Abstract
The aim of this study was to compare an aliskiren/amlodipine combination with high‐dose amlodipine monotherapy on ambulatory blood pressure monitoring (ABPM) and organ protection. The study was a prospective, randomized, multicenter, open‐label trial in elderly essential hypertensive patients. A total of 105 patients with clinic BP (CBP) ≥140/90 mm Hg with amlodipine 5 mg were randomly allocated to aliskiren (150–300 mg)/amlodipine (5 mg) (ALI/AML group, n=53) or high‐dose amlodipine (10 mg) (h‐dAML group, n=52) and treated for 16 weeks. Each patient's CBP, ABPM, urine albumin‐to‐creatinine ratio (UACR), and brachial‐ankle pulse wave velocity (baPWV) were measured at baseline and at the end of the study. The ALI/AML and h‐dAML groups showed similarly reduced mean 24‐hour SBP, daytime SBP, nighttime SBP, and baPWV. However, UACR reduction was significantly greater in the ALI/AML group (P=.02). ALI/AML was significantly less effective in reducing early‐morning BP (P=.002) and morning BP surge (P=.001) compared with h‐dAML.
For the management of hypertension, not only the evaluation of blood pressure (BP) but also the screening of asymptomatic organ damage as a surrogate marker for cardiovascular outcomes has been recommended in hypertension treatment guidelines in many countries.1, 2, 3 In hypertensive patients, urine albumin to creatinine ratio (UACR) and brachial‐ankle pulse wave velocity (baPWV) are associated with cardiovascular events and are used as surrogate markers of such events.4, 5, 6, 7, 8
Aliskiren is the first in a new class of orally effective direct renin inhibitors. The agent has been reported to control the entire renin‐angiotensin‐aldosterone system (RAAS).9, 10 Aliskiren as an add‐on therapy to another antihypertensive drug was also reported to reduce the risk of asymptomatic organ damage.11, 12 However, a recent clinical trial showed that for patients with type 2 diabetes, the addition of aliskiren to standard therapy including RAAS inhibitors, angiotensin‐converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs) did not reduce cardiovascular and renal events and in fact increased adverse events.13 As a result, the European Society of Hypertension and European Society of Cardiology guidelines contraindicated the use of aliskiren as combination therapy with another RAAS inhibitor in type 2 diabetes.2 Even in hospitalized heart failure patients, the initiation of aliskiren in addition to standard therapy did not reduce the rate of cardiovascular death or heart failure rehospitalization.14 It is not clear why aliskiren add‐on therapy to standard antihypertensive treatment has shown inconsistent results concerning the effect of asymptomatic organ damage and cardiovascular events, and although BP reduction has the most important role in asymptomatic organ damage protection and the reduction of adverse cardiovascular events, this issue was not sufficiently debated in these previous studies.
There is substantial evidence that the prognostic power of ambulatory BP monitoring (ABPM) for cardiovascular and renal diseases is higher than that of clinic BP monitoring.15, 16 In a relatively large patient population, the combination of aliskiren and amlodipine, which is commonly used as a first choice of hypertensive treatment, was reported to produce BP reduction on ABPM that was similar to that of high‐dose amlodipine monotherapy.17 However, to our knowledge, there is no published information about the relationship between the change in BP level on ABPM and asymptomatic organ damage.
In the present study, in uncontrolled hypertensive patients receiving the commonly used dose of amlodipine (5 mg), we evaluated the difference in the BP‐lowering effect on ABPM, and we investigated the effect on renal protection and aortic stiffness between patients taking aliskiren/amlodipine (ALI/AML) and those taking high‐dose amlodipine (h‐dAML).
Methods
Study Patients
Study patients were recruited from the outpatient department of internal medicine at Fukushima Prefectural Miyashita Hospital (Fukushima, Japan) and Yanai Municipal Heigun Clinic (Yamaguchi, Japan). The entry period was from April 2011 to December 2012. All patients were Japanese hypertensive patients who were untreated or were treated with 5 mg of amlodipine. During the 4‐week run‐in period, all patients received a once‐daily 5‐mg dosage of amlodipine. At the end of the run‐in period, patients with a clinic or average home systolic BP (SBP) ≥140 mm Hg and/or diastolic BP (DBP) ≥90 mm Hg were eligible for the study.
Patients were excluded if they had secondary hypertension, moderate to severe symptoms of heart failure (higher than New York Heart Association class III heart failure), severe arrhythmia, renal insufficiency (estimated glomerular filtration rate <30 mL/min/1.73 m2), severe noncardiovascular disease (eg, cancer or liver cirrhosis), severe diabetes (hemoglobin A1c ≥12%), bilateral renal artery stenosis, allergy, or hypersensitivity to drugs used in the study, or a recent history (≤6 months) of myocardial infarction, stroke, coronary intervention, or coronary artery bypath graft.
