Abstract
The authors assessed the association between the ratio of urinary activity of N‐acetyl‐β‐D‐glucosaminidase (NAG) to creatinine and the brachial‐ankle pulse wave velocity (baPWV) in patients without overt diabetes mellitus (DM). This was a cross‐sectional study of 233 patients who had an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2 and no history of kidney disease. Patients were divided into two groups: high NAG group (>5.8 U/g creatinine) and low NAG group (≤5.8 U/g creatinine). Mean baPWVs of the high NAG group were significantly higher than those of the low NAG group in both the eGFR ≥30 and <60 tertiles and the eGFR ≥60 and <90 tertiles. The baPWV was positively correlated with NAG in all patients (r=0.341, P<.001). Stepwise multivariate regression analysis showed that the baPWV was significantly related with NAG, age, and systolic blood pressure. Elevated NAG is related to elevated arterial stiffness in patients without DM.
Increased arterial stiffness is associated with an increased risk of cardiovascular (CV) disease.1 Pulse wave velocity (PWV), an index of arterial stiffness, can be measured with noninvasive means. Although the aortic (carotid‐femoral) PWV is currently the gold standard for measuring arterial stiffness,2 the brachial‐ankle PWV (baPWV) is also a good marker that can be measured without specialized technical skills and is more applicable for studies in the general population.3, 4 In addition, the baPWV has been shown to be related to Framingham 10‐year coronary heart disease risk.5
On the other hand, the risk of sudden cardiac death is increased by renal insufficiency, which is also, reportedly, a predictor of CV events.6, 7 Furthermore, the risks of morbidity and mortality are increased in patients with heart failure and signs of renal tubular damage.8, 9 Urinary N‐acetyl‐β‐D‐glucosaminidase (NAG) has been used as a marker of renal tubular damage. The lysosomal enzyme NAG has a molecular weight of 140 kDa and is widely distributed, with highest concentrations in the proximal tubules.10 Thus, increased urinary levels of NAG indicate damage to the proximal tubules. In the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI‐Prevenzione) trial, the urinary level of NAG was more consistently and more strongly related to poor outcomes in patients with chronic heart failure than were urinary levels of neutrophil gelatinase‐associated lipocalin (NGAL) or kidney injury molecule 1 (KIM‐1).8 A study of elderly patients with type 2 diabetes has found a positive predictive trend of NAG excretion for the development of myocardial infarction and peripheral vascular disease.11 Thus, the importance of urinary NAG as a clinical marker of renal tubular function has been confirmed in several studies.
Recent findings have confirmed the value of arterial stiffness for predicting CV events and the progression of kidney disease,12 and a previous study has shown that baPWV is associated with the progression of chronic kidney disease.13 Several studies have found an association between baPWV and albuminuria.14, 15, 16 However, to our knowledge, the association of renal tubular function to baPWV or to PWV has not been investigated in detail. Additionally, no studies have evaluated the association between PWV and urinary NAG. Therefore, the aim of the present cross‐sectional study was to examine the association between baPWV and urinary NAG by means of multivariate analysis.
Research Design and Methods
Study Patients
The patients included 79 men and 154 women (total of 233; age range, 25–90 years) who were outpatients of our hospital of geriatrics. Some of these patients had undergone routine periodic health examinations, which yielded abnormal findings, ie, increases in blood pressure (BP) and serum levels of glucose, cholesterol, triglycerides, and gamma‐glutamyl transferase. Thereafter, the patients came to our hospital as outpatients for further evaluation of these findings. Some outpatients had been found to have hypertension, dyslipidemia, or impaired glucose tolerance and were being treated for these conditions. The patients had no severe renal dysfunction (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2) and no history of kidney disease or proteinuria. Patients were excluded if they had been transported to our department by ambulance. All patients were able to walk without support and had neither anorexia nor stress conditions that might have affected glycemic conditions.
Other exclusion criteria were pregnancy; severe illness; previous gastrectomy; anemia; overt diabetes (glycated hemoglobig [HbA1c] ≥6.9% or fasting plasma glucose level ≥126 mg/dL); renal glucosuria; urine protein test ≥2+ (equivalent to ≥1.0 g/L); history of CV disease, other vascular disease, liver cirrhosis, or chronic hepatitis; and the use of oral hypoglycemic agents, insulin, or steroids. Patients were considered to have hypertension if they had previously received a diagnosis of hypertension (systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg or both) or were being treated with antihypertensive agents.
Before the study started, each patient received detailed information regarding the study protocol and then provided their informed consent for participation. The study protocol conformed to the ethics regulations described by the Declaration of Helsinki.
Measurement of PWV and Ankle Brachial Index
baPWV and ankle‐brachial index (ABI) were measured with an automated PWV/ABI analyzer (Colin Co, Ltd., Komaki, Japan), with the patients in a supine position after having rested for at least 5 minutes.16 The PWV/ABI analyzer was able to simultaneously record PWV, BP, electrocardiograms, and heart sounds. Electrocardiography electrodes were placed on the patients' wrists, a microphone for detecting heart sounds placed on the left edge of the sternum, and cuffs wrapped around each of the four extremities. The volume waveforms for the brachium and ankle were stored, and the sampling times were 10 seconds with automatic gain analysis and quality adjustment. The mean baPWV was used as a marker for arterial stiffness. The ABI was calculated by dividing the ankle systolic BP by the upper extremity systolic BP. If the ABI was <0.9, the patient was considered to have peripheral artery disease and was excluded from the study.
