Abstract
Osteoprotegerin (OPG) has been implicated in the process of vascular stiffness. The aim of this study was to evaluate the relationship between fasting serum OPG concentration and carotid‐femoral pulse wave velocity (c‐f PWV) in hypertensive patients. Fasting blood samples were obtained from 184 participants with or without hypertension. c‐f PWV were performed by SphygmoCor system. Serum OPG levels were measured using a commercially available enzyme‐linked immunosorbent assay. Hypertensive patients who had diabetes had higher c‐f PWV levels than those without diabetes (P=.031). The univariable linear regression analysis showed that age (P<.001), systolic blood pressure (P=.003), pulse pressure (r=0.287; P=.003), log‐BUN (P=.011), Cre (P<.001), and log‐OPG concentration (P<.001) were positively correlated with c‐f PWV levels, while the glomerular filtration rate (P=.005) and HDL‐C level (P=.024) was negatively correlated with c‐f PWV levels among the hypertensive patients. Multivariable forward stepwise linear regression analysis of the significant variables also showed that log‐OPG (β=0.312, regression coefficient: 1.736; 95% confidence interval, 0.809–2.663; P<.001) was still an independent predictor of c‐f PWV levels in hypertensive patients. Serum OPG levels positively associated with c‐f PWV levels in hypertensive patients.
Arterial stiffness has been recognized as an independent risk factor for cardiovascular morbidity and mortality.1 Carotid‐femoral pulse wave velocity (c‐f PWV) is a direct measurement of aortic stiffness and has been recommended as the gold standard measurement for arterial stiffness.2 An increase of 1 m/s in c‐f PWV is associated with an increase of 14% of total cardiovascular events, 15% cardiovascular mortality, and 15% all‐cause mortality in a systematic review and meta‐analysis study.1 In large numbers of healthy patients or hypertensive patients, c‐f PWV has been demonstrated to be a predictor of future cardiovascular events.3
Arterial stiffness is defined by a reduction in arterial distensibility.3, 4 Recent science suggests that arterial stiffness is associated with endothelial dysfunction, the expression of modified vascular wall matrix proteins, altered vascular smooth muscle cell number, structure and functions, inflammation, and potential genetic determinants.5 Vascular calcification in blood vessels is an active, cell‐regulated process, which may lead to increased arterial stiffness.6 Osteoprotegerin (OPG) is one of the vascular calcification inhibitors and may reflect endothelial dysfunction.7 In recent studies, OPG has been associated with increased PWV, progression of arterial calcification, and increased mortality in both end‐stage renal failure patients and the general population.8, 9 c‐f PWV is a strong and independent predictor of overall risk and cardiovascular risk in hypertension.10 Aortic stiffness is also an independent predictor of primary coronary events in patients with essential hypertension.11 The aim of the current study was to determine the relationship between the fasting serum OPG level and c‐f PWV among hypertensive patients.
Methods
Patients
Between January 2012 and December 2012, 184 participants from a medical center located in Hualien, Taiwan, were enrolled in this study (106 had hypertension and 78 did not). Trained personnel measured blood pressure (BP) in the morning for all participants, using standard mercury sphygmomanometers with appropriate cuff sizes, after the participants had been sitting for at least 10 minutes. Systolic BP (SBP) and diastolic BP (DBP) were taken at the points of appearance and disappearance, respectively, of the Korotkoff sounds. SBP and DBP were taken 3 times at 5‐minute intervals and were averaged for analysis. In the prevalence survey, hypertension was defined as SBP ≥140 mm Hg, and/or DBP ≥90 mm Hg, or prescription of antihypertensive medication in the past 2 weeks. A person was regarded as diabetic if their fasting plasma glucose was either ≥126 mg/dL or if he/she was using diabetes medication (oral or insulin).12 Congestive heart failure was defined by the American College of Cardiology Foundation and the American Heart Association 2005 guidelines.13 Coronary artery disease was defined as >50% stenosis in any segment on coronary angiography. The Protection of the Human Subjects Institutional Review Board of Tzu‐Chi University and Hospital approved this study. Patients were excluded if they had an acute infection, acute myocardial infarction, and pulmonary edema at the time of blood sampling or if they declined to provide informed consent for the study. All participants provide written informed consent to participate in this study.
