Abstract
The authors tested the hypothesis that high salt intake is associated with hypertensive target organ damage (TOD) independent of blood pressure (BP), and oxidative stress is a modifying factor of this association. A total of 369 community‐dwelling Japanese adults (mean age, 67.5 years; 56.6% women) were examined in this observational study. At the patients' annual health check‐ups, urinary salt excretion (U‐SALT), 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG), and albumin‐creatinine ratio (UACR) were measured from first morning urine. U‐SALT (β=0.14, P=.016) and 8‐OHdG (β=0.13, P=.018) were both independently associated with logUACR. U‐SALT was associated with TOD independent of BP level, and oxidative stress may be a modifying factor in the association between high salt intake and TOD. The elevation of 8‐OHdG may be involved in the pathophysiology of TOD induced by salt intake.
Eastern Asia, including Japan, is a region well‐known for high salt intake, and the region's salt‐related cardiovascular mortality is the highest in the world.1 The International Cooperative Study on Salt, Other Factors, and Blood Pressure (INTERSALT) reported in 1988 that high salt intake increases blood pressure (BP).2 Some later epidemiological studies also reported an association between high salt intake and increased rate of cardiovascular events.3, 4 A number of clinical trials such as the Dietary Approaches to Stop Hypertension (DASH) trial also demonstrated that salt restriction was associated with a reduction in BP.5, 6
Excessive salt intake is associated not only with high BP but also target organ damage (TOD). In contrast to the long‐standing notion that high salt intake causes TOD via high BP, the direct association of high salt intake and TOD has been shown in a recent review article.7 In previous studies, high salt intake was associated with arterial stiffness8 and cardiac hypertrophy9 in healthy normotensive patients and with the urinary albumin creatinine ratio (UACR) in diabetic overweight patients.10 However, these studies are not conclusive because of their small sample size8, 9 and completely different populations,10 and to our knowledge the results have never been reported in a general population. In addition, the mechanism of the effect of high salt intake on TOD independent of BP is still unclear. In a rat model, it was reported that high salt intake caused endothelial dysfunction from an increase in reactive oxygen species (ROS).11 Another animal study found that high salt intake increased oxidative stress in the kidney and resulted in increased UACR.12 An association between ROS and UACR in humans was reported,13 but there are no studies regarding salt intake, ROS, and UACR in humans.
We thus performed the present study to clarify two hypotheses: (1) that there is an association between high salt intake and UACR independent of BP in general populations, and (2) oxidative stress is a modifying factor between high salt intake and UACR.
Methods
Study Design and Participants
This was a cross‐sectional and observational study in a general population. We enrolled 369 community‐dwelling residents of Munakata Ohshima Island, a small and remote island in Japan with a total of 780 residents. Patients 18 years or older who agreed to participate in this study were enrolled. The study was approved by the ethics committee of the internal review board at Jichi Medical University, Tochigi, Japan (Clinical, #13–17), and written informed consent was obtained from each participant. Individuals undergoing hemodialysis were not included in this study.
The residents of Munakata Ohshima Island are known to consume even more salt than the residents of other regions in Japan. In addition, the higher proportion of hypertensive residents on Munakata Ohshima Island, based on medical records, has been an issue in Munakata City. The patients' body mass index (BMI) was calculated as weight (kg)/height2 (m2). Current smoking was defined as smoking currently or within the past 1 year.
BP Measurements
At baseline, clinic BP was measured with a validated BP monitor (HEM‐7051; Omron Health Care, Kyoto, Japan).14 In addition, at least two clinic BP readings were taken after the patient rested for at least 5 minutes while seated. Hypertension was diagnosed when the clinic systolic BP (SBP) was ≥140 mm Hg and/or diastolic BP (DBP) was ≥90 mm Hg on at least two separate occasions based on Japanese Society of Hypertension (JSH) 2009 guidelines15 or by a previous diagnosis of hypertension with current antihypertensive medication use.
Urine Examinations
Urine samples were collected by asking the patients to bring a sample of their first morning urine. These urine samples were used to measure the urinary concentrations of salt, creatinine (cre), urinary albumin, and 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG). We estimated the patients' dietary salt intake based on the urinary excretion of salt (U‐SALT) in the first morning urine, adjusted by the estimated 24‐hour urinary cre excretion by applying the Tanaka's formula.16, 17 Predicted 24‐hour cre excretion is given as: [−2.04 × age (years)] + [14.89 × weight (kg)] + [16.14 × height (cm)] − 2244.45 (mg/d). The Tanaka formula is given as: 23 × (21.98 × xNa0.392), where xNa = [spot Na (mmol/L)/spot creatinine (mg/dL) × 10] × [predicted 24‐hour urinary cre (mg/d)]. The validity of this method was confirmed.16, 17, 18 We used the urinary albumin to cre ratio (UACR) (mg/g‧cre) as a surrogate marker of hypertensive renal TOD.