Classification of Renal Tubular Function
To measure urinary levels of NAG, we used a synthetic substrate, 4‐hydroxymethyl‐2‐pyridinyl 2‐(acetylamino)‐2‐deoxy‐1‐thio‐β‐D‐glucopyranoside (L‐type NAG [4HP‐NAG substrate method]; Wako Pure Chemical Industries, Ltd, Osaka, Japan). When saline was assayed, the absorbance change was ≤0.01 (⊿E/min), and when a sample was assayed, the coefficient of variation for the results was ≤5%. Furthermore, when the activity of a sample was known, the assayed value was accurate to within 6%.
Urinary NAG, which is the ratio of the urinary activity of NAG to creatinine (Cr), was calculated with spot urine specimens. In Japanese patients, the normal range for urinary NAG has been reported to be 1.6 U/g Cr to 5.8 U/g Cr17 and is reported in the manufacturer's instructions for the L‐type NAG assay substrate to be 1.0 U/g Cr to 6.3 U/g Cr.18 Therefore, in the present study we determined the urinary NAG in samples of second morning urine specimens and considered it to be elevated when >5.8 U/g Cr. Our patients were divided into two groups on the basis of urinary NAG levels: patients with low levels (≤5.8 U/g Cr; n=133) and patients with high levels (>5.8 U/g Cr; n=100).
Classification of eGFR
To determine the eGFR, we used the following three‐variable equation modified for Japanese patients, as recently proposed by the Japanese Society of Nephrology:19 eGFR (mL/min/1.73 m2)=194×serum Cr (mg/dL)−1.094×age (years)−0.287×0.739 (if the patient was a woman). Patients were then divided on the basis of eGFR into low, middle, and high eGFR tertiles with the following eGFR ranges: ≥30 and <60, ≥60 and <90, and ≥90 mL/min/1.73 m2, respectively.
Analysis of Biochemical Variables
White blood cell counts were determined with an automated hematology analyzer (XE‐5000; Sysmex Co, Ltd, Kobe, Japan). Serum levels of total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, albumin, uric acid, and Cr were measured with an enzymatic assay and an automatic analyzer (JCA‐BM6070; Nihon Denshi Co, Ltd, Tokyo, Japan). HbA1c levels (normal range, 4.1%–5.9%) were measured with a high‐performance liquid chromatograph (Auto A1C analyzer; Arkray, Inc, Kyoto, Japan) and a method recommended by the Japan Diabetes Society (JDS). To convert the HbA1c (JDS) (%) to the internationally used HbA1c (%) defined by the National Glycohemoglobin Standardization Program (NGSP), ie, HbA1c (NGSP) (%), 0.3% was added when HbA1c (JDS) was ≤4.9%, and 0.4% was added when HbA1c (JDS) was ≥5.0% and <6.5%.20 Specimens of blood and urine were obtained after the patients had fasted overnight. Second morning urine specimens were used to determine urine pH and concentrations of protein by means of urine test strips (Uropaper α III; Eiken Chemical Co, Ltd, Tokyo, Japan). Urine analyses were performed with a fully automated urine analyzer (US‐3100R Plus; Eiken Chemical Co, Ltd). Renal tubular epithelial cells were counted with urine flow cytometry and measured with an automated device for microscopic urinalysis (UF‐1000i; Sysmex Co, Ltd). The UF‐1000i, using two stains with fluorescent dye, stains formed elements in urine. The staining agent was a fluorescent polymethine dye that binds to DNA. After staining, the particles are transported to a flow cell and irradiated by a laser. The UF‐1000i uses a semiconductor laser instead of an argon laser, and uses both the forward and the sideward scattered light. There are two reaction chambers, one for sediment analysis and one for microbial counting. Particles were counted with an impedance method that also provides information regarding the size of the particles. Results for cells were considered positive when the cell count was ≥1 per five high‐power fields or ≥0.1 cells/μL.
Statistical Analysis
Significance was indicated by P<.05. Data are presented as means±standard deviations. All analyses were performed with the statistical software program SPSS version 12 (SPSS Inc, Chicago, IL). Characteristics were compared between the low and high urinary NAG groups by means of chi‐square test and the Kruskal‐Wallis H test. The association between the urinary NAG and baPWV level was analyzed with the Spearman correlation coefficient. Elderly and nonelderly patients were compared by means of the regression equation and analysis of covariance. The associations between the baPWV level and patient characteristics, including age, sex, body mass index (BMI), systolic BP, heart rate, white blood cell count, serum Cr, serum uric acid, total cholesterol, high‐density lipoprotein cholesterol, serum albumin, HbA1c, 1,5‐anhydroglucitol (1,5‐AG), hypertension, statins, and urinary pH were evaluated by means of multivariate regression analysis.
Results
The characteristics of patients, divided into two groups on the basis of urinary NAG, are shown in Table 1. Characteristics that differed significantly between the groups were age, 1,5‐AG, serum albumin, eGFR, and urine pH. The association between the urinary NAG and the baPWV was a weak positive correlation (r=0.341, P<.001). Furthermore, the finding that the slopes of the regression lines did not differ between elderly and nonelderly patients supports the null hypothesis (elderly patients; y=17.2x+1756.3, nonelderly patients; y=17.5x+1385.5) (analysis of covariance).