Study Design
This study was a 16‐week, prospective, randomized, open‐label, parallel‐group study with two treatment arms evaluating the effects of ALI/AML and h‐dAML on ambulatory BP, UACR, and baPWV. Figure 1 shows the study protocol. At the end of the 4‐week run‐in period with 5 mg of amlodipine, 105 eligible patients were registered and randomized to either the group that would additionally receive aliskiren (150 mg) in combination therapy or to the group that would be doubled to 10 mg of amlodipine monotherapy. Dose titration was permitted only in the ALI/AML group: aliskiren was optimally titrated to 300 mg when the patient's clinic or average home SBP was ≥140 mm Hg and/or DBP was ≥90 mm Hg after 8 weeks of treatment.
Figure 1.
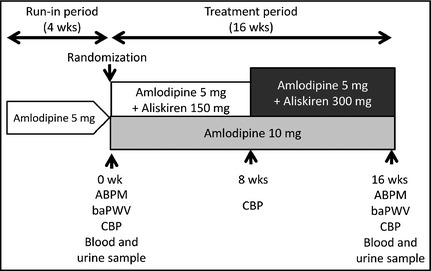
Study design. ABPM indicates ambulatory blood pressure monitoring; baPWV, brachial‐ankle pulse wave velocity; CBP, clinic blood pressure.
The patients were instructed to take their medications after breakfast and were not permitted to take any antihypertensive medications other than the study medications. Other drugs that had the potential to interfere with the safety and efficacy of the study drugs were also not allowed. At baseline and at the end of the study, each patient's clinic BP, ambulatory BP, blood and urine parameters including UACR, and baPWV were measured.
This study was approved by the institutional review board of Jichi Medical University. The study was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN000010163) and written informed consent was obtained from all of the patients.
BP Measurement
Brachial BP at the clinic was recorded as the average of triplicate measurements taken at 15‐second intervals using a validated oscillometric device (HEM‐5041; Omron Healthcare Co, Lake Forest, IL) at each visit after an initial 5 minutes of seated rest. ABPM was carried out twice on two separate weekdays with an automatic ABPM device (TM2425 or TM2431; A&D Co, Tokyo, Japan), which recorded BP and pulse rate every 30 minutes for 24 hours by the oscillometric method. ABPM was performed at baseline and at the end of the study.
We defined 24‐hour BP as the average of all BP readings throughout a given 24‐hour period. Nighttime BP was defined as the average of BP from the time when the patient went to bed until the time the patient got out of bed, and daytime BP was defined as the average of BP values recorded during the rest of the day. Early‐morning BP was defined as the average of BPs during the first 2 hours after awaking (four BP readings). The lowest nighttime BP was defined as the average BP of three readings centered on the lowest nighttime reading. Morning BP surge was calculated as early‐morning BP minus the lowest nighttime BP.
ABPM data were analyzed in 86 patients (40 in the ALI/AML group and 46 in the h‐dAML group) who completed the ABPM measurements.
Blood and Urine Examination
Blood and urine samples for laboratory evaluations (hematology, blood chemistry, and urine measurements) were obtained from the patients in the morning in a fasting state at baseline and at the end of the study. The blood samples were centrifuged at 3000 × g for 15 minutes at room temperature. Plasma/serum and urine samples were stored at 4°C in refrigerated containers and sent to a commercial laboratory (SRL Inc, Tokyo, Japan) within 24 hours. All assays were performed within 24 hours of sample collection at this single laboratory. Serum creatinine was measured by enzymatic methods and then quantified by a photometric method. Plasma renin activity was measured using a radioimmunoassay kit (TFB Inc, Tokyo, Japan).
baPWV Measurement
Aortic stiffness was assessed by baPWV. Each patient's baPWV was measured with a baPWV device (BP‐203RPE III form; Omron Colin Co, Kyoto, Japan) in a quiet and temperature‐controlled laboratory after a 5‐minute rest in the supine position by trained investigators who did not know the patients' characteristics. The measurement of baPWV was performed at baseline and at the end of the study. The average of the right and left baPWV values was used for the analysis.
