Abstract
Overactivity of the epithelial sodium channel (ENaC) is considered to be one mechanism underlying obesity‐related blood pressure (BP) elevation. In an open‐labeled, nonplacebo‐controlled clinical trial (Clinicaltrials.gov: NCT‐01308983), the authors aimed to comprehensively evaluate the effects of amiloride monotherapy, an ENaC blocker, on BP and cardiovascular risk in young adults with prehypertension (n=17). The mean body mass index of participants was 28.45±1.30 kg/m2. Following 10 mg daily amiloride for 4 weeks, peripheral systolic BP (SBP), central SBP, and carotid‐radial pulse wave velocity were significantly reduced by −7.06±2.25 mm Hg, −7.68±2.56 mm Hg, and −0.72±0.33 m/s, respectively, whereas flow‐mediated dilation was significantly increased by 2.2±0.9%. Following amiloride monotherapy for 4 weeks, a significant increase in serum aldosterone was observed (5.85±2.45 ng/dL). ENaC inhibition by amiloride may be used as an early intervention to halt the progression to full hypertension and cardiovascular disease in young adults with prehypertension.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) introduced the new term prehypertension for adults with systolic blood pressure (SBP) of 120 mm Hg to 139 mm Hg and/or diastolic blood pressure (DBP) of 80 mm Hg to 89 mm Hg to recognize the negative impact of BP elevation on cardiovascular disease (CVD) risk.1 Utilizing the National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2006, Gupta and colleagues2 reported the prevalence of prehypertension in disease‐free adults to be 36.3%. It is estimated that approximately 40% of individuals with prehypertension progress to hypertension within 2 years.3 As such, it is critical to intervene early to stop the progression of prehypertension to hypertension, and hypertension to complicated hypertension later in life.
The JNC 7 recommends a lifestyle modification approach including weight loss, dietary changes, and regular aerobic exercise for the treatment of prehypertension. In fact, several randomized controlled trials have demonstrated the effectiveness of weight loss and dietary changes. Specifically, reductions in sodium intake and increased consumption of fruits and vegetables, ie, adoption of the Dietary Approaches to Stop Hypertension (DASH) diet, have been shown to lower BP over time and thus reduce the future risk of hypertension and CVD.4, 5, 6 However, the effectiveness of these lifestyle interventions has been inconsistent outside of the controlled environment of clinical trials.7 There was low accordance with DASH dietary pattern among adults with known hypertension in the United States,8 suggesting that lifestyle modification is not only hard to adopt, but also difficult to develop into habits in daily life. To date, two clinical trials using pharmacologic interventions have shown benefits of preventing and/or delaying de novo hypertension in individuals with prehypertension. Julius and colleagues3 showed a 66% relative risk reduction in the incidence of new‐onset hypertension following 2 years of treatment with the angiotensin receptor blocker (ARB) candesartan as compared with placebo in adults with prehypertension. Using a similar pharmacologic approach, Luders and colleagues9 reported a 34% relative risk reduction after 3 years in the development of hypertension in prehypertensive patients who were randomly assigned to take an angiotensin‐converting enzyme (ACE) inhibitor, ramipril, vs patients assigned to placebo.
Amiloride, a well‐tolerated potassium‐sparing diuretic, blocks the epithelial sodium channel (ENaC). ENaC, comprised of α, β, and γ subunits and primarily expressed on the apical membrane of epithelial cells in the renal cortical collecting duct, is known to play an important role in BP homeostasis.10, 11, 12 A clinical trial evaluating the usefulness of pharmacologic intervention using diuretics, chlorthalidone plus amiloride, in individuals with prehypertension to reduce incidence of hypertension and adverse CV events is currently underway in Brazil.7 In the clinical trial, amiloride (2.5 mg/d) is used to prevent the deleterious hypokalemia induced by chlorthalidone. However, the effects of pharmacologic blockade of ENaC by amiloride as monotherapy remain unknown in individuals with prehypertension.