Anthropometric Analysis
Participant weight was measured in light clothing and without shoes to the nearest 0.5 kg, and height was measured to the nearest 0.5 cm. Waist circumference was measured using a tape measurement around the waist from the point between the lowest ribs and the hip bones with the hands on the hips. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared.14, 15
Biochemical Investigations
Fasting blood samples (approximately 5 mL) were immediately centrifuged at 3000 g for 10 minutes. Serum levels of blood urea nitrogen (BUN), creatinine (Cre), fasting glucose, total cholesterol (TCH), triglycerides (TG), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), total calcium, phosphorus, and C‐reactive protein (CRP) were measured using an autoanalyzer (COBAS Integra 800; Roche Diagnostics, Basel, Switzerland).14, 15 Serum OPG levels (eBioscience Inc., San Diego, CA) were measured using a commercially available enzyme‐linked immunosorbent assay. The calculation of estimated glomerular filtration rate (GFR) used the Modification of Diet in Renal Disease equation.
c‐f PWV Measurements
Measurements of c‐f PWV were performed using applanation tonomentry (SphygmoCor system; AtCor Medical, West Ryde, Australia) by transcutaneously recording the pressure pulse waveform in the underlying artery as previously described.16 All measurements were performed in the morning in the supine position after a minimum of 10 minutes of rest in a quiet, temperature‐controlled room. Records were made simultaneously with an electrocardiographic (ECG) signal, which provided an R‐timing reference. Pulse wave recordings were performed consecutively at two superficial artery sites (carotid‐femoral segment). Integral software was used to process each set of pulse wave and ECG data to calculate the mean time difference between R wave and pulse wave on a beat‐to‐beat basis, with an average of 10 consecutive cardiac cycles. The c‐f PWV was calculated using the distance and mean time difference between the two recorded points. Quality indices included in the software were set to ensure uniformity of data.
Statistical Analysis
Data are expressed as mean±standard deviation (SD) and were tested for normal distribution using Kolmogorov‐Smirnov statistics. Categorical variables were analyzed by chi‐square test. Comparison variables between normotensive participants and hypertensive patients were performed using the Student independent t test (two‐tailed) for normally distributed data or the Mann‐Whitney U test for parameters that presented a non‐normal distribution (fasting glucose, BUN, CRP, and OPG). Because the glucose, BUN, CRP, and OPG were not normally distributed, they underwent base 10 logarithmic transformations to achieve normality. Clinical variables that correlated with c‐f PWV levels in normotensive participants or hypertensive patients were evaluated by univariable linear regression analysis. Variables that were significantly associated with c‐f PWV in hypertensive patients were tested for independency in multivariable forward stepwise regression analysis (adopted factors: diabetes, age, systolic blood pressure, pulse pressure, HDL‐C, log‐BUN, creatinine, GFR, and log‐OPG). Data were analyzed using SPSS for Windows (version 19.0; SPSS Inc, Chicago, IL). A P value <.05 was considered statistically significant.
Results
The clinical and laboratory characteristics of participants with or without hypertension are presented in Table 1. Hypertensive patients had higher body weight (P<.001), BMI (P<.001), waist circumference (P=.009), TGs (P=.027), fasting glucose (P=.039), CRP (P=.011), SBP (P<.001), and DBP (P<.001) than normotensive participants. The c‐f PWV levels, serum OPG levels, sex, and diabetes did not differ statistically in participants with or without hypertension.
Table 1.
Clinical and Analytical Characteristics of 184 Participants With or Without Hypertension
| Items | No Hypertension (n=78) | Hypertension (n=106) | P Value |
|---|---|---|---|
| Age, y | 65.64±8.62 | 64.93±10.14 | .619 |
| Height, cm | 160.28±7.56 | 161.54±8.68 | .308 |
| Body weight, kg | 63.67±9.39 | 70.28±12.36 | <.001[Link] |