Table 1.
Clinical Characteristics of Study Patients
| Clinical Characteristics | All | Low Urinary NAG (≤5.8 U/g Cr) | High Urinary NAG (>5.8 U/g Cr) | P Value |
|---|---|---|---|---|
| No. | 233 | 133 | 100 | – |
| Age, y | 68.3±11.3 | 64.7±12.0 | 73.1±8.0 | <.001a |
| Female, % | 66.1 | 66.2 | 66.0 | .979 |
| Body mass index, kg/m2 | 23.6±3.6 | 23.6±3.7 | 23.6±3.4 | .828 |
| Systolic blood pressure, mm Hg | 129.2±14.0 | 128.2±12.7 | 130.4±15.5 | .535 |
| Diastolic blood pressure, mm Hg | 78.2±9.9 | 78.6±9.3 | 77.7±10.7 | .493 |
| Mean blood pressure, mm Hg | 103.8±10.5 | 103.4±9.8 | 104.2±11.4 | .851 |
| Hypertension, % | 63.3 | 59.2 | 68.4 | .162 |
| Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, % | 33.2 | 28.7 | 39.0 | .100 |
| Heart rate, beats per min | 67.9±10.9 | 67.5±9.6 | 68.6±12.4 | .582 |
| HbA1c, % | 5.82±0.34 | 5.79±0.31 | 5.87±0.36 | .076 |
| 1,5‐Anhydroglucitol, μg/mL | 19.9±6.8 | 21.1±6.8 | 18.5±6.6 | .003b |
| Total cholesterol, mg/dL | 207.2±30.2 | 208.9±31.7 | 205.1±28.1 | .521 |
| High‐density lipoprotein cholesterol, mg/dL | 60.6±17.4 | 62.5±19.9 | 58.0±12.9 | .322 |
| Low‐density lipoprotein cholesterol, mg/dL | 120.4±26.5 | 120.6±27.7 | 120.2±24.9 | .939 |
| Statin, % | 52.8 | 50.4 | 56.0 | .399 |
| White blood cell count, /μL | 5973.0±1307.3 | 5871.2±1279.9 | 6110.2±1337.8 | .243 |
| Serum albumin, g/dL | 4.42±0.27 | 4.47±0.25 | 4.36±0.28 | .001b |
| Uric acid, mg/dL | 5.29±1.38 | 5.45±1.50 | 5.06±1.17 | .074 |
| Serum creatinine, mg/dL | 0.72±0.17 | 0.70±0.16 | 0.75±0.18 | .143 |
| eGFR, mL/min/1.73 m2 | 71.5±15.9 | 74.1±15.4 | 68.0±16.1 | .003b |
| Urinary pH | 6.26±0.88 | 6.16±0.91 | 6.38±0.82 | .025b |
| Urinary NAG, mg/g·Cr | 6.01±3.68 | 3.72±1.32 | 9.09±3.60 | <.001a |
Abbreviations: Cr, creatinine; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; NAG, N‐acetyl‐β‐D‐glucosaminidase. a P<.001. b P<.05. Data are expressed as mean±standard deviation.
Mean baPWVs differed significantly between eGFR tertiles in both the low and high urinary NAG groups (P<.001 and .010, respectively) (Figure 1). Mean baPWVs of the high urinary NAG group were significantly higher than those of the low urinary NAG group in both the eGFR ≥30 and<60 tertile and the eGFR ≥60 and<90 tertile (P=.023 and .005, respectively). However, mean baPWVs did not differ significantly between the eGFR ≥90 tertiles of the two urinary NAG groups. On the other hand, mean ABI did not differ significantly between eGFR tertiles in either the low or high urinary NAG group (Figure 2).
Figure 1.
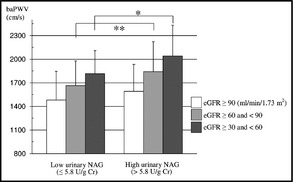
Association between urinary N‐acetyl‐β‐D‐glucosaminidase (NAG) groups (low urinary NAG group, ≤5.8 U/g Cr; and high urinary NAG group, >5.8 U/g Cr) and the brachial‐ankle pulse wave velocity (baPWV) (cm/s). Data are expressed as means±standard deviation. *P<.05, **P<.005 by the Mann‐Whitney U test.
Figure 2.
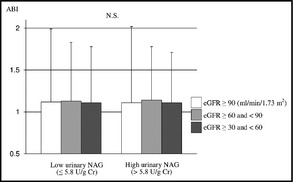
Association between urinary N‐acetyl‐β‐D‐glucosaminidase (NAG) groups (low urinary NAG group, ≤5.8 U/g Cr; and high urinary NAG group, >5.8 U/g Cr) and the ankle‐brachial index (ABI). Data are expressed as means±standard deviation. N.S. indicates not significant by the Mann‐Whitney U test and the Kruskal‐Wallis H test.
