bpm, and standing HR 100 to 110/70 to 75 mm Hg 95 bpm. She was accordingly instructed to maintain a low‐sodium diet of <2400 mg/d, monitor BP multiple times per day, and present these values 3 days later at a follow‐up clinic appointment that revealed better control with midodrine. However, she remained orthostatic with a sitting BP of 160/80 mm Hg, HR 90 bpm, and standing BP 130 to 140/70 to 75 mm Hg, HR 95 bpm. Because she remained hypertensive during sleep and at morning readings, we reduced midodrine to 5 mg twice a day, increased verapamil to 240 qHS, and switched her evening central sympatholytic to the more α2 selective agent guanfacine 2 mg as needed. Following these adjustments, she had fewer orthostatic episodes. One month following treatment initiation, she remained orthostatic; however, sitting BP improved to 120/70 mm Hg at 90 bpm, with a standing BP of 100 to 110/70 to 75 mm Hg at 95 bpm. The patient had independently discontinued her evening guanfacine because of daytime sleepiness and dry mouth. We reduced her noon midodrine dose from 5 mg to 2.5 mg and directed her to augment sodium intake to 4 g at both breakfast and lunch. Following these adjustments, the patient was instructed to measure morning pre‐medication BPs for the ensuing 2 weeks. Upon review of these values, the patient was no longer orthostatic. Mean home BPs were found to be 120/70 mm Hg while standing, with HR 77 bpm, and 105/65 mm Hg while sitting, with HR 74 bpm.
Case 2: Orthostatic Hypotension Following Neck Irradiation
A 63‐year‐old man diagnosed with tongue and neck cancer who received chemotherapy and radiation therapy following a neck dissection in 2001 was referred to the ASH Comprehensive Hypertension Center at the UCMC for consultation on refractory BP lability. Prior to radiation therapy, baseline BPs averaged 130/80 mm Hg. Within months following neck irradiation, he noticed increased variability of his systolic BP (SBP), although he remained generally normotensive. Approximately 1 year later, he was started on fludrocortisone 0.1 mg after a positive result on tilt‐table test. In 2009, he began to experience occasional symptoms of blurred vision and headache with SBP ranging from 90 mm Hg to 200 mm Hg. The patient complained of recently worsening episodes of chest pain, headache, dry mouth, and blurry vision. At our clinic, his BP was 170/100 mm Hg and he reported SBPs ranging from 50 mm Hg to 200 mm Hg. Neither his symptoms nor his pressures were related to body posture, head rotation, or diet. In addition to fludrocortisone, the patient's antihypertensive regimen included toprol 50 mg to be used specifically when SBP exceeded 200 mm Hg. Orthostatic measurements in the office demonstrated a supine BP of 185/105 mm Hg with an HR of 62 bpm and a standing BP of 155/76 mm Hg with an HR of 68 bpm. Recent available chemistry showed that levels of electrolytes and kidney function were normal, allowing us to rule out adrenal pathology as a possible secondary source. We therefore assessed that this patient's autonomic dysregulation was attributable to bilateral carotid body tissue damage with subsequent baroreceptor failure resulting from trauma related to neck irradiation. Fludrocortisone was discontinued and the patient was placed on a regimen of atenolol 50 mg at bedtime. The patient was further instructed to record a log of BP measurements taken in the supine and standing positions at 2‐minute intervals upon awakening. At a 2‐week follow‐up appointment, mean home BPs were found to range from 130 to 220/70 to 120 mm Hg supine with an HR of 70 to 100 beats per minute, 110 to 120/60 to 100 mm Hg sitting with an HR of 70 to 110 bpm, and 50 to 190/60 to 80 mm Hg sitting with an HR of 70 to 80 bpm. Based on these results, he was given verapamil 180 mg and metoprolol 200 mg at bedtime and midodrine 5 mg in the morning as needed. However, within 4 months, the midodrine was causing hypertension tension during the day and made BP more difficult to control, hence it was stopped. Upon awakening, he was to wear thigh‐high compression stockings (30 mm Hg), keep his legs elevated while seated, and increase salt intake to 4000 mg/d focused between 8 am and noon. After approximately 1 year taking this regimen, the patient was found to have dramatically reduced BP fluctuations with little to no symptomatic orthostasis and a mean home supine BP of 152/82 mm Hg with an HR of 58 bpm and standing BP of 146/60 mm Hg with an HR of 60 bpm.