| Waist circumference, cm | 89.51±9.47 | 93.27±9.70 | .009[Link] |
| Body mass index, kg/m2 | 24.73±2.76 | 26.82±3.50 | <.001[Link] |
| Total cholesterol, mg/dL | 170.46±40.04 | 171.97±38.20 | .795 |
| Triglyceride, mg/dL | 123.21±58.17 | 157.99±128.30 | .027[Link] |
| HDL‐C, mg/dL | 47.05±13.26 | 45.13±12.67 | .321 |
| LDL‐C, mg/dL | 101.82±30.34 | 101.27±29.88 | .903 |
| Fasting glucose, mg/dL | 120.64±42.19 | 137.79±59.04 | .039[Link] |
| Blood urea nitrogen, mg/dL | 15.62±4.21 | 17.28±6.26 | .097 |
| Creatinine, mg/dL | 1.05±0.26 | 1.12±0.34 | .134 |
| Glomerular filtration rate, mL/min | 73.15±14.94 | 69.67±21.18 | .216 |
| Total calcium, mg/dL | 9.07±0.35 | 9.15±0.36 | .141 |
| Phosphorus, mg/dL | 3.59±0.54 | 3.49±0.50 | .172 |
| Ca×P product, mg2/dL2 | 32.67±5.37 | 31.97±4.91 | .361 |
| Systolic blood pressure, mm Hg | 119.69±11.12 | 136.94±17.47 | <.001[Link] |
| Diastolic blood pressure, mm Hg | 68.69±8.41 | 74.92±10.72 | <.001[Link] |
| Pulse pressure, mm Hg | 51.00±12.25 | 62.03±16.35 | <.001[Link] |
| C‐reactive protein, mg/dL | 0.20±0.09 | 0.34±0.40 | .011[Link] |
| c‐f PWV, m/s | 9.24±3.51 | 9.56±2.69 | .485 |
| Osteoprotegerin, pg/L | 3.63±5.11 | 4.00±4.39 | .250 |
| Sex, % | |||
| Male | 74.4 | 67.0 | .280 |
| Female | 25.6 | 33.0 | |
| Diabetes, % | |||
| No | 61.5 | 47.2 | .054 |
| Yes | 38.5 | 52.8 | |
Abbreviations: c‐f PWV, carotid‐femoral pulse wave velocity; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol. Data are expressed as means±standard deviations and were analyzed by the Student t test or Mann‐Whitney U test (fasting glucose, blood urea nitrogen, C‐reactive protein, osteoprotegerin). Data are expressed as number of patients and analysis by chi‐square test. a P<.05 was considered statistically significant.
The clinical and laboratory characteristics of the hypertensive patients are presented in Table 2. The medical histories of the hypertensive patients included dyslipidemia (n=85 [80.2%]), coronary artery disease (n=70 [66.0%]), and congestive heart failure (n=15 [14.1%]). The medications prescribed to the hypertensive patients included angiotensin receptor blockers (ARBs; n=58 [54.7%]), angiotensin‐converting enzyme (ACE) inhibitors (n=36 [34.0%]), calcium channel blocker (CCBs; n=53 [50.0%]), β‐blockers (n=63 [59.4%]), statins (n=59 [55.7%]), fibrate (n=24 [22.6%]), aspirin (n=61 [57.5%]), and clopidogrel (n=26 [24.5%]). Normotensive participants (P=.040) or hypertensive patients (P=.031) who had diabetes had higher c‐f PWV levels than those without diabetes (Figure 1). The c‐f PWV levels did not differ statistically based on sex, coexisting dyslipidemia or coronary artery disease or congestive heart failure, and ARB, ACE inhibitor, CCB, β‐blocker, statin, fibrate, aspirin, or clopidogrel use.
Table 2.
Clinical Characteristics and Carotid‐Femoral Pulse Wave Velocity Levels of 106 Hypertensive Patients
| Characteristic | Number (%) | c‐f PWV, m/s | P Value |
|---|---|---|---|
| Sex | |||
| Male | 71 (67.0) | 9.80±2.83 | .193 |
| Female | 35 (33.0) | 9.07±2.34 | |
| Dyslipidemia | |||
| No | 21 (19.8) | 8.87±2.79 | .188 |
| Yes | 85 (80.2) | 9.73±2.65 | |
| CAD | |||
| No | 36 (34.0) | 9.34±2.39 | .556 |
| Yes | 70 (66.0) | 9.67±2.84 | |
| CHF | |||
| No | 91 (85.8) | 9.61±2.69 | .636 |
| Yes | 15 (14.2) | 9.25±2.78 | |
| ACE inhibitor | |||
| No | 70 (66.0) | 9.59±2.75 | .857 |
| Yes | 36 (34.0) | 9.49±2.61 | |
| ARB | |||
| No | 48 (45.3) | 9.44±2.40 | .682 |
| Yes | 58 (54.7) | 9.66±2.93 | |
| β‐Blocker | |||
| No | 43 (40.6) | 9.20±2.09 | .257 |
| Yes | 63 (59.4) | 9.81±3.02 | |
| CCB | |||
| No | 53 (50.0) | 9.15±2.21 | .121 |
| Yes | 53 (50.0) | 9.67±3.07 | |
| Statin | |||
| No | 47 (44.3) | 9.60±2.93 | .882 |
| Yes | 59 (55.7) | 9.53±2.51 | |
| Fibrate | |||
| No | 82 (77.4) | 9.40±2.38 | .255 |
| Yes | 24 (22.6) | 10.11±3.56 | |
| Aspirin | |||
| No | 45 (42.5) | 9.74±3.11 | .553 |
| Yes | 61 (57.5) | 9.43±2.36 | |
| Clopidogrel | |||
| No | 80 (75.5) | 9.54±2.58 | .912 |
| Yes | 26 (24.5) | 9.61±3.07 | |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CCB, calcium channel blocker; CHF, congestive heart failure; c‐f PWV, carotid‐femoral pulse wave velocity. Data are expressed as means±standard deviations.