Simple linear regression analysis (model 1) showed that baPWV was significantly and positively correlated with urinary NAG (Table 2A). Stepwise multivariate regression analysis (model 2, adjusted for age and sex) showed that the baPWV was significantly and positively correlated with both the urinary NAG (P=.032) and age (P<.001). Stepwise multivariate regression analysis (model 3) showed that the baPWV was significantly related with the urinary NAG (P=.016), age (P<.001), and systolic BP (P<.001). Table 2B shows simple linear regression analysis (model 1) and stepwise multivariate regression analysis (models 2 and 3), and Table 2B shows the subanalysis of patients without hypertension (n=88). Simple linear regression analysis (model 1) showed that baPWV was significantly and positively correlated with urinary NAG. Stepwise multivariate regression analysis (model 2, adjusted for age and sex) showed that the baPWV was significantly and positively correlated with both urinary NAG (P=.016) and age (P<.001). Stepwise multivariate regression analysis (model 3) showed that baPWV was significantly related with age (P<.001), sex (P=.009), systolic BP (P=.002), heart rate (P=.007), and serum levels of Cr (P<.001).
Table 2.
Multivariate Regression Analysis Assuming baPWV to be the Dependent Variable and Clinical Characteristics to be Independent Variables
| Independent Variables | Dependent Variable: baPWV | Full Model R 2 | |||||
|---|---|---|---|---|---|---|---|
| Unstandardized β Coefficient | Standard Error | Standardized β Coefficient | t | P Value | |||
| (A) All | |||||||
| Model 1 | Urinary NAG, U/g Cr | 31.241 | 6.400 | 0.306 | 4.882 | <.001a | 0.090 |
| Model 2 | Urinary NAG, U/g Cr | 12.877 | 5.962 | 0.126 | 2.146 | .032b | 0.310 |
| Age, y | 16.851 | 1.949 | 0.505 | 8.647 | <.001a | ||
| Model 3 | Urinary NAG, U/g Cr | 14.589 | 5.984 | 0.148 | 2.438 | .016b | 0.403 |
| Age, y | 15.307 | 1.918 | 0.497 | 7.983 | <.001a | ||
| Systolic blood pressure, mm Hg | 6.151 | 1.709 | 0.208 | 3.600 | <.001a | ||
| (B) Patients without HTN | |||||||
| Model 1 | Urinary NAG, U/g Cr | 40.780 | 8.919 | 0.442 | 4.572 | <.001a | 0.186 |
| Model 2 | Urinary NAG, U/g Cr | 20.668 | 8.392 | 0.224 | 2.463 | .016b | 0.406 |
| Age, y | 10.976 | 1.918 | 0.521 | 5.724 | <.001a | ||
| Model 3 | Urinary NAG, U/g Cr | – | – | – | – | .195 | 0.598 |
| Age, y | 10.634 | 1.727 | 0.526 | 6.158 | <.001a | ||
| Sex | 173.242 | 64.661 | 0.266 | 2.679 | .009a | ||
| Systolic blood pressure, mm Hg | 8.145 | 2.505 | 0.264 | 3.251 | .002a | ||
| Heart rate, beats per min | 5.739 | 2.057 | 0.211 | 2.790 | .007a | ||
| Serum creatinine, mg/dL | 759.585 | 199.049 | 0.369 | 3.816 | <.001a | ||
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; Cr, creatinine; NAG, N‐acetyl‐β‐D‐glucosaminidase. (A) All patients models. (B) Patients without hypertension (HTN) models. a P<.01. b P<.05. Model 1: uncorrected. Model 2: corrected for age and sex. Model 3: Model 2+ body mass index, systolic blood pressure, heart rate, white blood cell count, serum creatinine, serum uric acid, total cholesterol, high‐density lipoprotein cholesterol, serum albumin, glycated hemoglobin, 1,5‐AG, hypertension, statins, and urinary pH (patients without HTN models were excluded from the adjustment for HTN). The significant independent variables are shown.
The dependent variable was then changed from baPWV to urinary NAG and validated. Simple linear regression analysis (model 1) showed that urinary NAG was significantly and positively correlated with baPWV (Table 3A). Stepwise multivariate regression analysis (model 2; adjusted for age and sex) showed that the urinary NAG was significantly and positively correlated with both baPWV (P=.032) and age (P<.001). Stepwise multivariate regression analysis (model 3) showed that urinary NAG was significantly related with baPWV (P=.043), age (P=.013), white blood cell count (P=.043), serum level of uric acid (P=.006), and serum levels of albumin (P=.002). Table 3B shows simple linear regression analysis (model 1) and stepwise multivariate regression analysis (models 2 and 3), and Table 3B shows the subanalysis of patients without hypertension. Simple linear regression analysis (model 1) showed that urinary NAG was significantly and positively correlated with baPWV. Stepwise multivariate regression analysis (model 2; adjusted for age and sex) showed that urinary NAG was significantly and positively correlated with baPWV (P<.001). Stepwise multivariate regression analysis (model 3) showed that urinary NAG was significantly related with baPWV (P<.001), heart rate (P=.050), and serum levels of uric acid (P=.006).
Table 3.