Accumulating evidence indicates that central BP, measured noninvasively, may be a stronger and more accurate predictor of future CV events than peripheral BP.13, 14 Brachial artery flow‐mediated dilation (FMD) has been recognized as a valid and reliable noninvasive biomarker of nitric oxide–dependent vasodilation and endothelial function.15 Pulse wave velocity (PWV) is a noninvasive surrogate marker of arterial stiffness.16 In the present study, we aimed to investigate the effects of amiloride monotherapy on peripheral and central BP as well as on CVD risk including FMD and PWV in drug‐naive African American and Caucasian adults with prehypertension.
Methods
Experimental Design
This was an open‐labeled, nonplacebo‐controlled clinical trial (Clinicaltrials.gov: NCT01308983).
Study Participants
Participants were recruited from local communities of Augusta, Georgia, and surrounding areas via flyer distribution and word of mouth. The inclusion criteria were age between 18 and 35 years, Caucasian or African American confirmed by self‐report, and peripheral BP in the prehypertensive range (ie, 120–139/80–89 mm Hg). The exclusion criteria were history of any acute or chronic medical illnesses, use of any prescription or over‐the‐counter medications including vitamins or herbal supplements, pregnant and breast‐feeding women, and serum potassium >5.5 mEq/L and/or serum creatinine >1.4 mg/dL (or estimated glomerular filtration rate <60 mL/min/1.73m2) at baseline. The study was approved by the Human Assurance Committee of the Georgia Regents University (GRU). All participants provided written informed consent before testing. Of 61 participants screened, 17 prehypertensives were enrolled to take 10 mg of daily amiloride for 4 weeks. Amiloride was dispensed by the clinical research pharmacy at GRU. All participants were instructed to take amiloride in the morning with breakfast. Compliance with the amiloride monotherapy was assessed by pill counts at week 4.
Anthropometrics, Vitals, Blood, and Urine Collection
Height and body weight were measured to calculate body mass index (BMI). Waist and hip circumferences were assessed by a measuring tape at the level of the umbilicus and around the widest portion of the buttocks. Three peripheral BP and heart rate (HR) readings, each 1 minute apart, were obtained by automated Dinamap monitor (Critikon, Tampa, FL) after 5 to 10 minutes of rest with the use of an appropriately sized cuff wrapped on the nondominant arm in the supine position. Three peripheral BP and HR readings were averaged and used for final analysis. Fasting blood and spot urine samples were collected at baseline and at week 4, frozen at −80°C, and were later used for the biochemical measurement.
Central BP and PWV Assessment
Central BP, carotid femoral PWV (CF‐PWV), carotid radial PWV (CR‐PWV), and augmentation index adjusted for heart rate at 75 (AI%HR75) were measured noninvasively using applanation tonometry (Miller Instrument, Houston, TX) and SphygmoCor device (AtCor Medical, Sydney, Australia).17 The SphygmoCor device estimates the central BP by applying a generalized transfer function based on the tonometric readings of the radial pulse wave form.14
FMD Measurement
FMD was performed in accordance with the tutorial on the ultrasound assessment of FMD.18 As described previously,19 with the use of a 12‐MHz linear transducer, simultaneous B‐mode and blood velocity profiles (duplex mode) of the brachial artery were obtained (Logiq 7; GE Medical Systems, Milwaukee, WI). A forearm occlusion cuff (D.E. Hokanson, Bellevue, WA), placed immediately distal to the medial epicondyle, was rapidly inflated to 250 mm Hg for 5 minutes (E‐20 rapid cuff inflator; D.E. Hokanson, Bellevue, WA) to induce arterial occlusion and subsequent reactive hyperemia of the brachial artery. Electrocardiographic gating (Accusync 72; Accusync Medical Research, Milford, CT) was utilized to capture end‐diastolic arterial diameters for automated offline analysis of brachial artery vasodilation (Medical Imaging Applications, Coralville, IO).
Renin and Aldosterone Measurements
Plasma renin activity (PRA) was measured by radioimmunoassay (Mayo Medical Laboratories, Rochester, MN) and serum aldosterone concentration was measured by liquid chromatography‐tandem mass spectrometry (Mayo Medical Laboratories). Interassay coefficient of variations for PRA and serum aldosterone were 15% and 18.1%, respectively.
