Abstract
High dietary salt intake is known to contribute to hypertension and cardiovascular and cerebrovascular diseases. The authors investigated the association between dietary salt intake and development of hypertension or cardiovascular disease (CVD) in 243 patients with prehypertension. After a median follow‐up of 4.53 years (range, 3.1–8.7), 123 (50.6%) patients developed hypertension and 71 (29.2%) experienced cardiovascular events, including fatal and nonfatal myocardial infarctions. Adjusted hazard ratios for patients with a high salt diet (≥6 g/d) were 1.57 (95% confidence interval [CI], 1.17–3.31; P=.018) for hypertension and 1.97 (95% CI, 1.08–2.27; P=.011) for CVD. Multivariable‐adjusted analyses of subgroups showed a significant association between salt intake and CVD, but no such association was found in patients younger than 60 years, women, or patients with normal weight or normal cholesterol level. These results provide further research of prevention of hypertension and CVD in prehypertension.
Prehypertension was defined as a systolic blood pressure (BP) of 120 mm Hg to 139 mm Hg or a diastolic BP of 80 mm Hg to 89 mm Hg according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7).1 People with prehypertension are known to be at high risk for coronary atherosclerosis.2, 3, 4 A previous study showed that patients with prehypertension had increased common carotid artery intima‐media thickness compared with normotensive patients, which was measured as a subclinical sign of atherosclerosis.3 Coronary atherosclerosis, which plays an important role in the development of cardiovascular disease (CVD), develops through the interaction of multiple risk factors, including smoking, alcohol consumption, serum total cholesterol, triglycerides, and diabetes mellitus. Patients with prehypertension have higher levels of blood glucose, total cholesterol, low‐density lipoprotein (LDL) cholesterol, and triglycerides; a higher body mass index (BMI); and lower levels of high‐density lipoprotein (HDL) cholesterol.5, 6, 7 Higher BMI (≥30 kg/m2) and total cholesterol levels (>200 mg/dL) were shown to be more common among prehypertensive patients than normotensive patients.8
The harmful effects of dietary high salt intake on BP, and on the heart and cerebral vessels, are considered to be a serious issue. Previous studies have confirmed that long‐term high dietary salt intake might lead to the development of hypertension, and promote the progression of cardiovascular and cerebrovascular diseases.9, 10, 11 A meta‐analysis of 19 independent cohort samples including 177,025 participants who were followed‐up for 3.5 to 19 years assessed the relationship between the level of habitual salt intake and stroke or total CVD, and concluded that high salt intake was associated with increased risks of stroke and total CVD.12 Results of a randomized crossover study suggested that reducing dietary salt intake should form a part of the preventive treatment for resistant hypertension. Compared with a high sodium diet, a low‐sodium diet for 7 days reduced systolic and diastolic BPs by 22.7 mm Hg and 9.1 mm Hg, respectively.13 In a study aimed to assess the adverse cardiovascular effect of acute salt loading, results showed that urinary sodium excretion and 24‐hour ambulatory systolic BP significantly rose with salt loading. Compared with the placebo group, salt loading in young normotensive patients was significantly associated with reduction of endothelium‐dependent vasodilatation in response to acetylcholine. In conclusion, salt loading may lead to endothelial dysfunction, which is associated with atherosclerotic disease in young normotensive patients.14, 15 However, these studies were carried out in hypertensive or normotensive patients, and little is known about the association between dietary salt intake and the development of CVD in the prehypertensive population. Therefore, the present study aimed to assess the risk factors of coronary atherosclerosis associated with different levels of salt intake, and to analyze the relationship between salt intake and hypertension or CVD in patients with prehypertension.
Methods
Study Population
This study initially enrolled a total of 11,801 patients, aged 45 to 75 years, between 2003 and 2009. All participants presented to the Shenyang Northern Hospital with a diagnosis of coronary heart disease. Patients with hypertension or normotension (n=8353) were excluded from the study. Patients with a history of myocardial infarction (MI), coronary revascularization, congenital heart disease, or cerebrovascular diseases were also excluded (n=1745). All patients were examined by coronary angiography, and those with a degree of coronary artery stenosis 30% to 70% were included. A total of 243 patients were finally selected for the study. According to a detailed questionnaire, patients who consumed <6 g/d of salt were defined as the normal salt group, and those who consumed ≥6 g/d were defined as the high salt group.16 There were 120 patients in the normal salt group (69 men, 51 women) and 123 in the high salt group (71 men, 52 women).