Multivariate Regression Analysis Assuming Urinary NAG to be the Dependent Variable and Clinical Characteristics to be Independent Variables
| Independent Variables | Dependent Variable: urinary NAG | ||||||
|---|---|---|---|---|---|---|---|
| Unstandardized β Coefficient | Standard Error | Standardized β Coefficient | t | P Value | Full Model R 2 | ||
| (A) All | |||||||
| Model 1 | baPWV, cm/s | 0.003 | 0.001 | 0.306 | 4.882 | <.001a | 0.090 |
| Model 2 | baPWV, cm/s | 0.002 | 0.001 | 0.158 | 2.160 | .032b | 0.137 |
| Age, y | 0.088 | 0.024 | 0.270 | 3.691 | <.001a | ||
| Model 3 | baPWV, cm/s | 0.002 | <0.001 | 0.133 | 2.039 | .043b | 0.227 |
| Age, y | 0.065 | 0.026 | 0.207 | 2.508 | .013b | ||
| White blood cell count, /μL | <0.001 | <0.001 | 0.133 | 2.039 | .043b | ||
| Serum uric acid, mg/dL | −0.486 | 0.175 | −0.181 | −2.773 | .006a | ||
| Serum albumin, g/dL | −2.879 | 0.900 | −0.215 | −3.197 | .002a | ||
| (B) Patients without HTN | |||||||
| Model 1 | baPWV, cm/s | 0.005 | 0.001 | 0.442 | 4.572 | <.001a | 0.186 |
| Model 2 | baPWV, cm/s | 0.005 | 0.001 | 0.442 | 4.572 | <.001a | 0.186 |
| Model 3 | baPWV, cm/s | 0.005 | 0.001 | 0.436 | 4.314 | <.001a | 0.309 |
| Heart rate, beats per min | 0.060 | 0.030 | 0.203 | 1.996 | .050b | ||
| Serum uric acid, mg/dL | −0.628 | 0.221 | −0.277 | −2.842 | .006a | ||
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; NAG, N‐acetyl‐β‐D‐glucosaminidase. (A) All patient models. (B) Patients without hypertension (HTN) models. a P<.01. b P<.05. Model 1: uncorrected. Model 2: corrected for age and sex. Model 3: Model 2+ body mass index, systolic blood pressure, heart rate, white blood cell count, serum creatinine, serum uric acid, total cholesterol, high‐density lipoprotein cholesterol, serum albumin, glycated hemoglobin, 1,5‐AG, hypertension, statin, and urinary pH (patients without HTN models were excluded from the adjustment for HTN). The significant independent variables are shown.
In addition to these analyses, patients were divided into two groups in the subanalysis on the basis of the presence of renal tubular epithelial cells (≥0.1 cells/μL, n=44) or their absence (0 cells/μL, n=175). Urinary NAG was significantly higher in patients with renal tubular epithelial cells (6.68±3.05 U/g Cr) than in patients without these cells (5.88±3.84 U/g Cr; Mann‐Whitney U test, P=.039). Furthermore, the association between urinary NAG and baPWV was positively correlated in patients who were receiving no medications (r=0.457, P=.032 [n=22]) and in patients who did not have microalbuminuria (<30 mg/g·Cr, r=0.350, P=.001 [n=85]).
Discussion
The main finding of the present study was that urinary NAG, as a marker of renal tubules, is correlated with baPWV level, as an indicator of increased arterial stiffness, in patients without diabetes mellitus (DM).
One study showed the association of urinary NAG with macrovascular disease.11 This study investigated patients with type 2 DM during a median follow‐up of 7 years and showed that urinary NAG proved comparable to urinary albumin when analyzed with respect to preexistence and development of severe macrovascular disease. However, the mechanism underlying this association is unknown. A recent community study showed that urinary NGAL, as a biomarker of renal tubules, was associated with increased cardiovascular and all‐cause mortality independent of cardiovascular risk factors and glomerular filtration.21 It has been speculated that urinary NGAL in this population may reflect early chronic tubular damage that may be indicative of an increased mortality risk. Many previous studies have found that baPWV is related to both eGFR and chronic kidney disease (CKD).12, 22, 23, 24 The present study (Figure 1) found that the mean baPWV differed significantly between eGFR tertiles in both the low and high urinary NAG groups. In the progression of CKD, pathogenic mechanisms converge upon a common pathway that leads to progressive interstitial fibrosis, peritubular capillary loss, and destruction of functioning nephrons through tubular atrophy.25 Another study found that the progression of early CKD is mediated by a common pathophysiologic mechanism resulting in glomerulosclerosis and tubulointerstitial injury.26 Eddy and Neilson reported that the pathologic correlate of clinical progression in patients with CKD is the relentless expansion of interstitial fibrosis.27 The tubulointerstitium is believed to play a key role in the common progression of renal dysfunction. In addition, Hong and Lim28 have suggested that the increasing urinary NAG may be used to predict glomerular dysfunction in children with CKD. These reports might help explain the association between urinary NAG and baPWV.