Figure 1.
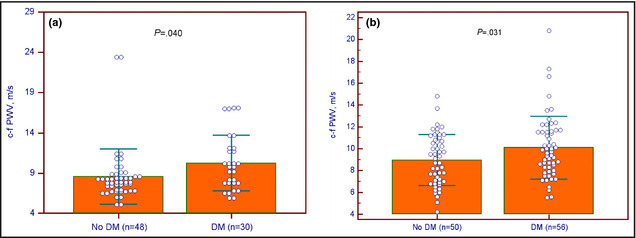
Clustered dots plot with means±standard deviations of carotid‐femoral pulse wave velocity (c‐f PWV) levels with or without diabetes mellitus (DM) among participants without (a) or with (b) hypertension.
The univariable linear analysis of c‐f PWV levels in normotensive participants or hypertensive patients is presented in Table 3. Age (r=0.349; P=.02) was positively correlated with c‐f PWV levels in normotensive participants. Age (r=0.370; P<.01), SBP (r=0.282; P=.003), pulse pressure (r=0.287; P=.003), log‐BUN (r=0.234; P=.011), Cre (r=0.360; P<.001), and the log‐OPG concentration (r=0.392; P<.001) were positively correlated with c‐f PWV levels, while the GFR (r=−0.273; P=.005) and HDL‐C level (r=−0.219; P=.024) was negatively correlated with c‐f PWV levels among the hypertensive patients. Two‐dimensional scattered plots of c‐f PWV levels among the 106 hypertensive patients are shown in Figure 2 and Figure 3.
Table 3.
Correlation of Carotid‐Femoral Pulse Wave Velocity and Clinical Variables by Univariable Linear Regression Analysis Among Participants With or Without Hypertension
| Variable | No Hypertension (n=78) | Hypertension (n=106) | ||
|---|---|---|---|---|
| r Value | P Value | r Value | P Value | |
| Age, y | 0.349 | .002b | 0.370 | <.001b |
| Height, cm | −0.168 | .141 | −0.030 | .757 |
| Body weight, kg | −0.087 | .447 | −0.047 | .629 |
| Waist circumference, cm | −0.188 | .099 | 0.070 | .474 |
| Body mass index, kg/m2 | 0.038 | .744 | −0.023 | .818 |
| Systolic blood pressure, mm Hg | 0.101 | .380 | 0.282 | .003b |
| Diastolic blood pressure, mm Hg | −0.026 | .820 | 0.022 | .824 |
| Pulse pressure, mm Hg | 0.110 | .340 | 0.287 | .003b |
| Total cholesterol, mg/dL | 0.058 | .612 | −0.091 | .356 |
| Triglyceride, mg/dL | −0.091 | .426 | −0.073 | .457 |
| HDL‐C, mg/dL | 0.070 | .544 | −0.216 | .024b |
| LDL‐C, mg/dL | 0.082 | .475 | −0.018 | .852 |
| Log‐glucose, mg/dLa | −0.071 | .535 | 0.001 | .993 |
| Log‐BUN, mg/dLa | −0.033 | .776 | 0.246 | .011b |
| Creatinine, mg/dL | −0.011 | .923 | 0.360 | <.001b |
| Glomerular filtration rate, mL/min | −0.131 | .255 | −0.273 | .005b |
| Log‐CRP, mg/dLa | 0.099 | .388 | 0.105 | .285 |
| Total calcium, mg/dL | −0.066 | .567 | −0.060 | .544 |
| Phosphorus, mg/dL | 0.202 | .076 | −0.053 | .590 |
| Ca×P product, mg2/dL2 | 0.163 | .153 | −0.062 | .528 |
| Log‐osteoprotegerin, pg/La | −0.020 | .864 | 0.392 | <.001b |
Abbreviations: BUN, blood urea nitrogen; CRP, C‐reactive protein; HDL‐C, high density lipoprotein‐cholesterol; LDL‐C, low density lipoprotein‐cholesterol. aData showed skewed distribution, and therefore were log‐transformed before analysis. b P<.05 is considered statistically significant in the univariable linear analyses.
Figure 2.