Statistical Analyses
Descriptive statistics are presented as mean±standard error of mean (SEM). Continuous data were checked for normality using the Shapiro‐Wilk test and quantile‐quantile plot. Following 4 weeks of amiloride monotherapy, changes in the outcome variables (peripheral BP, central BP, FMD, CF‐PWV, CR‐PWV, AI%HR75, serum electrolytes, urine electrolytes, PRA, and serum aldosterone) were evaluated by a paired t test if the variables were normally distributed or by Wilcoxon signed rank test. Pearson's correlation analysis was performed to assess correlations among changes (mean value at week 4 minus mean value at baseline) in FMD and CR‐PWV and changes in peripheral and central BP. All tests were conducted two‐sided and a P value ≤.05 was considered statistically significant. Statistical analyses were performed using SPSS software (version 19.0; IBM Inc, Chicago, IL).
Results
Baseline clinical characteristics of participants are presented in Table 1. The mean age and BMI were 26±1 years and 28.45±1.30 kg/m2, respectively. The average compliance by pill counts was 92±2%. No serious adverse event occurred during the course of a 4‐week study period.
Table 1.
Clinical Characteristics of Participants
| Baseline (N=17) | |
|---|---|
| Age, y | 26±1 |
| Men/women | 11/6 |
| Caucasians/African Americans | 9/8 |
| Height, cm | 170.69±2.59 |
| Weight, kg | 84.32±4.57 |
| BMI, kg/m2 | 28.45±1.30 |
| Waist circumference, cm | 99.87±3.02 |
| WHR | 0.89±0.01 |
Abbreviations: BMI, body mass index; WHR: waist to hip ratio. Values are expressed as mean±standard error of the mean.
Peripheral and Central BP
As shown in Table 2, there were significant reductions (Δ mean±SEM) in peripheral SBP (−7.06±2.25 mm Hg) (Figure 1A), peripheral DBP (−4.35±1.67 mm Hg), central SBP (−7.68±2.56 mm Hg) (Figure 1B), and central DBP (−4.49±1.78 mm Hg) after 4 weeks of amiloride monotherapy. No changes in HR and weight from baseline to posttest were observed. Reductions in peripheral and central BPs following amiloride monotherapy did not differ between Caucasians (N=9) and African Americans (N=8).
Table 2.
Effects of Amiloride on BP and CVD Risk
| Baseline (N=17) | 4‐Week (N=17) | P Value | |
|---|---|---|---|
| BP, HR, and weight | |||
| Peripheral SBP, mm Hg | 127.29±2.05 | 120.24±1.73 | <.01 |
| Peripheral DBP, mm Hg | 68.59±2.29 | 64.24±2.08 | .02 |
| Central SBP, mm Hg | 110.33±2.95 | 102.65±1.77 | .01 |
| Central DBP, mm Hg | 69.86±2.40 | 65.38±2.15 | .03 |
| HR, beats per min | 63.16±2.09 | 63.01±2.35 | .94 |
| Weight, kg | 84.33±4.57 | 83.87±4.81 | .50 |
| CVD risk | |||
| FMD, % | 5.3±0.8 | 7.4±0.8 | .03 |
| FMD/Shear, %/s−1, AUC | 0.12±0.02 | 0.18±0.04 | .05 |
| CF‐PWV, m/s | 6.58±0.35 | 6.57±0.21 | .96 |
| CR‐PWV, m/s | 8.21±0.39 | 7.50±0.33 | .04 |
| AI%HR75, % | 7.66±3.86 | 5.57±3.56 | .23 |
Abbreviations: AI%HR75, augmentation index adjusted for heart rate at 75; BP, blood pressure; CF‐PWV, carotid‐femoral pulse wave velocity; CR‐PWV, carotid‐radial pulse wave velocity; DBP, diastolic blood pressure; CVD, cardiovascular diseases; FMD, flow‐mediated dilation; HR, heart rate; SBP, systolic blood pressure. Values are expressed as mean±standard error of the mean. P value was obtained from paired t test or Wilcoxon signed rank test. Bold values indicate significance.
Figure 1.