Statistical Analysis
All statistical analyses were performed with SPSS version 21 software (SPSS, Chicago, IL). The normality of the data was assessed using the Kolmogorov‐Smirnov test before analysis. Statistical analyses were performed based on an intention‐to‐treat principal. Data are expressed as mean (±standard deviation) or percentage. Differences between the groups were analyzed with the unpaired Student t test for normally distributed variables. Categorical parameters were compared with the chi‐square test. Plasma renin activity (PRA), plasma aldosterone concentration (PAC), and UACR were log transformed because these variables were not normally distributed, and the results are expressed as geometric mean values with 95% confidence intervals (CIs). A two‐tailed paired t test was used to compare the mean values between baseline and after treatment for each treatment group. Two‐sided values of P<.05 were considered to indicate significance. The prevalence of male sex, current smokers, diabetes, and antidiabetic drugs, which were different between ALI/AML and h‐dAML groups at baseline, were selected as covariates in the analysis of covariance, and Bonferroni test was used for the comparison in ambulatory BP, UACR, and baPWV change between the two groups.
Results
Patient Characteristics
A total of 110 patients with essential hypertension were screened for this study. In total, 105 patients met the study eligibility criteria and entered the 4‐week run‐in period, of whom 105 were registered and randomized to treatment with aliskiren 150 mg/amlodipine 5 mg (ALI/AML group, n=53) or amlodipine 10 mg (h‐dAML group, n=52). At the end of 8 weeks of treatment, only in the ALI/AML group, patients with clinic sitting BP ≥140/90 mm Hg were allowed to receive optimal aliskiren uptitration from 150 mg to 300 mg.
During the 16‐week treatment period, 19 patients discontinued study treatment. One patient in the ALI/AML group discontinued as a result of an adverse event (AE) (dizziness) and no patients in the h‐dAML group discontinued as a result of an AE. There was one withdrawal consent in the h‐dAML group (0 in the ALI/AML group). One patient in the ALI/AML group was lost to follow‐up (vs 0 in the h‐dAML group). Sixteen patients were protocol deviators (11 in the ALI/AML group and five in the h‐dAML group). Eighty‐six patients (81.9%) completed the 16‐week treatment period (Figure 2).
Figure 2.
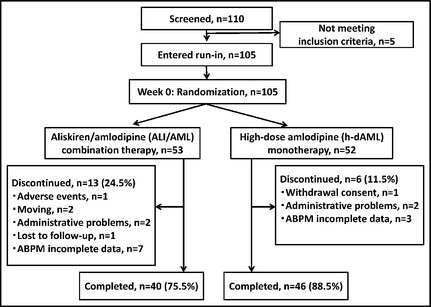
Patient flow diagram.
The mean age of all patients was 77.1 years, and 41.0% were male. The baseline characteristics of the ALI/AML and h‐dAML groups were similar except for the percentage of males (50.9% vs 30.8%, P=.04), current smokers (13.5% vs 0.0%, P=.01), diabetics (19.2% vs 5.8%, P=.04), and those taking antidiabetic drugs (19.2% vs 5.8%, P=.04) (Table 1). No significant differences were found between the ALI/AML and h‐dAML groups in clinic BP, 24‐hour BP, UACR, or baPWV at baseline (Table 2).
Table 1.
Comparison of Baseline Characteristics Between the ALI/AML and h‐dAML Groups
| Characteristic | ALI/AML Group (n=53) | h‐dAML Group (n=52) | P Value |
|---|---|---|---|
| Baseline | |||
| Age, y | 77.3±7.9 | 77.0±7.2 | .82 |
| Male, % | 50.9 | 30.8 | .04 |
| Body mass index, kg/m2 | 25.4±4.4 | 25.3±3.6 | .88 |
| Duration of hypertension, y | 9.0±7.6 | 10.0±7.6 | .51 |
| Family history of hypertension, % | 86.5 | 80.8 | .43 |
| Current smokers, % | 13.5 | 0.0 | .01 |
| Habitual drinkers, % | 32.7 | 23.1 | .27 |
| Dyslipidemia, % | 42.3 | 38.5 | .69 |
| Diabetes, % | 19.2 | 5.8 | .04 |
| Antidiabetic drugs, % | 19.2 | 5.8 | .04 |
| Statin, % | 40.4 | 38.5 | .84 |
| Total cholesterol, mg/dL | 179.5±35.7 | 182.7±30.2 | .63 |
| LDL cholesterol, mg/dL | 103.2±22.3 | 103.7±23.9 | .92 |
| Glucose, mg/dL | 105.9±35.0 | 103.1±18.7 | .60 |
| Creatinine, mg/dL | 0.7±0.2 | 0.7±0.2 | .28 |
| eGFR, mL/min/1.73 m2 | 71.7±18.4 | 71.8±17.8 | .99 |
| Proportion of eGFR <60, % | 22.6 | 26.9 | .61 |
| UACR, mg/g cr | 28.7 (20.2–40.8) | 22.2 (15.9–31.0) | .29 |
| Plasma renin activity, ng/mL/h | 0.86 (0.70–1.06) | 0.76 (0.63–0.92) | .39 |
| Plasma aldosterone concentration, pg/mL | 74.2 (64.2–85.9) | 83.7 (73.1–95.8) | .23 |
Abbreviations: ALI/AML, aliskiren/amlodipine; eGFR, estimated glomerular filtration rate; h‐dAML, high‐dose amlodipine; LDL, low‐density lipoprotein; UACR, urinary albumin to creatinine ratio. Data are expressed as mean±standard deviation or percentage or geometric mean (95% confidence interval).