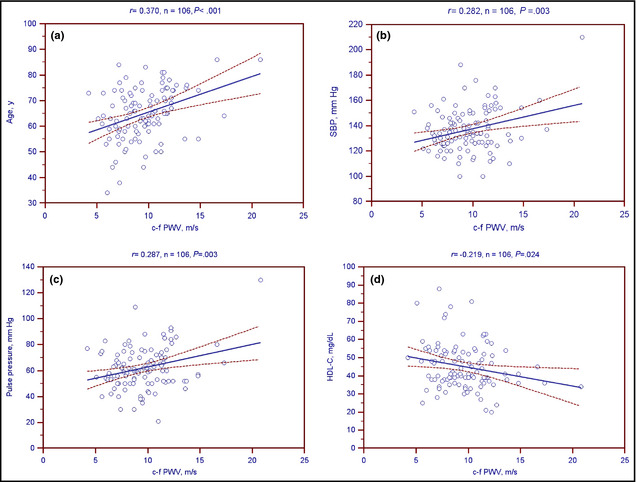
Two‐dimensional scattered plots of carotid‐femoral pulse wave velocity (c‐f PWV) levels with (a) age, (b) systolic blood pressure (SBP), (c) pulse pressure, and (d) high‐density lipoprotein cholesterol (HDL‐C) among 106 hypertensive patients. Dashed lines represent 95% confidence intervals.
Figure 3.
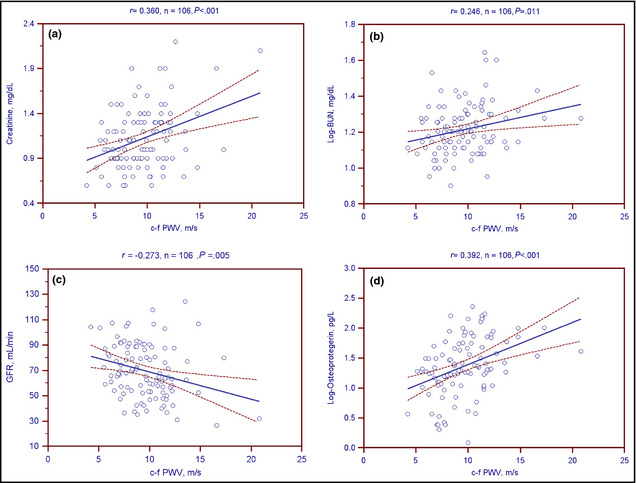
Two‐dimensional scattered plots of carotid‐femoral pulse wave velocity (cf PWV) levels with (a) creatinine, (b) log‐transformed blood urea nitrogen (BUN), (c) glomerular filtration rate (GFR), and (d) log‐transformed osteoprotegerin among 106 hypertensive patients. Dashed lines represent 95% confidence interval.
Multivariable forward stepwise linear regression analysis of the variables that were significantly associated with c‐f PWV levels (diabetes, age, SBP, pulse pressure, HDL‐C, log‐BUN, creatinine, GFR, and log‐OPG) among hypertensive patients showed that age (β=0.268, regression coefficient: 0.071; 95% confidence interval [CI], 0.023–0.119; P=.004), HDL‐C (β=−0.234, regression coefficient: 0.050; 95% CI, −0.086 to −0.013; P=.008), Cre (β=0.207; regression coefficient: 1.645; 95% CI, 0.254–3.036; P=.021), and log‐OPG (β=0.312; regression coefficient: 1.736; 95% CI, 0.809–2.663; P<.001) were the independent predictors of c‐f PWV levels in hypertensive patients (Table 4).
Table 4.
Correlation Between Carotid‐Femoral Pulse Wave Velocity Among Hypertensive Patients
| Variables | Correlation Coefficient (β) | Regression Coefficient (95% Confidence Interval) | P Value |
|---|---|---|---|
| Age, y | 0.268 | 0.071 (0.023–0.119) | .004a |
| HDL‐C, mg/dL | −0.234 | 0.050 (−0.086 to −0.013) | .008a |
| Creatinine, mg/dL | 0.207 | 1.645 (0.254–3.036) | .021a |
| Log‐osteoprotegerin, pg/L | 0.312 | 1.736 (0.809–2.663) | <.001a |
P<.05 was considered statistically significant in the multivariate logistic regression analysis (adopted factors: diabetes, age, systolic blood pressure, pulse pressure, high‐density lipoprotein cholesterol [HDL‐C]), log‐blood urea nitrogen, creatinine, glomerular filtration rate, and log‐osteoprotegerin).
Discussion
The results of our study showed that diabetes, age, SBP, BUN, Cre, and OPG concentration were positively correlated with c‐f PWV levels, while GFR and HDL‐C levels were negatively correlated with c‐f PWV levels among hypertensive patients. After adjusting for significant variables by multivariable forward stepwise linear regression analysis, it was shown that OPG was still an independent predictor of c‐f PWV levels in hypertensive patients.