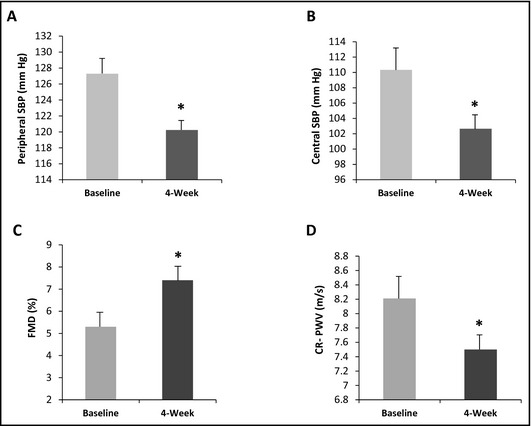
Effects of amiloride on peripheral systolic blood pressure (SBP), central SBP, flow‐mediated dilation (FMD), and carotid‐radial pulse wave velocity (CR‐PWV) in all participants (N=17). *P value <.05. (A) Effects of amiloride on peripheral SBP. (B) Effects of amiloride on central SBP. (C) Effects of amiloride on FMD. (D) Effects of amiloride on CR‐PWV.
FMD and PWV
A significant increase in FMD (2.2±0.9%) (Figure 1C) and reduction in CR‐PWV (−0.72±0.33 m/s) (Figure 1D) were observed at posttest (Table 2). FMD/shear was also improved (0.06±0.03%/s−1, AUC) at week 4. However, no significant changes were observed in CF‐PWV (−0.01±0.28 m/s) and AI%HR75 (−2.09± 1.67%) following amiloride monotherapy (Table 2).
Biochemical Variables
Biochemical changes after amiloride monotherapy are summarized in Table 3. At posttest, an increase in serum concentrations of aldosterone was observed (5.85±2.45 ng/dL). On the other hand, the magnitude of rise in PRA was small and not significant (0.16±0.18 ng/mL/h). There was a significant elevation of aldosterone‐renin‐ratio at week 4 (3.80±1.29 ng/dL/ng/mL/h). The concentrations of both serum potassium (0.21±0.05 mEq/L) and serum creatinine (0.11±0.05 mg/dL) were significantly elevated following 4 weeks of amiloride monotherapy.
Table 3.
Biochemical Changes After Amiloride Treatment
| Baseline (N=17) | Week 4 (N=17) | P Value | |
|---|---|---|---|
| RAAS | |||
| Serum aldosterone, ng/dL | 5.58±0.89 | 11.42±2.36 | .01 |
| Plasma renin activity, ng/mL/h | 1.05±0.18 | 1.21±0.21 | .41 |
| ARR, ng/dL per ng/mL/h | 6.04±1.19 | 9.84±1.61 | <.01 |
| Serum glucose | |||
| Fasting glucose, mg/dL | 88.94±1.90 | 91.82±1.84 | .17 |
| Serum electrolytes | |||
| Potassium, mEq/L | 4.12±0.05 | 4.33±0.06 | .02 |
| Sodium, mEq/L | 139.25±0.86 | 141.25±0.51 | .07 |
| Chloride, mEq/L | 108.06±1.04 | 107.82±0.46 | .83 |
| BUN, mg/dL | 12.76±0.78 | 12.76±0.88 | 1.00 |
| Creatinine, mg/dL | 0.91±0.04 | 1.02±0.06 | .05 |
| eGFR, mL/min/1.73 m2 | 107.15±5.08 | 97.56±4.97 | .17 |
| Urine electrolytes | |||
| Potassium, mEq/L | 33.91±6.09 | 33.73±6.23 | .98 |
| Sodium, mEq/L | 133.93±17.42 | 138.93±18.13 | .80 |
| Chloride, mEq/L | 147.40±16.50 | 150.13±16.69 | .88 |
| Sodium/potassium ratio | 4.67±0.67 | 5.96±1.06 | .35 |
| Creatinine, mg/dL | 154.25±26.97 | 129.43±20.06 | .35 |
Abbreviations: ARR, aldosterone renin ratio; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate‐calculated using the Modification of Diet in Renal Disease formula; RAAS, renin angiotensin aldosterone system. Values are expressed as mean±standard error of the mean. P value was obtained from paired t test or Wilcoxon signed rank test. Bold values indicate significance.