Table 2.
Changes in BP and Laboratory Data During the Treatment Period (Intention‐to‐Treat Populations)
| Change | ALI/AML Group (n=47) | h‐dAML Group (n=51) | P Value |
|---|---|---|---|
| Clinic SBP, mm Hg | |||
| Baseline | 147.6±20.7 | 147.7±18.9 | .99 |
| End of study | 135.7±16.4 | 138.8±15.0 | .33 |
| Change after 16 weeks | −11.9±20.4a | −8.9±17.0b | .43 |
| Clinic DBP, mm Hg | |||
| Baseline | 73.9±10.3 | 76.2±10.9 | .30 |
| End of study | 70.5±9.1 | 70.3±8.8 | .92 |
| Change after 16 weeks | −3.4±7.3b | −5.8±7.5a | .11 |
| Clinic pulse rate, beats per min | |||
| Baseline | 68.8±12.0 | 69.9±11.9 | .65 |
| End of study | 71.5±12.0 | 71.5±10.5 | .98 |
| Change after 16 weeks | 2.7±10.8 | 1.6±10.5 | .63 |
| 24‐hour SBP, mm Hg | |||
| Baseline | 134.2±12.1 | 133.8±12.6 | .88 |
| End of study | 127.2±13.2 | 127.8±12.9 | .84 |
| Change after 16 weeks | −7.0±13.2b | −6.0±9.7a | .69 |
| 24‐hour DBP, mm Hg | |||
| Baseline | 74.5±7.5 | 74.4±8.1 | .97 |
| End of study | 69.9±7.5 | 70.7±7.5 | .62 |
| Change after 16 weeks | −4.6±6.8a | −3.7±5.4a | .51 |
| 24‐hour pulse rate, beats per min | |||
| Baseline | 64.7±8.9 | 64.4±8.0 | .91 |
| End of study | 65.0±9.2 | 65.0±7.5 | .99 |
| Change after 16 weeks | 0.3±5.4 | 0.5±3.9 | .85 |
| Daytime SBP, mm Hg | |||
| Baseline | 139.8±12.6 | 136.8±13.2 | .29 |
| End of study | 132.2±14.0 | 131.6±14.0 | .84 |
| Change after 16 weeks | −7.6±15.7b | −5.2±12.3b | .44 |
| Daytime DBP, mm Hg | |||
| Baseline | 77.5±7.8 | 76.6±8.6 | .60 |
| End of study | 73.1±7.8 | 73.2±7.8 | .96 |
| Change after 16 weeks | −4.4±9.1b | −3.4±6.4b | .54 |
| Daytime pulse rate, beats per min | |||
| Baseline | 68.1±8.7 | 68.6±8.7 | .81 |
| End of study | 68.7±9.4 | 69.7±7.7 | .60 |
| Change after 16 weeks | 0.6±6.8 | 1.1±5.7 | .71 |
| Nighttime SBP, mm Hg | |||
| Baseline | 124.2±15.7 | 127.6±13.8 | .29 |
| End of study | 119.6±16.6 | 121.2±14.3 | .62 |
| Change after 16 weeks | −4.6±13.6b | −6.3±9.6a | .49 |
| Nighttime DBP, mm Hg | |||
| Baseline | 69.0±9.4 | 70.0±8.5 | .62 |
| End of study | 65.4±9.4 | 66.6±8.4 | .53 |
| Change after 16 weeks | −3.7±7.0b | −3.4±7.4a | .88 |
| Nighttime pulse rate, beats per min | |||
| Baseline | 58.5±9.9 | 56.8±7.0 | .36 |
| End of study | 59.4±9.0 | 57.5±7.8 | .28 |
| Change after 16 weeks, beats per min | 0.9±5.3 | 0.7±4.2 | .79 |
| Early‐morning SBP, mm Hg | |||
| Baseline, mm Hg | 137.5±15.9 | 144.4±19.2 | .07 |
| End of study, mm Hg | 141.5±19.5 | 133.6±17.2 | .05 |
| Change after 16 weeks, mm Hg | 4.0±21.2 | −10.8±22.1b | .002 |
| Early‐morning DBP, mm Hg | |||
| Baseline, mm Hg | 77.1±11.5 | 77.0±10.0 | .96 |
| End of study, mm Hg | 75.6±11.2 | 75.3±12.3 | .93 |
| Change after 16 weeks, mm Hg | −1.6±11.5 | −1.7±13.5 | .97 |
| Early‐morning pulse rate, beats per min | |||