To our knowledge, only a single recent study has investigated the association between PWV and renal markers of tubular damage.29 In this study of smokers with and without chronic obstructive pulmonary disease (COPD), aortic PWV was found to be independently related to urinary levels of albumin in patients with COPD but was not related to urinary levels of either NGAL or KIM‐1 in all patients or in those with COPD. In this study, urinary NAG and urinary β2‐microglobulin were not measured, but a study in Japanese patients found that increased serum levels of β2‐microglobulin are associated with increased baPWV.30 Furthermore, as a marker of GFR, serum β2‐microglobulin has been found to be as good as Cr clearance, regardless of sex, age, and renal function.31
On the other hand, the level of urinary NAG is non‐zero in normal patients. However, the reason for this is not clear at all. NAG is hydrolytic lysosomal enzyme, and is found in many tissues of the body. NAG cannot be filtered by the glomerulus in the kidney, because it is the high‐molecular weight form of NAG. Thus, it is said that urinary NAG originates from renal tubular cells and in the absence of the glomerular damage. One explanation for non‐zero is the necrosis and apoptosis of renal tubular epithelial cells, but likely other explanations for this exist as well. NAG exist in human tissues and body as two major isoenzymic forms (A and B), and several minor forms (I1, I2, As, and P), which are distinguished according to their different charge characteristics by chromatography on diethylaminoethyl cellulose.32, 33 The possibility to evaluate the different isoenzymatic profile of NAG has led to the notion of so‐called functional enzymuria mainly linked to preferential urinary excretion of A isoenzyme, and so‐called lesional enzymuria with preferential urinary release of B isoenzyme.33, 34, 35 Thereby, NAG isoenzyme A might be measured in urine as a result of exocytosis or leakage from stimulated renal tubular epithelial cells. The percentage of A isoform is the greatest in normal urine, and NAG isoenzymes A and B account for 80% to 90% and 10% to 20% of total NAG, respectively.36 In the past, we reported the association between urinary NAG and serum 1,5‐AG levels as a marker of postprandial glucose in patients with and without type 2 DM37, 38 and between urinary NAG and plasma glucose levels at 120 minutes of the oral glucose tolerance test in prediabetes patients.39 Possibily, we investigated the relations with functional enzymuria stronger than lesional enzymuria.
Age, hypertension, BP, and the white blood cell count have also been reported to be important correlates of greater baPWV.24, 40, 41, 42, 43 Heart rate and BMI have also been associated with baPWV.44, 45 We performed multivariate regression analyses, which were adjusted to include these characteristics, as referenced above. baPWV is highly correlated with BP or renal dysfunction or both. Therefore, we excluded patients with hypertension and performed stepwise multivariate regression analysis, which was adjusted for systolic BP and other markers (Tables 2B and 3B). Urinary NAG and baPWV were not associated in Table 2B, model 3, but were strongly related in Table 3B, model 3. Furthermore, we substituted the patients with hypertension for the patients without hypertension in the stepwise multivariate regression analysis; nevertheless, there were no associations between urinary NAG and baPWV in model 2 or 3 (data not shown). These results were difficult to interpret because of the small sample sizes. Our analysis included only cross‐sectional data, and we are unable to infer cause‐effect associations. However, we believe that these findings provide evidence for an association between urinary NAG and baPWV. There are several possibilities for mechanism‐based combinations. Yamanouchi and colleagues46 reported that the long‐term administration of total parenteral nutrition (free of 1,5‐AG) appeared to increase urinary excretion of NAG. One possibility is that some nutritional or chemical elements leak from renal tubular cells and vascular endothelial cells and induce inflammatory responses. Inoue and colleagues47 reported that serum NAG activity correlates with the severity of coronary artery disease. However, the relationship between urinary and serum NAG in healthy patients and patients with various diseases should be examined. In contrast, one study found that increased urinary excretion of NAG is associated with cardiovascular complications in patients with type 2 DM.48 The elevation of renal interstitial markers, such as urinary NAG and β2‐microglobulin, reportedly reflects the severity of interstitial changes, which result from atherosclerosis and subsequent ischemic injury.
We previously reported that urinary NAG is positively correlated with age in healthy patients.49 This finding suggests that the association between urinary NAG and baPWV is mediated by an age‐related factor. Therefore, in the present study the slopes of regression lines of the relationship between urinary NAG and baPWV were investigated separately in elderly and nonelderly patients. We found no significant difference in the slopes of the regression lines between elderly and nonelderly patients. Moreover, even after adjusting for age, this relationship between baPWV and urinary NAG remained on multivariate analysis. Therefore, we believe that urinary NAG is independently associated with baPWV but is dependent on age. Some studies, including our previous studies, have showed a negative correlation between serum 1,5‐AG and urinary NAG levels in patients with type 2 DM37, 50 and patients without DM.38 However, when adjusted for serum 1,5‐AG and other markers, a positive and significant correlation remained between urinary NAG levels and baPWV (model 3 in Tables 2A, 3A, and IIIB).
Study Limitations
The present study has several limitations. First, our analysis included only cross‐sectional data and we are therefore unable to infer cause‐effect associations. The present study did not examine morbidity and mortality related to CV disease. A second limitation was that all patients were outpatients in stable condition and that patients were excluded if they were unable to walk unsupported or were transported to the hospital by ambulance. Our study lacked healthy control patients, and data were derived from only a single study visit. A third limitation of the present study was that some demographic data, such as smoking habit and alcohol consumption, were not collected. Hence, our analysis was unable to adjust for these variables. Despite these limitations, this cross‐sectional study is, to our knowledge, the first to show, by means of multivariate analysis, an association between urinary NAG and baPWV in patients without DM.
Conclusions
The present study is, to our knowledge, the first to demonstrate that elevated arterial stiffness is related to elevated urinary NAG, a marker of renal tubules, in patients without diabetes. Further prospective long‐term studies of large cohorts in healthy elderly persons with no medication and/or patients with hypertension are required to confirm this presumed association.
Acknowledgments and Disclosures
The authors are grateful to Masao Okazaki, MD, of the Academic Information Center, The Jikei University School of Medicine, for his careful revision of the English of the manuscript. The authors report no specific funding in relation to this research and no conflicts of interest to disclosure.
J Clin Hypertens (Greenwich). 2015;17:290–297. DOI: 10.1111/jch.12492. © 2015 Wiley Periodicals, Inc.