Subgroup Analyses in Stage 2 Prehypertension
Participants with peripheral SBP of 130 mm Hg to 139 mm Hg and/or DBP of 85 mm Hg to 89 mm Hg (ie, stage 2 prehypertension, N=6) had significantly greater mean reductions in peripheral SBP (−14.5±4.60 mm Hg, P<.01, Figure 2A) and central SBP (−16.15±5.44 mm Hg, P<.01, Figure 2B) compared with participants with peripheral SBP of 120 to 129 mm Hg and/or DBP of 80 mm Hg to 84 mm Hg (ie, stage 1 prehypertension, N=11). Moreover, after 4 weeks of amiloride monotherapy in stage 2 vs stage 1 prehypertensive participants, improvements were also greater in CF‐PWV (−1.15±0.49 m/s, P=.01) and AI%HR75 (−7.07±2.19%, P=.02).
Figure 2.
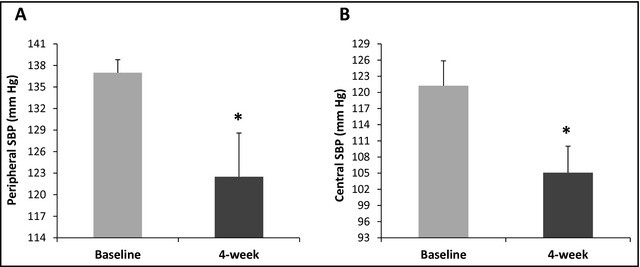
Effects of amiloride on peripheral and central systolic blood pressure (SBP) reduction in stage 2 prehypertensive participants (N=6). *P value <.01. (A) Effects of amiloride on peripheral SBP in stage 2 prehypertensive participants. (B) Effects of amiloride on central SBP in stage 2 prehypertensive participants.
Discussion
To the best of our knowledge, this is the first clinical trial that comprehensively evaluated the effects of 10 mg daily amiloride monotherapy for 4 weeks on peripheral BP, central BP, and CVD risk in apparently healthy prehypertensive individuals. The major findings of this study include the following: (1) amiloride decreased peripheral as well as central BP; (2) amiloride improved endothelial function as evidenced by the increase in FMD; (3) amiloride lowered peripheral arterial stiffness as indicated by the reduction in CR‐PWV; (4) amiloride mediated improvements in FMD and PWV might be independent of its BP‐lowering effects; and (5) amiloride treatment was associated with an elevation of serum aldosterone.
Peripheral and Central BP
Overactivity of the ENaC has been considered as one mechanism underlying obesity‐related hypertension.10 Amiloride has been shown to produce variable effects on peripheral BP in patients with hypertension.20, 21, 22, 23, 24, 25 Previously, Saha and colleagues26 found that ENaC inhibition by amiloride effectively lowered BP in clinically obese patients with hypertension who were already receiving other antihypertensive medications. There is a scarcity of data on the peripheral BP‐lowering effects of amiloride monotherapy in individuals with prehypertension. In a study conducted by Pratt and colleagues27 involving young normotensive individuals, no significant reduction in peripheral BP was observed following 5 mg of daily amiloride for 4 weeks. In contrast, Stears and colleagues28 reported a 7/2 mm Hg reduction in peripheral SBP/DBP with amiloride in titrated dosing with 20 mg/d as the maximal dose for 4 weeks in elderly patients with essential hypertension. In our study, the mean BMI was 28.45±1.30 kg/m2, suggesting that on average, participants were overweight or obese. The extent of drop in peripheral BP (ie, 8/4 mm Hg) by 10 mg/d amiloride for 4 weeks in drug‐naive prehypertensive individuals was greater than what was reported by Stears and colleagues in elderly treated hypertensive patients. The greater decline in BP in our study could likely be the result of missing placebo subtraction.29 However, it is also possible that amiloride might be more effective for young adults who are drug‐naive vs elderly hypertensive patients who, in general, are taking multiple medications for a variety of chronic health issues. To the best of our knowledge, the effects of amiloride monotherapy on central BP in individuals with prehypertension have not been explored until now. Our finding that amiloride lowered central BP in individuals with prehypertension appears to be in line with that of Matthesen and colleagues who observed a reduction in central BP by 10 mg daily amiloride for 4 weeks in patients with arterial hypertension.30 Central BP, owing to its proximity to the heart and central vasculature, may be more important in the context of future CV events.14 Accordingly, reducing central BP using amiloride monotherapy may provide an added benefit for the treatment of prehypertension that is not recognized by measuring brachial peripheral BP alone. In addition, in our subgroup analysis, we observed almost 2‐fold greater reductions in peripheral and central SBP in individuals with stage 2 prehypertension compared with those with stage 1 prehypertension. Also, improvements in CVD risk such as PWV and augmentation index were noticeably higher in individuals with stage 2 vs stage 1 prehypertension. We postulate that efficacy of amiloride might be enhanced when BP is higher in drug‐naive young adults.