| Baseline, beats per min | 65.3±11.2 | 65.0±9.9 | .90 |
| End of the study, beats per min | 66.1±10.3 | 66.9±11.3 | .72 |
| Change after 16 weeks, beats per min | 0.8±9.8 | 1.9±9.8 | .59 |
| Lowest nighttime SBP, mm Hg | |||
| Baseline, beats per min | 109.9±15.2 | 113.9±13.2 | .19 |
| End of study, beats per min | 103.8±14.5 | 108.5±15.1 | .15 |
| Change after 16 weeks, beats per min | −6.0±10.8b | −5.4±10.2b | .79 |
| Morning BP surge, mm Hg | |||
| Baseline, mm Hg | 27.6±18.5 | 30.5±15.3 | .43 |
| End of study, mm Hg | 37.6±20.5 | 25.0±13.8 | .001 |
| Change after 16 weeks | 10.0±23.2b | −5.4±20.3 | .001 |
| SD of 24‐hour SBP, mm Hg | |||
| Baseline | 21.1±5.5 | 18.5±4.9 | .02 |
| End of study | 21.1±5.9 | 18.7±5.0 | .04 |
| Change after 16 weeks | 0.0±5.2 | 0.2±4.4 | .83 |
| SD of 24‐hour DBP, mm Hg | |||
| Baseline | 13.0±4.3 | 12.2±4.2 | .37 |
| End of study | 13.6±4.8 | 13.3±5.1 | .79 |
| Change after 16 weeks | 0.5±5.6 | 1.1±4.8 | .63 |
| SD of daytime SBP, mm Hg | |||
| Baseline | 21.0±5.9 | 19.3±5.9 | .20 |
| End of study | 21.7±6.1 | 19.2±5.8 | .05 |
| Change after 16 weeks | 0.7±6.4 | −0.2±6.2 | .51 |
| SD of daytime DBP, mm Hg | |||
| Baseline | 12.8±4.7 | 12.6±4.9 | .83 |
| End of study | 14.3±6.1 | 13.8±5.7 | .71 |
| Change after 16 weeks | 1.5±7.1 | 1.2±5.6 | .85 |
| SD of nighttime SBP, mm Hg | |||
| Baseline | 14.7±5.4 | 13.6±3.8 | .26 |
| End of study | 14.1±3.8 | 13.6±6.0 | .65 |
| Change after 16 weeks | −0.7±6.3 | 0.0±6.4 | .65 |
| SD of nighttime DBP, mm Hg | |||
| Baseline | 9.5±4.2 | 8.6±3.0 | .26 |
| End of study | 9.2±2.4 | 9.7±5.5 | .58 |
| Change after 16 weeks | −0.3±5.1 | 1.1±5.7 | .23 |
| Plasma renin activity, ng/mL/h | |||
| Baseline | 0.83 (0.66–1.04) | 0.74 (0.62–0.89) | .46 |
| End of study | 0.17 (0.13–0.22) | 1.27 (1.02–1.60) | <.001 |
| Change from baseline, % | −79.4 (−84.4 to −72.8)a | 71.3 (47.7–98.8)a | <.001 |
| Plasma aldosterone concentration, pg/mL | |||
| Baseline | 73.4 (62.2–86.7) | 84.1 (73.3–96.5) | .21 |
| End of study | 75.4 (63.7–89.3) | 109.4 (94.4–126.8) | .001 |
| Change from baseline, % | 2.7 (−13.6 to 22.1) | 30.1 (14.0–48.4)a | .03 |
Abbreviations: DBP diastolic blood pressure; SBP, systolic blood pressure. Data are expressed as mean±standard deviation (SD) or geometric mean (95% confidence interval). Clinic blood pressure (BP) was evaluated in 47 patients in the aliskiren/amlodipine (ALI/AML) group and in 51 patients in the high‐dose amlodipine (h‐dAML) group. Twenty‐four‐hour BP was evaluated in 40 patients in the ALI/AML group and in 46 patients in the h‐dAML group. Laboratory data were evaluated in 45 patients in the ALI/AML group and in 51 patients in the h‐dAML group. a P<.001 and b P<.05 vs baseline by paired t test.