Baseline Measurements
BP was measured after admission using an automated oscillometric BP device after the patient rested for 5 minutes in the sitting position without discomfort and application of medication. The mean of 3 results was recorded, according to JNC 7 guidelines. Patients with a systolic BP 120 mm Hg to 139 mm Hg or diastolic BP 80 mm Hg to 89 mm Hg were categorized as having prehypertension. General information (age, sex, height, and weight) and results of laboratory examinations, echocardiography, and coronary angiography were acquired from hospital records. Information on salt intake, exercise training, smoking, family history of hypertension or coronary heart disease, and antihypertensive medication use were obtained from a detailed questionnaire by phone call follow‐up.
BMI was calculated from body weight and height. All patients were asked whether they currently smoked, and current smoking was defined as at least one cigarette per day for at least 1 year.17 Exercise training was defined as walking for at least 60 min/d more than 5 times a week. A sedentary lifestyle was defined as doing exercise less than once a week. Information on these parameters was obtained as part of the questionnaire. To assess salt ingestion, the patients were asked how much salt they consumed per month in their diet, such as in pickles and miso marinated in salt, and whether they preferred eating salted food.18 Serum total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides were detected in the clinical laboratory using standard enzymatic methods. Patients who reported physician‐diagnosed diabetes mellitus or who were taking antidiabetic medication were considered to be diabetic. The Gensini score, determined by coronary angiography, was considered to be a direct sign of coronary artery stenosis, and thus reflected the degree of coronary atherosclerosis.
Information on daily drug use after discharge, morbidity status, and diagnoses of outcomes after re‐admission to the hospital was collected during follow‐up. Reports of fatal events were validated by review of death certificates from the hospital, and as much information as possible was gathered regarding the cause of death. Patients were followed up for a median of 4.53 years (25th and 75th percentiles: 3.16 and 6.26 years, respectively). The outcomes of this study included hypertension, nonfatal and fatal cardiovascular MI, stroke, and cerebrovascular death. A diagnosis of MI was based on symptoms, electrocardiographic signs, and levels of cardiac enzymes (creatinine kinase, creatine kinase isoenzyme, and troponin T or I), and the occurrence of confirmed nonfatal acute MI and cardiovascular death after the baseline examination. All the outcomes were predefined.
Statistical Analysis
Continuous variables were presented as mean±standard deviation, categorical variables were given as percentages, and t tests were used to compare continuous variables between different salt intake groups. Chi‐square tests were used to compare the rates of outcomes for different levels of salt intake. Multivariate Cox regression models, including sex, age, BMI, triglycerides, cholesterol, family history of hypertension and CVD, and history of diabetes, were used to assess the relationship between different levels of salt intake and occurrence of outcomes. Unadjusted and adjusted hazard ratios (HRs) and corresponding 95% confidence intervals (CI) were calculated by Cox regression analysis. We also estimated the incidence of hypertension and CVD associated with different salt diets by using Kaplan‐Meier survival functions. All analyses were performed using SPSS version 17.0 (SPSS Inc, Chicago, IL). Two‐tailed values of P<.05 were considered statistically significant.
Results
A total of 243 adult patients (aged 45–75 years) who met our inclusion and exclusion criteria were considered to represent the salt intake conditions in northeast China at the time of study entry. In our study, 49.3% (57.5% men, 42.5% women) prehypertensive patients with a diagnosis of coronary heart disease consumed a diet high in salt. Table 1 shows the baseline characteristics of the study population according to dietary salt intake and the characteristics associated with coronary atherosclerosis. Compared with the normal salt group, patients with a high salt diet (salt intake ≥6 g/d) were more likely to have a higher BMI, higher alcohol consumption, and a family history of CVD. Conversely, prehypertensive patients with dietary salt intake <6 g/d were more likely to be older. There were no significant differences in triglyceride or cholesterol levels between the high and normal salt diet groups. Similarly, there were no differences in Gensini scores between the two groups. There were no differences in the use of medications to prevent CVD, such as aspirin, β‐blockers, statins, angiotensin‐converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs) between the two groups (Table 1).