FMD and PWV
In the present study, amiloride in a daily dose of 10 mg for 4 weeks improved endothelial function assessed by FMD in participants with prehypertension. Earlier, Farquharson and Struthers31 observed no improvement in stimulated endothelial function assessed by forearm venous occlusion plethysmography with 1 month of treatment with 5 mg daily amiloride. In contrast to our findings, the disparity in their findings could be explained by the differences in the doses of amiloride, the techniques of assessing endothelial function, and clinical characteristics of study participants. While all participants in our study were apparently healthy and medication‐naive, Farquharson and Struthers studied patients with mild to moderate congestive heart failure who were taking other medications. In addition to improving FMD, amiloride also lowered CR‐PWV. The study by Matthesen and colleagues30 also reported a reduction in PWV with 10 mg of amiloride daily for 4 weeks in patients with arterial hypertension. The exact mechanisms by which amiloride improves vascular function remain unknown. Evidence from in vitro studies indicates that the ENaC that is present on vascular smooth muscle cells mediates vasoconstriction,32, 33 while the endothelial ENaC exerts its deleterious effects on the endothelial cells by increasing cell swelling and stiffness under the influence of aldosterone and also by inhibiting nitric oxide production stimulated by shear stress.34, 35 Moreover, these actions of ENaC on the endothelium were shown to be effectively inhibited by amiloride. Taken together, we postulate that amiloride improves FMD and PWV by blocking vascular ENaC. However, the inhibitory effects of amiloride on other ion channels that are present in the vasculature such as Na+/H+ exchanger and Na+/Ca+ exchanger cannot be fully excluded.36 Nonetheless, the evidence suggests that amiloride, in a concentration ≤1 μmol/L (~5 mg/d amiloride), selectively and more efficiently inhibits ENaC than other ion channels.36, 37 It is also possible that the decrease in BP by amiloride caused by inhibition of tubular ENaC (ie, ENaC present in kidney) might have accounted for the improvement in FMD and PWV. However, in the correlation analysis, mean changes in FMD and PWV did not correlate with mean changes in either peripheral BP or central BP, suggesting that amiloride‐mediated improvement in FMD and PWV could be independent of reduction in BP. In other words, inhibition of vascular ENaC could be independent of inhibition of tubular ENaC. Another speculation is that increase in body potassium concentration may improve endothelial dysfunction.38 However, Farquharson and Struthers31 did not notice a decrease in endothelial dysfunction by amiloride despite a 0.4 mEq/L rise in serum potassium.
Biochemistry
The level of serum aldosterone increased significantly after 4 weeks of daily amiloride monotherapy, but the elevation in PRA was small and insignificant. While elevation in both serum aldosterone and PRA appears to have been mediated through the renin‐angiotensin‐aldosterone system, an additional direct stimulating effect of potassium retention may contribute to a greater increase in serum aldosterone vs PRA.39 Even though we noted an increase in serum potassium concentrations at posttest, the values remained within normal physiological range. This indicates that short‐term amiloride monotherapy might be safe and well‐tolerable for treatment in drug‐naive prehypertensive participants. The possibility that accompanied by the increase of aldosterone and the resultant activation of the renin‐angiotensin‐aldosterone system (RAAS), amiloride may still be able to lower BP and improve CVD risk could open up new RAAS‐independent therapeutic discovery opportunities. In addition, whereas the direct effect of amiloride to block ENaC limits potassium secretion, the subsequent increase of potassium enhances aldosterone secretion. Future studies are warranted to tease apart the effects of amiloride and amiloride analogs on aldosterone stimulation to see if they would be distinguished from their direct effect on ENaC.40
Strengths and Limitations
The novelty of this study includes comprehensive assessments of amiloride monotherapy on peripheral and central BP as well as CVD risk such as FMD and PWV in drug‐naive prehypertensives. There were limitations of our study. First, our study is limited by the absence of a placebo arm or other active treatments for comparison. Second, our sample size was relatively small. Third, ambulatory BP measurement, which might be very useful for correct interpretation of the results, was not included in our study.