Changes in Ambulatory BP
Both ALI/AML and h‐dAML significantly and similarly lowered the patients' mean 24‐hour BP, daytime BP, and nighttime BP (Table 2). Patients in the h‐dAML group had significantly decreased early‐morning SBP from baseline and had no significant change in morning BP surge, whereas patients in the ALI/AML group had no significant change in early‐morning SBP and significantly increased morning BP surge from baseline. The reduction of early‐morning SBP was significantly greater in the h‐dAML group than in the ALI/AML group (Table 2). ALI/AML significantly increased morning BP surge compared with h‐dAML (Table 2). Changes in standard deviations of 24‐hour, daytime, and nighttime systolic and diastolic BP were similar between the two groups.
Changes in the UACR
ALI/AML did not provide change in UACR, whereas h‐dAML tended to increase UACR, but both changes were not significant (ALI/AML −15.0% [95% CI, −33.3 to 8.4], P=.19 and h‐dAML 27.9% [95% CI, −0.4 to 64.1], P=.05 vs baseline) (Table 3). h‐dAML significantly increased UACR compared with ALI/AML (P=.02).
Table 3.
Changes in UACR, eGFR, and baPWV During the Treatment Period (Intention‐to‐Treat Populations)
| Change | ALI/AML Group (n=45) | h‐dAML Group (n=51) | P Value |
|---|---|---|---|
| UACR, mg/g cr | |||
| Baseline | 26.3 (18.6–37.2) | 21.4 (15.4–29.8) | .39 |
| End of study | 22.4 (16.3–30.7) | 27.4 (19.1–39.2) | .40 |
| Change after 16 weeks, % | −15.0 (−33.3 to 8.4) | 27.9 (−0.4 to 64.1) | .02 |
| eGFR, mL/min/1.73 m2 | |||
| Baseline | 70.4±19.2 | 71.0±17.2 | .86 |
| End of study | 67.8±18.4 | 69.3±18.0 | .64 |
| Change after 16 weeks | −2.5±7.2a | −1.7±6.3 | .54 |
| baPWV, cm/s | |||
| Baseline | 1894.5±327.8 | 2016.2±360.9 | .10 |
| End of study | 1837.7±326.6 | 1927.1±318.3 | .20 |
| Change after 16 weeks | −56.8±204.7 | −89.1±239.6a | .50 |
Abbreviations: eGFR, estimated glomerular filtration rate; UACR, urine albumin to creatinine ratio. Data are expressed as mean±standard deviation or geometric mean (95% confidence interval). Laboratory data were evaluated in 45 patients in the aliskiren/amlodipine (ALI/AML) group and in 51 patients in the high‐dose amlodipine (h‐dAML) group. Brachial‐ankle pulse wave velocity (baPWV) was evaluated in 41 patients in the ALI/AML group and in 48 patients in the h‐dAML group. a P<.05 vs baseline by paired t test.
Changes in baPWV
ALI/AML tended to reduce baPWV from baseline, but the reduction was not significant. In contrast, h‐dAML significantly reduced baPWV from baseline. The difference in the magnitude of baPWV reduction was insignificant between the two groups (Table 3).
Changes in Laboratory Data
Biomarkers related to the RAAS were evaluated. Table 2 shows the geometric means for these biomarker measurements at baseline and at the endpoint and the geometric mean ratio between the endpoint and baseline. ALI/AML significantly decreased PRA, whereas h‐dAML significantly increased PRA. The PAC did not change significantly in the ALI/AML group, in contrast to the significant increase in the PAC in the h‐dAML group (30.1%). The percent change of PAC with h‐dAML was significantly higher than that with ALI/AML (Table 2).
Discussion
The results of the present study showed that compared with h‐dAML monotherapy, the ALI/AML combination therapy provided a similar BP‐lowering effect in mean 24‐hour BP, daytime BP, and nighttime BP. ALI/AML provided superior reduction in albuminuria compared with h‐dAML. However, ALI/AML was significantly less effective in lowering early‐morning BP and morning BP surge compared with h‐dAML.