Discussion
Rapid cardiovascular adaptations activated by upright posture are regulated by the autonomic nervous system. In healthy individuals, standing activates afferent autonomic neural pathways to induce baroreceptor unloading, and subsequent increases in efferent sympathetic outflow and vasoconstriction, to increase venous return and maintain resting BP.3 Autonomic dysfunction leads to impairment of these compensatory mechanisms and clinically results in orthostatic hypotension, defined as a reduction in SBP ≥20 mm Hg or diastolic BP ≥10 mm Hg within 3 minutes of standing or head‐up tilt to an angle of at least 60°.4 In hypertensive patients, a reduction of SBP >30 mm Hg is more appropriate.
Chronic orthostatic hypotension often with supine hypertension is a condition that commonly affects the elderly and frequently causes disabling presyncopal symptoms, syncope, and impaired quality of life. In many patients, orthostatic hypotension with supine hypertension can remain undetected for long periods because BP is often measured only in the seated position when it can be normal. Supine hypertension is frequently present in patients with autonomic dysfunction possibly because of desensitization of baroreflex, inappropriate natriuresis, increased intravascular volume, and residual sympathetic tone.5
The diagnosis can be easily performed at the bedside by measuring BP and HR in the patient while in the supine position after minimally 5 minutes of rest, as well as between 1 and 3 minutes after standing. The test is required in all who experience syncope/presyncope or signs and/or symptoms of cerebral hypoperfusion such as dizziness and lightheadedness (especially in the morning). The diagnosis of orthostatic hypotension may require multiple measurements on different days, and maintenance of a BP diary with recordings of supine and standing BP in the morning and after particular activities (medication intake, meals, exercise). Symptoms are frequently worse on awakening because of nighttime pressure natriuresis. Therefore, morning orthostatic measurements are more sensitive to detect orthostatic hypotension. Ambulatory automated BP monitoring may be useful in the detection of supine hypertension.
In addition to unpleasant and disabling symptoms, orthostatic hypotension is associated with an increased risk of falls, cognitive dysfunction,6 coronary arterial disease,7 chronic kidney disease,8 stroke,9 and cardiovascular and all‐cause mortality.10, 11 The mechanisms behind them are unclear, and whether orthostatic hypotension is a cause or consequence of comorbidities is still uncertain. Orthostatic hypotension is associated with nondipping or reverse‐dipping pattern of diurnal BP, while the cardiovascular risk remained even after adjustment for such diurnal BP patterns.12
Once orthostatic hypotension is diagnosed, clinicians should take the patient's medical history and physical examination carefully. Certain medications such as antihypertensive drugs (eg, α‐blockers, diuretics, and vasodilators) and antidepressants, and changes of daily life (eg, dehydration, weight loss, diet, infection, stress, sleep problems) are common causes of orthostatic hypotension. Assessments of other manifestations of autonomic neuropathy or neurodegenerative diseases can be helpful in the diagnosis of neurogenic orthostatic hypotension. Laboratory tests such as anemia, glucose, electrolyte, renal function, hormones (eg, thyroid and adrenal), and proteinuria might be helpful.
Treatment
The primary target of treating orthostatic hypotension is to improve the patient's functional status by ameliorating symptoms, reducing the risk of falls, and preventing end‐organ damage by supine hypertension. Achievement of BP targets used for the general population should not be one of the primary goals of treatment.