Obesity and weight gain are major risk factors for hypertension.17 Metabolic syndrome has been shown to be a risk factor for cardiovascular disease and, as the prevalence of obesity increases, the prevalence of hypertension with its associated cardiovascular risk increases as well.18 CRP is significantly predictive of incident hypertension.19 Our study also showed that hypertensive patients had higher body weight, BMI, waist circumference, fasting glucose, TGs, and CRP than normotensive participants.
Contraction of the left ventricle generates a pulse wave that is propagated throughout the arterial tree. PWV is calculated as the distance traveled by the pulse wave divided by the time taken to travel the distance.3 c‐f PWV is a direct measurement of aortic stiffness, and has been recommended as the gold standard measurement for arterial stiffness.2 Aging of the arterial system is accompanied by progressive structural changes, consisting of fragmentation and degeneration of elastin, increases in collagen, thickening of the arterial wall, endothelium damage, and progressive dilation of the arteries.20 Diabetes is a disease of accelerated arterial ageing, as shown by stiffer arteries and consequently steeper increases in pulse pressure with age in these patients.21 Our results show that age and diabetes are associated with c‐f PWV levels in normotensive and hypertensive patients. Patients with lecithin‐cholesterol acyltransferase mutation had low HDL‐C levels that increased arterial stiffness.22 HDL‐C was inversely associated with c‐f PWV in community‐dwelling individuals and healthy adults undergoing a general health examination in China.23, 24 The possible mechanisms of HDL‐C of anti‐atheromatous effects on the arterial wall by promoting macrophage cholesterol efflux and reverse cholesterol transport activity and nonatheromatous effects on the arterial wall, such as the stimulation of endothelial nitric oxide production and repair, as well as anti‐apoptotic, anti‐inflammatory, and anti‐oxidant effects that may improve arterial stiffness.25 Arterial stiffness leads to an increase in SBP because a heart pumping blood into a stiffer arterial bed must generate higher end‐systolic pressures for the same net stroke volume.21 This leads to increased decay of arterial pressure and volume during systole, causing reduced arterial volume at the onset of diastole, which, in turn, causes an enhanced fall in DBP. The increase in SBP, which subsequently increases left ventricular afterload, and the decrease in DBP may reduce coronary perfusion.4 Therefore, arterial stiffness increases peak‐ and end‐systolic pressures in the ascending aorta, thereby raising myocardial pressure load and oxygen consumption, and decreasing DBP and subendocardial blood flow and increasing pulse pressure.26 Individuals with metabolic syndrome also have increased arterial stiffness.21 Patients with decreased GFR exhibited a significant reverse association with c‐f PWV in women with normal to mildly impaired renal function.27 Schillaci and colleagues28 reported that the decreased GFR was a major determinant of aortic stiffness in hypertensive patients with normal renal function. Reduced GFR were associated with central arterial stiffness by higher augmentation index.29 Our study showed that SBP, log‐BUN, and Cre levels were positively correlated, while the GFR and HDL‐C levels were negatively correlated with c‐f PWV levels among hypertensive patients. In our study, age, Cre, and HDL‐C levels were also independent predictors of c‐f PWV levels in hypertensive patients after multivariable analysis.
Arterial stiffness is associated with vascular calcification.6 The mechanisms underlying the postulated role of OPG in arterial stiffness may involve endothelial and ventricular dysfunction, inflammation, and calcification.30 The vascular role of OPG is multifaceted and depends on the interplay with its ligands, receptor activator of NF‐κB ligand, and tumor necrosis factor–related apoptosis‐inducing ligand, and a bidirectional modulation involving osteogenic, inflammatory, and apoptotic responses.30 OPG is expressed in vivo by endothelial cells, vascular smooth muscle cells, and osteoblasts.7 The production of OPG is enhanced by inflammatory cytokines and may reflect endothelial dysfunction. Additionally, the failing myocardium, plaque rupture, and other inflamed tissues could contribute to elevated circulating OPG concentrations.7, 30 Studies in vitro and in animal models suggest that OPG inhibits vascular calcification.30 In addition to inhibiting apoptotic passive calcification, the ability of OPG to inhibit alkaline phosphatase–mediated osteogenic differentiation of vascular cells is also likely to contribute to the protective role of OPG.30 Clinical studies also suggest that an increase in serum OPG levels is associated with vascular calcification, coronary artery disease, stroke, and future cardiovascular events.30 Serum OPG was positively associated with c‐f PWV in healthy patients, patients with peripheral artery disease, patients with chronic kidney disease, hemodialysis patients, and with the extent of coronary artery disease.31, 32, 33, 34 Serum OPG level was significantly related to severity and 10‐year progression of carotid atherosclerosis.8 Elevated serum concentrations of OPG are found in a range of cardiovascular pathologies, suggesting the potential value of OPG as a biomarker of vascular risk and prognosis.30 Our study showed that serum log‐OPG concentrations were positively correlated with c‐f PWV levels among hypertensive patients. Multivariable forward stepwise linear regression analysis of the significant variables also showed that serum log‐OPG concentration was also an independent predictor of c‐f PWV levels in the current study.