Conclusions
For the first time, we observed a significant improvement in peripheral BP along with central BP, FMD, and PWV following 10 mg of daily amiloride as monotherapy in young adults with prehypertension, particularly stage 2 prehypertension. Effects of amiloride on FMD and PWV (ie, inhibition of vascular ENaC) appear to be independent of its BP‐lowering effects (ie, inhibition of tubular ENaC). Amiloride is well‐tolerated and devoid of any major side effects, which make its use desirable as early intervention to halt the progression to full hypertension and CVD. Larger placebo‐controlled, randomized clinical trials of amiloride with dose‐ranging regimen, longer‐term treatment, and inclusion of ambulatory BP measurement are warranted.
Acknowledgments and disclosures
The authors would like to thank all of the participants and the GRU clinical research pharmacy staff (Marjorie Phillips and Darla Irwin) who made this study possible. Funding was provided by Diabetes and Obesity Discovery Institute of GRU and National Institutes of Health grants L077230. The authors declare no conflicts of interest.
J Clin Hypertens (Greenwich). 2014;16:47–53. ©2013 Wiley Periodicals, Inc.
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Gupta AK, McGlone M, Greenway FL, Johnson WD. Prehypertension in disease‐free adults: a marker for an adverse cardiometabolic risk profile. Hypertens Res. 2010;33:905–910. [DOI] [PubMed] [Google Scholar]
- 3. Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin‐receptor blocker. N Engl J Med. 2006;354:1685–1697. [DOI] [PubMed] [Google Scholar]
- 4. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high‐normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 5. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 6. He J, Whelton PK, Appel LJ, et al. Long‐term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35:544–549. [DOI] [PubMed] [Google Scholar]
- 7. Fuchs FD, Fuchs SC, Moreira LB, et al. Prevention of hypertension in patients with pre‐hypertension: protocol for the PREVER‐prevention trial. Trials. 2011;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168:308–314. [DOI] [PubMed] [Google Scholar]
- 9. Luders S, Schrader J, Berger J, et al. The PHARAO study: prevention of hypertension with the angiotensin‐converting enzyme inhibitor ramipril in patients with high‐normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens. 2008;26:1487–1496. [DOI] [PubMed] [Google Scholar]
- 10. Bubien JK. Epithelial Na+ channel (ENaC), hormones, and hypertension. J Biol Chem. 2010;285:23527–23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pratt JH. Central role for ENaC in development of hypertension. J Am Soc Nephrol. 2005;16:3154–3159. [DOI] [PubMed] [Google Scholar]
- 12. Su YR, Menon AG. Epithelial sodium channels and hypertension. Drug Metab Dispos. 2001;29(4 Pt 2):553–556. [PubMed] [Google Scholar]
- 13. Nelson MR, Stepanek J, Cevette M, et al. Noninvasive measurement of central vascular pressures with arterial tonometry: clinical revival of the pulse pressure waveform? Mayo Clin Proc. 2010;85:460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. [DOI] [PubMed] [Google Scholar]
- 15. Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow‐mediated dilation for incident cardiovascular events in a population‐based study: the multi‐ethnic study of atherosclerosis. Circulation. 2009;120:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mattace‐Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. [DOI] [PubMed] [Google Scholar]
- 17. Dong Y, Stallmann‐Jorgensen IS, Pollock NK, et al. A 16‐week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25‐hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95:4584–4591. [DOI] [PubMed] [Google Scholar]
- 18. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow‐mediated dilation. Hypertension. 2010;55:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris RA, Pedersen‐White J, Guo DH, et al. Vitamin D3 supplementation for 16 weeks improves flow‐mediated dilation in overweight African‐American adults. Am J Hypertens. 2011;24:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Multiclinic comparison of amiloride, hydrochlorothiazide, and hydrochlorothiazide plus amiloride in essential hypertension. Multicenter Diuretic Cooperative Study Group. Arch Intern Med. 1981;141:482–486. [PubMed] [Google Scholar]