Table 1.
Distribution of Risk Factors for Coronary Atherosclerosis and Medication Use According to Dietary Salt Intake
| Baseline Characteristics | High Salt (≥6 g/d) | Normal Salt (<6 g/d) | P Value |
|---|---|---|---|
| (n=120) | (n=123) | ||
| Men, % | 69 (57.5) | 71 (57.7) | .1 |
| Age, y | 58.7±7.5 | 60.8±7.4 | .025 |
| BMI, kg/m2 | 25.5±2.9 | 24.7±2.7 | .032 |
| Serum sodium, mmol/L | 141.4±2.7 | 142.0±2.6 | .076 |
| Serum potassium, mmol/L | 4.09±0.3 | 4.07±0.3 | .631 |
| LVEDD, mm | 45.7±3.4 | 45.7±4.3 | .974 |
| EF, % | 65.3±7.2 | 65.9±7.7 | .546 |
| Triglycerides, mmol/L | 2.0±1.6 | 1.7±1.4 | .259 |
| Total cholesterol, mmol/L | 4.7±1.1 | 4.4±1.1 | .140 |
| LDL cholesterol, mmol/L | 2.6±0.7 | 2.5±0.6 | .272 |
| HDL cholesterol, mmol/L | 1.4±0.3 | 1.4±0.3 | .105 |
| Serum creatinine, μmol/L | 82.6±14.4 | 86.3±17.9 | .076 |
| Current smoker, % | 58 (48.3) | 48 (39.0) | .156 |
| Alcohol use, % | 16 (13.3) | 7 (5.7) | .042 |
| Sedentary lifestyle, % | 35 (29.2) | 23 (18.7) | .071 |
| Diabetes, % | 18 (15) | 18 (14.6) | .936 |
| Family history of hypertension, % | 42 (35) | 30 (24.4) | .07 |
| Family history of CVD, % | 30 (25) | 18 (14.6) | .042 |
| Gensini score | 3.89±2.25 | 3.88±2.64 | .966 |
| Medications, % | |||
| Aspirin | 45 (37.5) | 48 (39.0) | .807 |
| β‐Blocker | 9 (7.5) | 10 (8.1) | .989 |
| Statins | 17 (14.2) | 15 (12.2) | .650 |
| ACE inhibitor/ARB | 3 (2.5) | 1 (0.8) | .305 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CVD, cardiovascular disease; EF, ejection fraction; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVEDD, left ventricular end‐diastolic dimension.
Table 2 shows the use of antihypertensive medications in the follow‐up period, such as diuretics, β‐blockers, calcium channel blockers (CCBs), ACE inhibitors, or ARBs, among others such as Chinese traditional medicine. Four patients (3.3%) in the high salt group and 7 patients (5.7%) in the normal salt group took ≥2 antihypertensive drugs (P=.377), 21 patients (17.5%) in the high salt group and 14 patients (11.4%) in the normal salt group were not taking any antihypertensive medication (P=.174). Moreover, there was no significant difference in antihypertensive medication use between the two groups. During a mean follow‐up period of 4.53 years (range, 3.1–8.7), 4 patients died of cardiovascular events, 67 patients experienced nonfatal MIs, and 123 patients progressed to hypertension. The incidence of nonfatal MI events was significantly higher among patients with a high salt diet compared with those with a normal salt diet (P=.001) (Table 3). Analysis of independent risk factors for hypertension, after adjusting for age, sex, BMI, current smoking, and other risk factors, identified a high salt diet and family history of hypertension as being independently associated with hypertension. The HR for a high salt diet was 1.56 (95% CI, 1.07–2.26; P=.018) and for a family history of hypertension was 2.22 (95% CI, 1.51–3.26; P<.0001). Among the patients with prehypertension, only a high salt diet was independently associated with CVD, after adjusting for the all CVD risk factors (HR, 1.97; 95% CI, 1.17–3.31; P=.011) (Table 4).
Table 2.