References
- 1. Mattace‐Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. [DOI] [PubMed] [Google Scholar]
- 2. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 3. Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid‐femoral and brachial‐ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–2027. [DOI] [PubMed] [Google Scholar]
- 4. Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. [DOI] [PubMed] [Google Scholar]
- 5. Hung CS, Lin JW, Hsu CN, et al. Using brachial‐ankle pulse wave velocity to associate arterial stiffness with cardiovascular risks. Nutr Metab Cardiovasc Dis. 2009;19:241–246. [DOI] [PubMed] [Google Scholar]
- 6. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 7. Fried LF, Shlipak MG, Crump C, et al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–1372. [DOI] [PubMed] [Google Scholar]
- 8. Damman K, Masson S, Hillege HL, et al. Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J. 2011;32:2705–2712. [DOI] [PubMed] [Google Scholar]
- 9. Damman K, Voors AA, Hillege HL, et al. Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. Eur J Heart Fail. 2010;12:974–982. [DOI] [PubMed] [Google Scholar]
- 10. Le Hir M, Dubach UC, Schmidt U. Quantitative distribution of lysosomal hydrolases in the rat nephron. Histochemistry. 1979;63:245–251. [DOI] [PubMed] [Google Scholar]
- 11. Weitgasser R, Schnoell F, Gappmayer B, Kartnig I. Prospective evaluation of urinary N‐acetyl‐β‐D‐glucosaminidase with respect to macrovascular disease in elderly type 2 diabetic patients. Diabetes Care. 1999;22:1882–1886. [DOI] [PubMed] [Google Scholar]
- 12. Taal MW. Arterial stiffness in chronic kidney disease: an update. Curr Opin Nephrol Hypertens. 2014;23:169–173. [DOI] [PubMed] [Google Scholar]
- 13. Chen SC, Chang JM, Liu WC, et al. Brachial‐ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu CS, Pi‐Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects–a population‐based study (Taichung Community Health Study, TCHS). Atherosclerosis. 2010;211:315–321. [DOI] [PubMed] [Google Scholar]
- 15. Ishikawa T, Hashimoto J, Morito RH, et al. Association of microalbuminuria with brachial‐ankle pulse wave velocity: the Ohasama study. Am J Hypertens. 2008;21:413–418. [DOI] [PubMed] [Google Scholar]
- 16. Kohara K, Tabara Y, Tachibana R, et al. Microalbuminuria and arterial stiffness in a general population: the Shimanami Health Promoting Program (J‐SHIPP) study. Hypertens Res. 2004;27:471–477. [DOI] [PubMed] [Google Scholar]
- 17. Yuzawa Y, Ito I. Urinary NAG, β2 microglobulin — renal tubular injury, AKI (acute kidney injury) and biomarker. Nippon Naika Gakkai Zasshi. 2008;97:971–978 [Article in Japanese]. [Google Scholar]
- 18. L‐Type NAG [Package Insert]. Osaka, Japan: Wako Pure Chemical Industries, Ltd.; 2013. [Google Scholar]
- 19. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 20. Kashiwagi A, Kasuga M, Araki E, et al; Committee on the Standardization of Diabetes Mellitus Related Laboratory Testing of Japan Diabetes Society . International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helmersson‐Karlqvist J, Larsson A, Carlsson AC, et al. Urinary neutrophil gelatinase‐associated lipocalin (NGAL) is associated with mortality in a community‐based cohort of older Swedish men. Atherosclerosis. 2013;227:408–413. [DOI] [PubMed] [Google Scholar]
- 22. Yoon HE, Shin DI, Kim SJ, et al. Brachial‐ankle pulse wave velocity predicts decline in renal function and cardiovascular events in early stages of chronic kidney disease. Int J Med Sci. 2013;10:1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawamoto R, Kohara K, Tabara Y, et al. An association between decreased estimated glomerular filtration rate and arterial stiffness. Intern Med. 2008;47:593–598. [DOI] [PubMed] [Google Scholar]
- 24. Liu IT, Wu JS, Yang YC, et al. Mild chronic kidney disease associated with greater risk of arterial stiffness in elderly adults. J Am Geriatr Soc. 2013;61:1758–1762. [DOI] [PubMed] [Google Scholar]
- 25. Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:353–365. [DOI] [PubMed] [Google Scholar]
- 26. Metcalfe W. How does early chronic kidney disease progress? A background paper prepared for the UK Consensus Conference on early chronic kidney disease. Nephrol Dial Transplant. 2007;22(suppl 9):ix26–ix30. [DOI] [PubMed] [Google Scholar]
- 27. Eddy AA, Neilson EG. Chronic kidney disease progression. J Am Soc Nephrol. 2006;17:2964–2966. [DOI] [PubMed] [Google Scholar]