Pharmacologic interventions have been shown to influence c‐f PWV in humans. Specifically, a significantly greater reduction from baseline with valsartan/hydrochlorothiazide compared with amlodipine has been shown with aortic PWV.35 However, one of the interesting findings from the Conduit Artery Function Evaluation (CAFE]), the Preterax in Regression of Arterial Stiffness in a Controlled Double‐Blind Study (REASON), and the Effect of the Fixed Dose Combination Amlodipine/Valsartan on Central Aortic Blood Pressure in Uncontrolled Essential Hypertension With Amlodipine 5 mg (EXPLOR) trials is that different antihypertension regimens produced clear differential effects on central aortic systolic and pulse pressures but little difference in PWV between the treatments compared in the studies.36 In addition, a systematic review paper also could not safely conclude the effect of statins on arterial stiffness, as estimated by PWV measurements.37 Our results did not show a relationship between statins and fibrate or other medications (ARBs, ACE inhibitors, CCBs, β‐blockers, aspirins, or clopidogrel) and c‐f PWV levels among patients with hypertension. Further studies are therefore required to elucidate the relationship between medication and c‐f PWV levels in hypertensive patients.
Study Limitations
Our study has some limitations. First, this study had a cross‐sectional design. Therefore, our findings should be investigated in long‐term prospective studies before a causal relationship between serum OPG levels and c‐f PWV in hypertensive patients can be established. Second, although we take into account significant variables in the association of c‐f PWV, we cannot conclusively exclude residual confounding that may possibly affect our models. Further studies are needed to show the effects of OPG on c‐f PWV in patients with hypertension.
Conclusions
We showed an independent association of c‐f PWV with log‐OPG, age, Cre, and HDL‐C in hypertensive patients. OPG levels can be used in the future for the determination of hypertension in patients with arterial stiffness and requires further investigation.
Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
J Clin Hypertens (Greenwich). 2014;00:301–308. DOI: 10.1111/jch.12288. © 2014 Wiley Periodicals, Inc.
References
- 1. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 2. Tomiyama H, Yamashina A. Non‐invasive vascular function tests: their pathophysiological background and clinical application. Circ J. 2010;74:24–33. [DOI] [PubMed] [Google Scholar]
- 3. Tomlinson LA. Methods for assessing arterial stiffness: technical considerations. Curr Opin Nephrol Hypertens. 2012;21:655–660. [DOI] [PubMed] [Google Scholar]
- 4. Laurent S, Boutouyrie P. Arterial stiffness: a new surrogate end point for cardiovascular disease? J Nephrol. 2007;20(Suppl 12):S45–S50. [PubMed] [Google Scholar]
- 5. Wang X, Keith JC Jr, Struthers AD, et al. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc Ther. 2008;26:214–223. [DOI] [PubMed] [Google Scholar]
- 6. Persy V, D'Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15:405–416. [DOI] [PubMed] [Google Scholar]
- 7. Venuraju SM, Yerramasu A, Corder R, et al. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010;55:2049–2061. [DOI] [PubMed] [Google Scholar]
- 8. Speer G, Fekete BC, El Hadj Othmane T, et al. Serum osteoprotegerin level, carotid‐femoral pulse wave velocity and cardiovascular survival in haemodialysis patients. Nephrol Dial Transplant. 2008;23:3256–3262. [DOI] [PubMed] [Google Scholar]
- 9. Kiechl S, Schett G, Wenning G, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–2180. [DOI] [PubMed] [Google Scholar]
- 10. Blacher J, Asmar R, Djane S, et al. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. [DOI] [PubMed] [Google Scholar]
- 11. Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. [DOI] [PubMed] [Google Scholar]
- 12. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. I. Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 13. Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. [DOI] [PubMed] [Google Scholar]
- 14. Hsu BG, Chen YC, Lee RP, et al. Fasting serum level of fatty‐acid‐binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ J. 2010;74:327–331. [DOI] [PubMed] [Google Scholar]
- 15. Hsu BG, Ho GJ, Lee CJ, et al. Inverse association of serum long‐acting natriuretic peptide and bone mass density in renal transplant recipients. Clin Transplant. 2012;26:E105–E110. [DOI] [PubMed] [Google Scholar]