Nonpharmacologic measures are important components of therapy of orthostatic hypotension. These include removal of offending medications, patient education to avoid precipitating factors, physical and dietary interventions, and patient education. Recognition and removal of drugs, which can cause orthostatic hypotension, is crucial. The most common offending medications are diuretics, α‐adrenergic antagonists, and antidepressants. Patients should also be instructed to (1) maintain appropriate hydration throughout the day by drinking at least 1.5 L/d to 2 L/d of water during meals and before exercise and also rapid intake of water in the morning before getting out of bed; (2) arise slowly, in stages, from supine to sitting to standing, particularly in the morning when orthostatic hypotension is more pronounced; (3) avoid activities that reduce venous return such as walking in hot weather, or straining; (4) elevate the head of the bed 10° to 20° to decrease nocturnal diuresis and maintain intravascular volume; (5) prevent episodes of postprandial hypotension by avoiding large meals, minimizing alcohol intake, and avoiding standing immediately after eating; and (6) perform leg‐crossing maneuvers while actively standing to increase cardiac output and systemic BP.13
The use of compression stockings that produce at least 20‐ to 25‐mm Hg pressure or tight abdominal binders permits the application of graded pressure to the lower extremities and lower abdomen, thereby minimizing peripheral blood pooling in lower extremity and splanchnic circulation. It is essential that such stockings extend above the knees to the waist since most peripheral pooling occurs in the splanchnic circulation. Many patients, particularly those with peripheral neuropathies or those living in hot climates, poorly tolerate compression stockings. The efficacy of compression stockings and abdominal binders was validated in a small crossover study that showed reduced orthostatic BP decrease and symptoms compared with controls.14
Increased salt and water intake are essential parts of treatment. Rapid ingestion of 500 cc of water in <5 minutes can serve as a therapeutic measure in symptomatic patients. The BP effect is observed in the first 5 to 10 minutes and peaks around 30 minutes after ingestion and is mediated by a sympathetic reflex rather than a volume effect.15 Patients should be instructed to consume high‐sodium–containing foods. Salt tablets may also be prescribed. While the optimal dose will vary among patients, a target dose of 6 g/d to 10 g/d of sodium taken with breakfast and lunch, or a target urinary sodium level of 150 mEq to 200 mEq should be maintained. Night dosing of salt intake should be minimized to avoid worsening of supine hypertension.
In patients with more severe orthostatic hypotension refractory to nonpharmacologic treatments, pharmacologic interventions may be needed. Patients initiated on pharmacologic treatment should be instructed in BP recording and should provide to the clinician, for monitoring, a series of BP recordings taken over several days including when supine, sitting, and standing upon awakening as well as postprandial and bedtime BP recordings.
The first line of therapy for patients without baseline hypertension or heart failure is fludrocortisone (9‐alpha‐fluorohydrocortisone) initiated at 0.1 mg/d administered in the morning and increased up to 0.3 mg/d, a potent synthetic mineralocorticoid that increases intravascular volume by promoting renal sodium reabsorption.16 Dose adjustment should not be performed more rapidly than bi‐weekly. The volume expansion effects of this drug last for the first few weeks, with long‐term effects attributed to sensitization of the vasculature to norepinephrine and angiotensin II. Little benefit is obtained by further dosage increase above 0.3 mg/d. Patients treated with fludrocortisone must be carefully monitored for the development of edema or worsening supine hypertension, which may necessitate discontinuation or dosage reduction. Potassium levels should be monitored carefully after initiation of treatment. A small observational study showed that discontinuation of fludrocortisone because of side effects occurs in one third of elderly patients on treatment.17
If symptoms and BP variability is not adequately controlled with fludrocortisone, a direct sympathomimetic pressor agent such as midodrine may be added. The peripheral selective α1‐adrenergic agonist midodrine is effective in the treatment of orthostatic hypotension as shown in a randomized trial of 162 patients with orthostatic hypotension. Administration of midodrine 10 mg 3 times per day was associated with greater improvement in lightheadedness and in standing SBP compared with that in a control group.18 Midodrine is a short‐acting agent with a plasma half‐life of 30 minutes. The dose may be titrated from 2.5 mg to 10 mg 3 times a day. The main advantage of midodrine is the synergistic effects and allowance for lower dosages shown when combined with fludrocortisone.19 Potential side effects include pruritus, supine hypertension, gastrointestinal symptoms, and urinary retention. Supine hypertension, especially, often limits therapeutic use of midodrine. Dosing of the medication in the morning upon awaking and in the afternoon, but not in the evening, and raising the head of the bed helps with supine hypertension. Sympathomimetic side effects do not occur with midodrine, as it does not cross the blood brain barrier.