Antihypertensive Medications Use During Follow‐up According to Dietary Salt Intake
| Antihypertensive Medications, % | High Salt (≥6 g/d) | Normal Salt (<6 g/d) | P Value |
|---|---|---|---|
| (n=120) | (n=123) | ||
| Diuretic, % | 2 (1.7) | 4 (3.3) | .702 |
| CCB, % | 12 (10) | 7 (5.7) | .211 |
| β‐Blocker, % | 4 (3.3) | 4 (3.3) | .972 |
| ACE inhibitor/ARB, % | 12 (10) | 11 (8.9) | .778 |
| Others, % | 20 (16.7) | 25 (20.3) | .463 |
| None, % | 21 (17.5) | 14 (11.4) | .174 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
Table 3.
Incidence Rates of Outcomes According to Dietary Salt Intake
| Outcome, No., % | High Salt (n=120) | Normal Salt (n=123) | P Value |
|---|---|---|---|
| Hypertension | 67 (55.8) | 56 (45.5) | .108 |
| Cardiovascular disease | |||
| Nonfatal myocardial infarction | 45 (37.5) | 22 (17.9) | .001 |
| Fatal myocardial infarction | 3 (2.5) | 1 (0.8) | .301 |
| Stroke | 5 (4.2) | 8 (6.5) | .418 |
| Cerebrovascular death | 0 (0) | 0 (0) | 1.000 |
Table 4.
Risk Factors for Hypertension and CVD
| Outcome | Factor | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|---|
| Hypertension | High salt | 1.57 (1.08–2.27) | .018 |
| Age | 1.00 (0.97–1.03) | .826 | |
| Male | 1.20 (0.75–1.90) | .448 | |
| BMI | 1.03 (0.97–1.10) | .298 | |
| Cholesterol | 0.97 (0.64–1.49) | .906 | |
| Current smoker | 1.27 (0.80–2.01) | .305 | |
| Sedentary lifestyle | 1.00 (0.63–1.57) | .989 | |
| Family history of hypertension | 2.22 (1.51–3.26) | <.0001 | |
| Diabetes | 1.07 (0.69–1.65) | .778 | |
| CVD | High salt | 1.97 (1.17–3.31) | .011 |
| Age | 1.00 (0.97–1.04) | .986 | |
| Male | 1.68 (0.88–3.20) | .118 | |
| BMI | 1.04 (0.96–1.13) | .346 | |
| Cholesterol | 0.69 (0.38–1.25) | .222 | |
| LDL cholesterol | 1.04 (0.47–2.31) | .924 | |
| Current smoker | 1.45 (0.78–2.68) | .241 | |
| Sedentary lifestyle | 1.68 (0.99–2.85) | .054 | |
| Family history of CVD | 1.15 (0.66–2.00) | .619 | |
| Diabetes | 0.95 (0.54–1.65) | .843 |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; LDL, low‐density lipoprotein.
Figure 1 shows the Kaplan‐Meier survival function estimates for incidence of hypertension and cardiovascular events. The incidence rates of hypertension and cardiovascular events were significantly higher in patients with a high salt diet than in those with a normal salt diet. The incidence of hypertension in patients with a high salt diet was 56% (95% CI, 47–65) and in those with a normal salt diet was 46% (95% CI, 37–54) (P=.039). Forty‐five patients in the high salt group had CVD (incidence rate, 40%; 95% CI, 31–49) compared with 22 in the normal salt group (incidence rate, 19%; 95% CI, 12–26) (P=.001). Further analysis of the interactions between a high salt diet and risk factors for hypertension and coronary atherosclerosis by Cox regression models showed that the HRs for outcomes in patients with a high salt diet were significantly higher in those at high risk for hypertension and CVD. Subgroup analyses showed that among patients who were current smokers or who had a family history of hypertension, a diet high in salt was associated with a high risk for hypertension, after adjusting for age, sex, BMI, and risk factors for hypertension. The HRs for a high salt diet were 2.14 (95% CI, 1.18–3.88) in current smokers and 2.00 (95% CI, 1.11–3.60) in those with a family history of hypertension. An association between high salt intake and risk factors for cardiovascular atherosclerosis was observed to promote CVD. The adjusted HRs for a high salt diet were 2.78 (95% CI, 1.21–6.39) for advanced age, 2.30 (95% CI, 1.18–4.46) for men, 2.06 (95% CI, 1.01–4.24) for overweight, and 2.33 (95% CI, 1.30–4.19) for hypercholesterolemia. However, there was no apparent association between salt intake and CVD in patients younger than 60 years old, and associations were attenuated in those with BMI <25 kg/m2, nonsmokers, those with nonsedentary lifestyles, and those with lower or median cholesterol (Figure 2 and Figure 3).