- 21. Gombos EA, Freis ED, Moghadam A. Effects of MK‐870 in normal subjects and hypertensive patients. N Engl J Med. 1966;275:1215–1220. [DOI] [PubMed] [Google Scholar]
- 22. Katzman PL, Henningsen NC, Hulthen UL. Amiloride compared with nitrendipine in treatment of essential hypertension. J Hum Hypertens. 1988;2:147–151. [PubMed] [Google Scholar]
- 23. Laragh JH. The proper use of newer diuretics. Ann Intern Med. 1967;67:607–613. [PubMed] [Google Scholar]
- 24. Paterson JW, Dollery CT, Haslam RM. Amiloride hydrochloride in hypertensive patients. Br Med J. 1968;1:422–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas JP, Thomson WH. Comparison of thiazides and amiloride in treatment of moderate hypertension. Br Med J. 1983;286:2015–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saha C, Eckert GJ, Ambrosius WT, et al. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005;46:481–487. [DOI] [PubMed] [Google Scholar]
- 27. Pratt JH, Eckert GJ, Newman S, Ambrosius WT. Blood pressure responses to small doses of amiloride and spironolactone in normotensive subjects. Hypertension. 2001;38:1124–1129. [DOI] [PubMed] [Google Scholar]
- 28. Stears AJ, Woods SH, Watts MM, et al. A double‐blind, placebo‐controlled, crossover trial comparing the effects of amiloride and hydrochlorothiazide on glucose tolerance in patients with essential hypertension. Hypertension. 2012;59:934–942. [DOI] [PubMed] [Google Scholar]
- 29. Heran BS, Chen JM, Wang JJ, Wright JM. Blood pressure lowering efficacy of potassium‐sparing diuretics (that block the epithelial sodium channel) for primary hypertension. Cochrane Database of Systematic Reviews. 2012;11:CD008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matthesen SK, Larsen T, Vase H, et al. Effect of amiloride and spironolactone on renal tubular function and central blood pressure in patients with arterial hypertension during baseline conditions and after furosemide: a double‐blinded, randomized, placebo‐controlled crossover trial. Clin Exp Hypertens. 2013;35:313–324. [DOI] [PubMed] [Google Scholar]
- 31. Farquharson CA, Struthers AD. Increasing plasma potassium with amiloride shortens the QT interval and reduces ventricular extrasystoles but does not change endothelial function or heart rate variability in chronic heart failure. Heart. 2002;88:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol. 2005;289:F891–F901. [DOI] [PubMed] [Google Scholar]
- 33. Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension. 2004;44:643–648. [DOI] [PubMed] [Google Scholar]
- 34. Oberleithner H, Riethmuller C, Schillers H, et al. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA. 2007;104:16281–16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pérez FR, Venegas F, González M, et al. Endothelial epithelial sodium channel inhibition activates endothelial nitric oxide synthase via phosphoinositide 3‐kinase/Akt in small‐diameter mesenteric arteries. Hypertension. 2009;53:1000–1007. [DOI] [PubMed] [Google Scholar]
- 36. Teiwes J, Toto RD. Epithelial sodium channel inhibition in cardiovascular disease. A potential role for amiloride. Am J Hypertens. 2007;20:109–117. [DOI] [PubMed] [Google Scholar]
- 37. Kleyman TR, Cragoe EJ. Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1–21. [DOI] [PubMed] [Google Scholar]
- 38. Taddei S, Mattei P, Virdis A, et al. Effect of potassium on vasodilation to acetylcholine in essential hypertension. Hypertension. 1994;23:485–490. [DOI] [PubMed] [Google Scholar]
- 39. Bull MB, Laragh JH. Amiloride. A potassium‐sparing natriuretic agent. Circulation. 1968;37:45–53. [DOI] [PubMed] [Google Scholar]
- 40. Warnock DG, Bell PD. Improvement of blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005;46:469–470. [DOI] [PubMed] [Google Scholar]