Other available α1‐adrenoreceptor agonists such as ephedrine and pseudoephedrine have not been studied for use in patients with orthostatic hypotension; therefore, they are not frequently used. There have been single patient reports of their use in people with Parkinson's disease; however, these agents were not found to have a unique benefit over more traditional agents, and, given their propensity to increase HR in older patients with the lack of additional benefit, their use is not advised.20 Additional agents such as pyridostigmine, caffeine, and nonsteroidal anti‐inflammatory drugs can be used as second agents if fludrocortisone or midodrine are not tolerated or they do not help achieve adequate control of postural symptoms.
Concurrent treatment of supine hypertension is often required to prevent end‐organ damage but often renders more treatment. Antihypertensive medications may exacerbate orthostatic hypotension, while treatment of orthostatic hypotension may increase supine hypertension. In such patients, our approach is to maximize nonpharmacologic measures upon awaking and in the afternoon depending on the postural requirements, use intermediate‐acting β‐blockers (eg, atenolol) or short‐acting centrally acting α‐agonists such as clonidine titrated from 0.1 mg to 0.3 mg and dosed at bedtime. The addition of a 15° to 20° head tilt is also useful with these agents to lower pressure. Medications that raise the supine BP level such as fludrocortisone or midodrine should only be used to the degree that permits the patient to ambulate. It is uncertain what threshold of BP requires treatment in this setting. However, treatment should be targeted towards acceptable nocturnal BP control.
No treatment approach to supine hypertension has been extensively studied.21 Patients should be instructed to avoid lying down during the day; if tired, rest in a seated position, elevate the head of the bed at nighttime, and be extremely careful when arising at night, since orthostatic symptoms will be exacerbated. Treatment of supine hypertension with a transdermal nitroglycerin patch (0.025–0.1 mg/h), which is removed in the morning prior to the assumption of an upright position, has also been shown to be effective.5 Other short‐acting antihypertensive agents such as captopril and hydralazine may also be administered if treatment with short‐acting β‐blockers, clonidine, or nitrates is not tolerated or fails to control supine hypertension.
Our experience with patients who have orthostatic hypotension and supine hypertension, as presented above, suggests that patient education; strict adherence to nonpharmacologic measures with avoidance of offending agents, adequate hydration, high salt intake during the day; and use of compression stockings is usually effective in reducing orthostatic BP drop and improving symptoms. β‐Blockers and clonidine dosed at night are usually effective for treatment of supine hypertension to lenient BP control of <150/80 mm Hg and delay progression of end‐organ damage. Use of pharmacologic agents such as fludrocortisone or/and midodrine is warranted in patients with severe symptoms. Physicians should take into consideration age‐related comorbidities when selecting an appropriate therapeutic approach.
Conclusions
Our center experience suggests a therapeutic approach for patients with orthostatic hypotension and supine hypertension caused by autonomic failure. A number of reviews and guidelines have been published offering recommendations on the optimal management of these patients. These are, however, based on small cross‐sectional trials with poor quality and high risk of bias as indicated by a recent systematic review.2 Changes in postural BP drop and symptoms are frequently inconsistent and recruited patients often experience primary autonomic failure and severe orthostatic hypotension. Thus, these findings do not necessarily clinically reflect the more common cases, such as elderly with multiple comorbidities and those with diabetes, as the ones included in our cohort. Thus, a stepwise care approach based on individual comorbidities and emphasizing nonpharmacologic measures is warranted and physicians should share such an approach.
J Clin Hypertens (Greenwich). 2014;16:141–148. DOI: 10.1111/jch.12258. ©2014 Wiley Periodicals, Inc.