Figure 1.
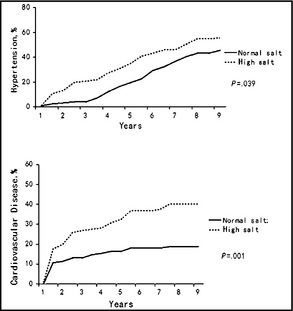
Kaplan‐Meier survival function estimates for hypertension and cardiovascular disease (fatal and nonfatal myocardial infarction) events.
Figure 2.
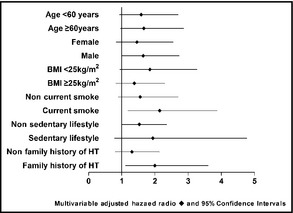
Hazard ratios for hypertension in high salt–intake subgroups estimated by Cox regression after adjusting for sex, age, body mass index (BMI), current smoking, sedentary lifestyle, triglycerides, cholesterol, family history of hypertension (HT), and diabetes, except for the factor defining the specific subgroup.
Figure 3.
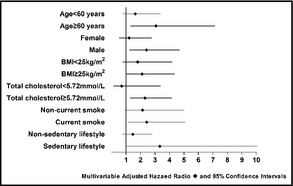
Hazard ratios for cardiovascular disease, including fatal and nonfatal myocardial infarction and coronary revascularization in high salt subgroups estimated by Cox regression after adjusting for sex, age, body mass index, current smoking, sedentary lifestyle, triglycerides, cholesterol, family history of cardiovascular disease, and diabetes, except for the factor defining the specific subgroup.
Discussion
Prehypertension is a condition that precedes hypertension, which is associated with a higher level of inflammatory markers such as tumor necrosis factor α, C‐reactive protein, and homocysteine levels, and higher common artery intima‐thickness and left ventricular mass compared with normotension.2, 3, 4 Salt intake also plays an important role in hypertension and CVD.10, 11 In this study, we assessed the relationship between different levels of dietary salt intake and development of hypertension or CVD in patients with prehypertension and showed that high dietary salt intake is a high risk factor for hypertension and CVD in prehypertensive patients, especially in current smokers, in those with a family history of hypertension, in men, and in overweight individuals.
As noted in previous studies, our results confirm an association between salt intake and hypertension. Patients who consume a diet high in salt were at greater risk for hypertension than those who consume a diet with normal salt, especially in the case of smokers and individuals with a family history of hypertension. The Intersalt Cooperative Research Group found that a reduction in sodium intake of 100 mmol/d reduced systolic BP by 3 mm Hg to 6 mm Hg at 40 years of age, and reduced the rise in systolic pressure between 25 and 55 years of age by 9 mm Hg to 11 mm Hg.9 The Dietary Approaches to Stop Hypertension (DASH‐Sodium) study was a randomized trial that included 412 participants with and without hypertension, of whom about two thirds were prehypertensive. All participants received 3 levels of sodium (141 mmol/d, 106 mmol/d, and 64 mmol/d). Reducing the sodium intake from the highest to the lowest level decreased systolic BP by 3 mm Hg to 7 mm Hg and diastolic BP by 2 mm Hg to 3 mm Hg, with larger reductions among patients with hypertension.19
Atherosclerosis is the primary cause of CVD and is the result of complex interactions among multiple risk factors. A study performed as part of the Heinz Nixdorf Recall study showed that prehypertensive participants had a higher prevalence of risk factors for coronary atherosclerosis, such as older age, higher BMI, and higher cholesterol and triglyceride levels, and these differences were found in both men and women.20 Coronary artery stenosis, which provides a direct sign of coronary artery atherosclerosis, was assessed according to the degree of hypertension. The results showed that fewer women than men had coronary artery stenosis, while hypertension, diabetes mellitus, dyslipidemia, and current smoking were more commonly seen in patients with coronary artery stenosis than in those without.2 The results of our study showed that patients with a high salt diet were at increased risk of CVD compared with patients with a normal salt diet, especially in men. These results are supported by those of a study in Finland that gathered data on 24‐hour urinary sodium excretion and cardiovascular risk factors in 1173 men and 1263 women aged 25 to 64 years. They found that 24‐hour urinary sodium excretion was higher in men than women. However, serum cholesterol levels and the percentage of smokers did not differ across quartiles of 24‐hour sodium excretion in the overall population. They also analyzed the relationship between weight and salt intake on cardiovascular events. Consistent with the conclusions of our study, overweight individuals with a high intake of salt were at greater risk of CVD, but this association was not reflected in the population with normal weight.21