- 28. Hong JD, Lim IS. Correlation between glomerular filtration rate and urinary N‐acetyl‐β‐D‐glucosaminidase in children with persistent proteinuria in chronic glomerular disease. Korean J Pediatr. 2012;55:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. John M, Hussain S, Prayle A, et al. Target renal damage: the microvascular associations of increased aortic stiffness in patients with COPD. Respir Res. 2013;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saijo Y, Utsugi M, Yoshioka E, et al. Association of β2‐microglobulin to arterial stiffness in Japanese subjects. Hypertens Res. 2005;28:505–511. [DOI] [PubMed] [Google Scholar]
- 31. Jovanović D, Krstivojević P, Obradović I, et al. Serum cystatin C and β2‐microglobulin as markers of glomerular filtration rate. Ren Fail. 2003;25:123–133. [DOI] [PubMed] [Google Scholar]
- 32. Ellis BG, Tucker SM, Thompson AE, Price RG. Presence of serum and tissue forms of N‐acetyl‐β‐glucosaminidase in urine from patients with renal disease. Clin Chim Acta. 1975;64:195–202. [DOI] [PubMed] [Google Scholar]
- 33. Capodicasa E, Angelini A, Tassi C. Isoenzyme A and urinary N‐acetyl‐β‐D‐glucosaminidase activity in normal pregnancy. Ren Fail. 2011;33:650–653. [DOI] [PubMed] [Google Scholar]
- 34. Paigen K, Peterson J. Coordinacy of lysosomal enzyme excretion in human urine. J Clin Invest. 1978;61:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gibey R, Dupond JL, Peltier H, et al. An early and specific indicator of aminoglycoside nephrotoxicity: isoenzyme B of urinary N‐acetyl‐beta‐D‐glucosaminidase (NAG). Pathol Biol (Paris). 1986;34:342–345 [Article in French]. [PubMed] [Google Scholar]
- 36. Morita A, Numata Y, Kosugi Y, et al. Stabilities of N‐acetyl‐β‐D‐glucosaminidase (NAG) isoenzymes in urine: advantage of NAG isoenzyme B measurement in clinical applications. Clin Chim Acta. 1998;278:35–43. [DOI] [PubMed] [Google Scholar]
- 37. Ouchi M, Oba K, Motoyama M, et al. Postprandial glycemic control conditions in relation to urinary N‐Acetyl‐β‐D‐glucosaminidase in patients with type 2 diabetes mellitus without low glomerular filtration rate. Diabetes Technol Ther. 2014;16:41–47. [DOI] [PubMed] [Google Scholar]
- 38. Ouchi M, Oba K, Ohara M, et al. Change in urinary N‐acetyl‐β‐D‐glucosaminidase levels relevant to postprandial glycemic control conditions in subjects without diabetes mellitus. Clin Chim Acta. 2014;433:88–92. [DOI] [PubMed] [Google Scholar]
- 39. Ouchi M, Suzuki T, Hashimoto M, et al. Urinary N‐acetyl‐β‐D‐glucosaminidase levels are positively correlated with 2‐hr plasma glucose levels during oral glucose tolerance testing in prediabetes. J Clin Lab Anal. 2012;26:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tomiyama H, Hashimoto H, Matsumoto C, et al. Effects of aging and persistent prehypertension on arterial stiffening. Atherosclerosis. 2011;217:130–134. [DOI] [PubMed] [Google Scholar]
- 41. Lee YJ, Lee JW, Kim JK, et al. Elevated white blood cell count is associated with arterial stiffness. Nutr Metab Cardiovasc Dis. 2009;19:3–7. [DOI] [PubMed] [Google Scholar]
- 42. Yambe M, Tomiyama H, Yamada J, et al. Arterial stiffness and progression to hypertension in Japanese male subjects with high normal blood pressure. J Hypertens. 2007;25:87–93. [DOI] [PubMed] [Google Scholar]
- 43. Tomiyama H, Arai T, Koji Y, et al. The age‐related increase in arterial stiffness is augmented in phases according to the severity of hypertension. Hypertens Res. 2004;27:465–470. [DOI] [PubMed] [Google Scholar]
- 44. Lantelme P, Mestre C, Lievre M, et al. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–1087. [DOI] [PubMed] [Google Scholar]
- 45. Tomiyama H, Koji Y, Yambe M, et al. Elevated C‐reactive protein augments increased arterial stiffness in subjects with the metabolic syndrome. Hypertension. 2005;45:997–1003. [DOI] [PubMed] [Google Scholar]
- 46. Yamanouchi T, Minoda S, Ogata N, et al. Prolonged hyperalimentation as a possible cause of renal tubular dysfunction: evaluation of 1,5‐anhydro‐D‐glucitol resorption and N‐acetylglucosaminidase excretion in humans. Clin Sci (Lond). 1995;88:203–210. [DOI] [PubMed] [Google Scholar]
- 47. Inoue T, Matsunaga R, Morooka S, Uehara Y. Serum N‐acetyl‐beta‐D‐gulucosaminidase activity increases in association with insulin resistance in patients with coronary artery disease. Atherosclerosis. 2000;149:117–122. [DOI] [PubMed] [Google Scholar]
- 48. Yoshikawa R, Wada J, Seiki K, et al. Urinary PGDS levels are associated with vascular injury in type 2 diabetes patients. Diabetes Res Clin Pract. 2007;76:358–367. [DOI] [PubMed] [Google Scholar]
- 49. Oba K, Hirai M, Ajiro Y, et al. Effect of age on urinary excretion of N‐acetyl‐β‐D‐glucosaminidase. J Nippon Med Sch. 1999;66:33–36. [DOI] [PubMed] [Google Scholar]
- 50. Yamanouchi T, Kawasaki T, Yoshimura T, et al. Association between serum 1,5‐anhydroglucitol and urinary excretion of N‐Acetyl‐β‐D‐glucosaminidase and albumin determined at onset of NIDDM with 3‐year follow‐up. Diabetes Care. 1998;21:619–624. [DOI] [PubMed] [Google Scholar]