References
- 1. Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. 2007;120:841–847. [DOI] [PubMed] [Google Scholar]
- 2. Shapiro MH, Ruiz‐Ramon P, Fainman C, Ziegler MG. Light‐headedness and defective cardiovascular reflexes after neck radiotherapy. Blood Press Monit. 1996;1:81–85. [PubMed] [Google Scholar]
- 3. Smit AA, Halliwill JR, Low PA, Wieling W. Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol. 1999;519(Pt 1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46–48. [DOI] [PubMed] [Google Scholar]
- 5. Shannon J, Jordan J, Costa F, et al. The hypertension of autonomic failure and its treatment. Hypertension. 1997;30:1062–1067. [DOI] [PubMed] [Google Scholar]
- 6. Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108:106–111. [DOI] [PubMed] [Google Scholar]
- 7. Fedorowski A, Stavenow L, Hedblad B, et al. Orthostatic hypotension predicts all‐cause mortality and coronary events in middle‐aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franceschini N, Rose KM, Astor BC, et al. Orthostatic hypotension and incident chronic kidney disease: the atherosclerosis risk in communities study. Hypertension. 2010;56:1054–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eigenbrodt ML, Rose KM, Couper DJ, et al. Orthostatic hypotension as a risk factor for stroke: the atherosclerosis risk in communities (ARIC) study, 1987–1996. Stroke. 2000;31:2307–2313. [DOI] [PubMed] [Google Scholar]
- 10. Rose KM, Eigenbrodt ML, Biga RL, et al. Orthostatic hypotension predicts mortality in middle‐aged adults: the Atherosclerosis Risk In Communities (ARIC) Study. Circulation. 2006;114:630–636. [DOI] [PubMed] [Google Scholar]
- 11. Masaki KH, Schatz IJ, Burchfiel CM, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290–2295. [DOI] [PubMed] [Google Scholar]
- 12. Fagard RH, De CP. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension. 2010;56:56–61. [DOI] [PubMed] [Google Scholar]
- 13. Krediet CT, van Lieshout JJ, Bogert LW, et al. Leg crossing improves orthostatic tolerance in healthy subjects: a placebo‐controlled crossover study. Am J Physiol Heart Circ Physiol. 2006;291:H1768–H1772. [DOI] [PubMed] [Google Scholar]
- 14. Podoleanu C, Maggi R, Brignole M, et al. Lower limb and abdominal compression bandages prevent progressive orthostatic hypotension in elderly persons: a randomized single‐blind controlled study. J Am Coll Cardiol. 2006;48:1425–1432. [DOI] [PubMed] [Google Scholar]
- 15. Shannon JR, Diedrich A, Biaggioni I, et al. Water drinking as a treatment for orthostatic syndromes. Am J Med. 2002;112:355–360. [DOI] [PubMed] [Google Scholar]
- 16. Chobanian AV, Volicer L, Tifft CP, et al. Mineralocorticoid‐induced hypertension in patients with orthostatic hypotension. N Engl J Med. 1979;301:68–73. [DOI] [PubMed] [Google Scholar]
- 17. Hussain RM, McIntosh SJ, Lawson J, Kenny RA. Fludrocortisone in the treatment of hypotensive disorders in the elderly. Heart. 1996;76:507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Low PA, Gilden JL, Freeman R, et al. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double‐blind multicenter study. Midodrine Study Group. JAMA. 1997;277:1046–1051. [PubMed] [Google Scholar]
- 19. Kaufmann H, Brannan T, Krakoff L, et al. Treatment of orthostatic hypotension due to autonomic failure with a peripheral alpha‐adrenergic agonist (midodrine). Neurology. 1988;38:951–956. [DOI] [PubMed] [Google Scholar]
- 20. Lipp A, Sandroni P, Low PA. Systemic postganglionic adrenergic studies do not distinguish Parkinson's disease from multiple system atrophy. J Neurol Sci. 2009;281:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Logan IC, Witham MD. Efficacy of treatments for orthostatic hypotension: a systematic review. Age Ageing. 2012;41:587–594. [DOI] [PubMed] [Google Scholar]