Some studies have suggested that individuals with high BMI (>25 kg/m2) are more sensitive to CVD than people with normal weight. The First National Health and Nutrition Examination Survey (NHANES I) showed an independent association between sodium intake and risk of CVD in 14,407 participants, aged 25 to 75 years, who were followed up for 19 years. Dietary sodium intake was estimated at baseline by 24‐hour dietary recall. Incidence and mortality data for CVD were obtained from medical records and death certificates. The results did not differ according to sex but were affected by weight; there was no significant association between dietary sodium intake and CVD risk in nonoverweight people, but sodium intake was associated with increased occurrence of stroke and mortality from coronary heart disease in overweight individuals.22
Our study also found that prehypertensive patients who eat a diet high in salt had higher BMI values than those who eat a normal salt diet, and evidence support an association between salt intake and obesity. From the results of a previous study in 1104 untreated hypertensive patients with high or low salt diets, 24‐hour urinary volume in individuals with a high salt diet was 2.2 L, which decreased to 1.3 L in those with a low salt diet. It was also demonstrated that 24‐hour urinary volume was significantly related to urinary sodium in hypertensive and normotensive patients.23 Although the relationship has been demonstrated in adults, a similar relationship in children aged 4 to 18 years was detected. As a promoter of childhood obesity, a difference of 27 g/d sugar in sweetened soft drink consumption was associated with a difference of 1 g/d in terms of salt intake. Reducing salt intake could also reduce childhood obesity.24
Because of the dietary habits and culture of the area, our study did not include any prehypertensive patients with low dietary salt intake. However, the NHANE III study included 8699 noninstitutionalized US adults 30 years and older with no history of CVD events. Dietary sodium intake was estimated from a single 24‐hour dietary recall. The lower sodium quartile group (<2060 mg/d) in the study was more likely to be older and to have higher systolic BP, while the higher quartile sodium group (4048–9946 mg/d) was more likely to be male, to weigh more, to smoke, and to have higher diastolic BP. There was a significant inverse association between sodium intake and CVD mortality after a 8.7±2.3‐year follow‐up period, comparing the lowest with the highest sodium intake quartile.25 The results of the study by Martin and colleagues also support this view. Among 28,880 participants, those with a 24‐hour urinary sodium excretion of <3 g were at higher risk for CVD and chronic heart failure, compared with those with a 24‐hour urinary sodium excretion of 4 g to 5.99 g.26 Further studies are required to clarify the effects of a low salt diet on coronary atherosclerosis.
Study Limitations
Our study assessed a population‐based sample from northeast China with a relatively high prevalence of salt intake and coronary atherosclerosis, and therefore lacked information on the association between low salt intake and CVD. The median follow‐up period of the study was only 4.53 years, which may have limited the ability of the study to detect differences in some factors, such as serum creatinine, between the two salt intake groups. Additionally, the sample size of 243 patients was relatively small, and it is possible that more significant results could have been obtained with a larger sample size.
Conclusions
Previous studies have failed to reach a consensus regarding the treatment of prehypertension with recommended lifestyle changes to prevent the development of hypertension and coronary heart disease. The current study suggests that high salt intake contributes to the progression of hypertension and cardiovascular events in patients with prehypertension, especially in current smokers, those with a family history of hypertension, and in individuals with other CVD risk factors such as male sex and being overweight. These results provide valuable information for future research in preventing the progression of prehypertension.
Acknowledgment and disclosures
The authors wish to thank Dr Zhiming Zhu from The Third Military Medical University of PLA for his many helpful suggestions. This work was supported by grants from the National Basic Research Program of China (grant No. 2012CB517805 to Z.Z. and 2012CB517800 to Y.H.).