- 16. Norton GR, Majane OH, Maseko MJ, et al. Brachial blood pressure‐independent relations between radial late systolic shoulder‐derived aortic pressures and target organ changes. Hypertension. 2012;59:885–892. [DOI] [PubMed] [Google Scholar]
- 17. Sonne‐Holm S, Sørensen TI, Jensen G, et al. Independent effects of weight change and attained body weight on prevalence of arterial hypertension in obese and non‐obese men. BMJ. 1989;299:767–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity‐related hypertension: pathogenesis, cardiovascular risk, and treatment‐A position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich). 2013;15:14–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chuang SY, Hsu PF, Chang HY, et al. C‐reactive protein predicts systolic blood pressure and pulse pressure but not diastolic blood pressure: the cardiovascular disease risk factors two‐township study. Am J Hypertens. 2013;26:657–664. [DOI] [PubMed] [Google Scholar]
- 20. Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. [DOI] [PubMed] [Google Scholar]
- 21. Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–539. [DOI] [PubMed] [Google Scholar]
- 22. van den Bogaard B, Holleboom AG, Duivenvoorden R, et al. Patients with low HDL‐cholesterol caused by mutations in LCAT have increased arterial stiffness. Atherosclerosis. 2012;225:481–485. [DOI] [PubMed] [Google Scholar]
- 23. Wang F, Ye P, Luo L, et al. Association of serum lipids with arterial stiffness in a population‐based study in Beijing. Eur J Clin Invest. 2011;41:929–936. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Du Y, Fan L, et al. Relationships between HDL‐C, hs‐CRP, with Central Arterial Stiffness in Apparently Healthy People Undergoing a General Health Examination. PLoS ONE. 2013;8:e81778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Besler C, Lüscher TF, Landmesser U. Molecular mechanisms of vascular effects of high‐density lipoprotein: alterations in cardiovascular disease. EMBO Mol Med. 2012;4:251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Briet M, Boutouyrie P, Laurent S, et al. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82:388–400. [DOI] [PubMed] [Google Scholar]
- 27. Bian SY, Guo HY, Ye P, et al. Association of glomerular filtration rate with arterial stiffness in Chinese women with normal to mildly impaired renal function. J Geriatr Cardiol. 2012;9:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schillaci G, Pirro M, Mannarino MR, et al. Relation between renal function within the normal range and central and peripheral arterial stiffness in hypertension. Hypertension. 2006;48:616–621. [DOI] [PubMed] [Google Scholar]
- 29. Andrade J, Er L, Ignaszewski A, Levin A. Exploration of association of 1, 25‐OH2D3 with augmentation index, a composite measure of arterial stiffness. Clin J Am Soc Nephrol. 2008;3:1800–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Campenhout A, Golledge J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis. 2009;204:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zagura M, Serg M, Kampus P, et al. Association of osteoprotegerin with aortic stiffness in patients with symptomatic peripheral artery disease and in healthy subjects. Am J Hypertens. 2010;23:586–591. [DOI] [PubMed] [Google Scholar]
- 32. Scialla JJ, Leonard MB, Townsend RR, et al. Correlates of osteoprotegerin and association with aortic pulse wave velocity in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2612–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakashima A, Carrero JJ, Qureshi AR, et al. Plasma osteoprotegerin, arterial stiffness, and mortality in normoalbuminemic Japanese hemodialysis patients. Osteoporos Int. 2011;22:1695–1701. [DOI] [PubMed] [Google Scholar]
- 34. Tousoulis D, Siasos G, Maniatis K, et al. Serum osteoprotegerin and osteopontin levels are associated with arterial stiffness and the presence and severity of coronary artery disease. Int J Cardiol. 2013;167:1924–1928. [DOI] [PubMed] [Google Scholar]
- 35. Karalliedde J, Smith A, DeAngelis L, et al. Valsartan improves arterial stiffness in type 2 diabetes independently of blood pressure lowering. Hypertension. 2008;51:1617–1623. [DOI] [PubMed] [Google Scholar]
- 36. Williams B. Evaluating interventions to reduce central aortic pressure, arterial stiffness and morbidity–mortality. J Hypertens. 2012;30(Suppl):S13–S18. [DOI] [PubMed] [Google Scholar]
- 37. Rizos EC, Agouridis AP, Elisaf MS. The effect of statin therapy on arterial stiffness by measuring pulse wave velocity: a systematic review. Curr Vasc Pharmacol. 2010;8:638–644. [DOI] [PubMed] [Google Scholar]