Changes in Ambulatory BP
Similar to a previous report,17 the aliskiren add‐on to amlodipine therapy provided mean 24‐hour, daytime, and nighttime BP reduction on ABPM, as did h‐dAML. However, to the best of our knowledge, there is no report on the effect of aliskiren on early‐morning BP and morning BP surge on ABPM. Although it is not clear why the ALI/AML combination was significantly less effective in reducing early‐morning BP and morning BP surge than h‐dAML monotherapy, a previous meta‐analysis might support our findings. The result of meta‐analysis regarding the association between BP variability and antihypertensive drug treatment revealed that calcium channel blockers are significantly more effective in reducing interindividual BP variability than other RAAS inhibitors such as ACE inhibitors and ARBs.18 Rothwell and colleagues19 reported that interindividual BP variability is partly associated with intraindividual BP variability. 19
The morning period is the time when BP variability is likely to increase compared with other times of the day, especially in elderly patients.20 The patients enrolled in the present study were significantly older than those in any of the other studies that investigated the effect of aliskiren on BP evaluated by ABPM. Concerning BP variability associated with the morning, the add‐on of aliskiren to calcium channel blocker therapy might be inferior to high‐dose calcium channel blocker therapy, especially in elderly patients. It was reported that aortic stiffness is positively associated with early‐morning BP and morning BP surge.21 In our study, h‐dAML significantly reduced baPWV from baseline, whereas this association was not found with ALI/AML. It is possible that the reduction in early‐morning BP and morning BP surge shown by h‐dAML is caused partly by the improvement of aortic stiffness.
Changes in UACR
In the present study, h‐dAML significantly increased UACR compared with ALI/AML. ALI/AML decreased UACR from baseline, but it was not significant. In the Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) study,11 aliskiren significantly reduced the UACR by 20% from baseline independent of its office BP‐lowering effect, a finding which supports the present study's result. In the AVOID study, the baseline UACR was high (513 mg/g cr), which was significantly higher than the baseline UACR in the ALI/AML group (26.3 mg/g cr), and this might be the reason why there was no significant difference in the UACR between baseline and follow‐up in the ALI/AML group in the present study.
BP‐Lowering Effect on ABPM and Target Organ Protection
In many previous clinical investigations, aliskiren consistently reduced UACR.11, 13, 22, 23, 24 Nevertheless, in clinical trials, the addition of aliskiren did not improve hard endpoints such as cardiovascular events, heart failure, and renal failure compared with standard therapy.13, 14 In the Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints (ALTITUDE), the addition of aliskiren compared with standard therapy with RAAS blockade in patients with type 2 diabetes who were at high risk for cardiovascular and renal events also reduced UACR, but it did not improve the rate of cardiovascular events or renal failure.13 Among patients hospitalized for heart failure in the Aliskiren Trial on Acute Heart Failure Outcome (ASTRONAUT), the initiation of aliskiren in addition to standard therapy did not reduce the rate of cardiovascular death or heart failure rehospitalization. On the contrary, it increased renal failure compared with standard therapy.14 In these two clinical studies, BP was evaluated as clinic BP only, and neither home BP nor ABPM were performed. In our study, although ALI/AML provided a similar BP‐lowering effect in mean 24‐hour BP, daytime BP, and nighttime BP, and although it was superior in reducing UACR, ALI/AML did not successfully suppress the patients' early‐morning BP level or morning surge compared with h‐dAML. Morning BP surge was first found in 2003 to be a predictor of cerebrovascular events independently of clinic and 24‐hour BP levels.25 In that study, the relative risk of stroke events per 10‐mm Hg increase in sleep‐through morning BP surge was 1.24 (95% CI, 1.07–1.43). The reason why aliskiren did not reduce cardiovascular events in some previous clinical trials under conditions that could protect organ damage might be explained by the unsuccessful suppression of early‐morning BP and morning BP surge in select patients who had a high risk for cardiovasucular events, such as elderly patients. Our findings suggest that h‐dAML might be better than ALI/AML for elderly hypertensives with progressed atherosclerosis to reduce morning BP, while ALI/AML might be better than h‐dAML for hypertensives with high UACR and without progressed atherosclerosis to protect renal function.
Limitations
Our study has several limitations. First, the sample size was relatively small; therefore, the difference of change in baPWV between the two groups should be tested in a larger number of patients. Second, the results may not be applicable to younger populations or other racial populations (all of our patients were Japanese and mostly elderly). Third, the treatment period was relatively short and there were no outcome data. However, our findings might support the hypothesis that the add‐on of aliskiren to another antihypertensive drug is effective for organ damage protection, but may not help prevent adverse cardiovascular outcomes. Fourth, although the current study was a randomized controlled trial, the ALI/AML group had a significantly higher ratio of men, current smokers, diabetics, and use of antidiabetic drugs than the h‐dAML group at baseline. However, after adjusting for these factors, the results were similar (data not shown).
Conclusions
ALI/AML combination therapy is as effective as h‐dAML monotherapy for reducing patients' mean 24‐hour BP, daytime BP, and nighttime BP. Although UACR was significantly reduced in the ALI/AML group compared with the h‐dAML group, ALI/AML was not sufficiently effective in reducing early‐morning BP or morning BP surge compared with h‐dAML in elderly Japanese hypertensive patients.