J Clin Hypertens (Greenwich). 2014;16:575–580. © 2014 Wiley Periodicals, Inc.
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Washio M, Tokunaga S, Yoshimasu K, et al. Role of prehypertension in the development of coronary atherosclerosis in Japan. J Epidemiol. 2004;14:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manios E, Tsivgoulis G, Koroboki E, et al. Impact of prehypertension on common carotid artery intima‐media thickness and left ventricular mass. Stroke. 2009;40:1515–1518. [DOI] [PubMed] [Google Scholar]
- 4. Arima H, Murakami Y, Lam TH, et al. Effects of prehypertension and hypertension subtype on cardiovascular disease in the Asia‐Pacific Region. Hypertension. 2012;59:1118–1123. [DOI] [PubMed] [Google Scholar]
- 5. Ferguson TS, Younger NO, Tulloch‐Reid MK, et al. Prevalence of prehypertension and its relationship to risk factors for cardiovascular disease in Jamaica: analysis from a cross‐sectional survey. BMC Cardiovasc Disord. 2008;28:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenlund KJ, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000. Arch Intern Med. 2004;164:2113–2118. [DOI] [PubMed] [Google Scholar]
- 7. Grotto I, Grossman E, Huerta M, Sharabi Y. Prevalence of prehypertension and associated cardiovascular risk profiles among young Israeli adults. Hypertension. 2006;48:254–259. [DOI] [PubMed] [Google Scholar]
- 8. Liszka HA, Mainous AG III, King DE, et al. Prehypertension and cardiovascular morbidity. Ann Fam Med. 2005;3:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Intersalt Cooperative Research Group . Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1988;297:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gleiberman L. Blood pressure and dietary salt in human populations. Ecol Food Nutr. 1973;2:143–156. [Google Scholar]
- 11. Jackson FLC. An evolutionary perspective on salt, hypertension, and human genetic variability. Hypertension. 1991;17(suppl 1):129–132. [DOI] [PubMed] [Google Scholar]
- 12. Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta‐analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pimenta E, Gaddam KK, Oparil S. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tzemos N, Lim PO, Wong S, et al. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008;51:1525–1530. [DOI] [PubMed] [Google Scholar]
- 15. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. [DOI] [PubMed] [Google Scholar]
- 16. U.S. Department of Health and Human Services , U.S. Department of Agriculture . Dietary guidelines for Americans. http://www.health.gov/dietaryguidelines/dga2005/document/. Accessed March 10, 2005.
- 17. Zheng L, Sun Z, Zhang X, et al. Predictors of progression from prehypertension to hypertension among rural Chinese adults: results from Liaoning Province. Eur J Cardiovasc Prev Rehabil. 2010;17:217. [DOI] [PubMed] [Google Scholar]
- 18. Tian HG, Hu G, Dong QN, et al. Dietary sodium and potassium, socioeconomic status and blood pressure in a Chinese population. Appetite. 1996;26:235–246. [DOI] [PubMed] [Google Scholar]
- 19. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 20. Erbel R, Lehmann N, Mohlenkamp S, et al. Subclinical coronary atherosclerosis predicts cardiovascular risk in different stages of hypertension: result of the Heinz Nixdorf Recall Study. Hypertension. 2012;59:44–53. [DOI] [PubMed] [Google Scholar]
- 21. Tuomilehto J, Jousilahti P, Rastenyte D, et al. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet. 2001;357:848–851. [DOI] [PubMed] [Google Scholar]
- 22. He J, Ogden LG, Vupputuri S, et al. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282:2027–2034. [DOI] [PubMed] [Google Scholar]
- 23. He FJ, Markandu ND, Sagnella GA, MacGregor GA. Effect of salt intake on renal excretion of water in humans. Hypertension. 2001;38:317–320. [DOI] [PubMed] [Google Scholar]
- 24. He FJ, Marrero NM, MacGregor GA. Salt intake is related to soft drink consumption in children and adolescents: a link to obesity? Hypertension. 2008;51:629–634. [DOI] [PubMed] [Google Scholar]
- 25. Cohen HW, Hailpern SM, Alderman MH. Sodium intake and mortality follow‐up in the third national health and nutrition examination survey (NHANES III). J Gen Intern Med. 2008;23:1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. [DOI] [PubMed] [Google Scholar]