Disclosure
The authors declare no conflict of interest. This study was not sponsored by any companies. The coauthors did not receive funding from other sources.
Acknowledgments
H.M. analyzed the data from this study and was responsible for the writing of this article. S.H. and K.K. were the advisors for the conception and design of this study, and they assisted in conducting the statistical analyses. H.M. and M.F. recruited the study patients.
J Clin Hypertens (Greenwich). 2016;18:70–78. DOI: 10.1111/jch.12618. © 2015 Wiley Periodicals, Inc.
Clinical Trial Registry Number: UMIN000010163
References
- 1. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens. 2014;16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 3. Shimamoto K, Ando K, Fujita T, et al. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension: the Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253–390. [DOI] [PubMed] [Google Scholar]
- 4. Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. [DOI] [PubMed] [Google Scholar]
- 5. Salles GF, Cardoso CR, Fiszman R, Muxfeldt ES. Prognostic importance of baseline and serial changes in microalbuminuria in patients with resistant hypertension. Atherosclerosis. 2011;216:199–204. [DOI] [PubMed] [Google Scholar]
- 6. Holtkamp FA, De Zeeuw D, De Graeff PA, et al. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32:1493–1499. [DOI] [PubMed] [Google Scholar]
- 7. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 8. Blacher J, Safar ME, Guerin AP, et al. Aortic pulse wave velocity index and mortality in end‐stage renal disease. Kidney Int. 2003;63:1852–1860. [DOI] [PubMed] [Google Scholar]
- 9. Nussberger J, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39:E1–E8. [DOI] [PubMed] [Google Scholar]
- 10. Azizi M, Ménard J, Bissery A, et al. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II‐renin feedback interruption. J Am Soc Nephrol. 2004;15:3126–3133. [DOI] [PubMed] [Google Scholar]
- 11. Parving HH, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. [DOI] [PubMed] [Google Scholar]
- 12. McMurray JJ, Pitt B, Latini R, et al; Aliskiren Observation of Heart Failure Treatment (ALOFT) Investigators . Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circ Heart Fail. 2008;1:17–24. [DOI] [PubMed] [Google Scholar]
- 13. Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators . Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213. [DOI] [PubMed] [Google Scholar]
- 14. Gheorghiade M, Böhm M, Greene SJ, et al; ASTRONAUT Investigators and Coordinators . Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. [DOI] [PubMed] [Google Scholar]
- 15. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressure compared with office blood pressure in the general population: follow‐up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. [DOI] [PubMed] [Google Scholar]
- 16. Clement DL, De Buyzere ML, De Bacquer DA, et al; Office versus Ambulatory Pressure Study Investigators . Prognostic value of ambulatory blood‐pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. [DOI] [PubMed] [Google Scholar]
- 17. Littlejohn TW 3rd, Jones SW, Zhang J, et al. Efficacy and safety of aliskiren and amlodipine combination therapy in patients with hypertension: a randomized, double‐blind, multifactorial study. J Human Hypertens. 2013;27:321–327. [DOI] [PubMed] [Google Scholar]
- 18. Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive‐drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta‐analysis. Lancet. 2010;375:906–915. [DOI] [PubMed] [Google Scholar]
- 19. Rothwell PM, Howard SC, Dolan E, et al; ASCOT‐BPLA and MRC Trial Investigators . Effects of beta blockers and calcium‐channel blockers on within‐individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. [DOI] [PubMed] [Google Scholar]
- 20. Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56:765–773. [DOI] [PubMed] [Google Scholar]
- 21. Polónia J, Amado P, Barbosa L, et al. Morning rise, morning surge and daytime variability of blood pressure and cardiovascular target organ damage. A cross‐sectional study in 743 subjects. Rev Port Cardiol. 2005;24:65–78. [PubMed] [Google Scholar]
- 22. Persson F, Rossing P, Schjoedt KJ, et al. Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int. 2008;73:1419–1425. [DOI] [PubMed] [Google Scholar]
- 23. Morishita Y, Yasui T, Numata A, et al. Aliskiren suppresses the renin‐angiotensin‐aldosterone system and reduces blood pressure and albuminuria in elderly chronic kidney disease patients with hypertension. Int J Nephrol Renovasc Dis. 2012;5:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suzuki H, Okada K, Abe M, et al. Aliskiren reduces home blood pressure and albuminuria in patients with hypertensive nephrosclerosis. Clin Exp Nephrol. 2013;17:386–395. [DOI] [PubMed] [Google Scholar]
- 25. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. [DOI] [PubMed] [Google Scholar]