Urinary 8‐OHdG is a biomarker of oxidative DNA damage induced by ROS.19 We used 8‐OHdG (ng/mg‧cre) as a marker of oxidative stress, because the association between salt intake and 8‐OHdG has been reported in salt‐sensitive animal models.20 Urine samples were immediately transferred to a freezer set at −18°C, transported to a commercial laboratory (SRL Inc, Tokyo, Japan), and measured by an enzyme‐linked immunosorbent assay. To ensure the stability of the samples, the 8‐OHdG levels were measured within 2 months after collection.21
Statistical Analysis
All statistical analyses were carried out with SPSS Windows software, version 22.0 (SPSS, Armonk, NY). The data are expressed as mean (±standard deviation [SD]) (continuous variables) or percentages (categorical variables). Data with a skewed distribution, such as the UACR values, were analyzed after natural logarithmic transformation. An unpaired t test was used to compare continuous variables, and the chi‐square test was used to compare categorical variables between two groups. Pearson's correlation coefficients were used to calculate the correlations between parameters.
To assess the independent predictive utility of the different factors associated with UACR, we performed multiple linear regression analyses using the forced entry method. We set age, sex, BMI, current smoking, mean clinic SBP, presence of diabetes mellitus (DM), presence of dyslipidemia, U‐SALT, and 8‐OHdG as independent variables, and the UACR as a dependent variable. In addition, the improvement of the goodness‐of‐fit of the linear relationship between clinical parameters and the UACR was computed to determine whether the goodness‐of‐fit of model 1 (age, sex, BMI, current smoking, mean SBP, presence of DM, presence of dyslipidemia) could be improved by the addition of U‐SALT (model 2) and the further addition of 8‐OHdG (model 3). P values <.05 were considered significant.
Results
We studied a total of 369 patients. Their mean age was 67.5±15.2 years, and 209 (56.6%) were women. We divided the patients into two groups by the quartiles of their U‐SALT values, and we compared the baseline characteristics of the fourth quartile (≥10.4 g/d) vs the first to third quartiles (<10.4 g/d) (Table 1) based on the value of the amount of average daily salt consumption in Japan (ie, 10 g).1
Table 1.
Baseline Characteristics of the 369 Patients
| Low U‐SALT (n=277) | High U‐SALT (n=92) | P Value | |
|---|---|---|---|
| Age, y | 67.0±15.7 | 69.2±13.3 | .22 |
| Female sex, No. (%) | 168 (60.6) | 41 (44.6) | .005 |
| Body mass index, kg/m2 | 23.3±3.8 | 25.4±4.1 | <.001 |
| Current smokers, No. (%) | 29 (10.5) | 10 (10.9) | .52 |
| Clinic systolic BP, mm Hg | 140±22 | 144±24 | .21 |
| Clinic diastolic BP, mm Hg | 77±11 | 79±14 | .16 |
| Clinic pulse rate, bpm | 72±11 | 69±13 | .057 |
| Hypertension, No. (%) | 191 (71.3) | 80 (87.9) | .001 |
| Ischemic heart disease, No. (%) | 28 (10.4) | 7 (7.8) | .31 |
| Stroke, No. (%) | 21 (7.8) | 7 (7.8) | .60 |
| Diabetes mellitus, No. (%) | 22 (9.1) | 15 (18.5) | .021 |
| Dyslipidemia, No. (%) | 111 (47.0) | 46 (59.7) | .035 |
| Antihypertensive medications, No. (%)a | 137 (52.9) | 58 (70.7) | .003 |
| Antihypertensive medications, No.b | 1.7±0.9 | 1.7±0.8 | .85 |
| Calcium channel blockers, No. (%)c | 100 (47.2) | 40 (55.6) | .14 |
| Angiotensin II receptor blockers, No. (%)c | 81 (38.4) | 30 (41.7) | .36 |
| Angiotensin‐converting enzyme inhibitors, No. (%)c | 9 (4.3) | 8 (11.1) | .039 |
| Diuretics, No. (%)c | 29 (17.3) | 6 (10.0) | .13 |
| α‐Blockers, No. (%)c | 10 (4.7) | 7 (9.7) | .11 |
| β‐Blockers, No. (%)c | 21 (10.0) | 8 (11.1) | .47 |
Abbreviations: BP, blood pressure; bpm, beats per minute; U‐SALT, urinary salt excretion. Data are expressed as number (percentage) or mean±standard deviation (SD). aThe percentages in hypertensive patients. bMean±SD of the patients taking antihypertensive medications. cThe percentage of patients among those taking antihypertension medications.
In the group with high U‐SALT (the fourth quartile), the BMI values and percentages of patients with hypertension, DM, and antihypertensive medication use were significantly higher compared with the low U‐SALT group (ie, first to third quartiles). We performed Pearson's correlation analyses between the estimated U‐SALT, clinic BP parameters, 8‐OHdG, and UACR in all patients. The U‐SALT data were significantly correlated with clinic SBP (r=0.14, P<.01), DBP (r=0.13, P<.05), and log UACR (r=0.21, P<.001) (Figure) but not with clinic pulse rate (PR).
Figure 1.
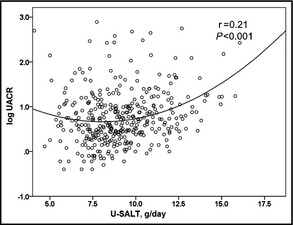
Association between urinary salt excretion (U‐SALT) and the urinary albumin‐to‐creatinine ratio (UACR) in all patients (N=369). U‐SALT was significantly correlated with log UACR (r=0.21, P<.01).
With regard to sex differences, the association between U‐SALT and 8‐OHdG (females, r=0.198, P=.004; males, r=0.099, P=.212) and between U‐SALT and UACR (females, r=0.247, P<.001; males, r=0.177, P=.025) were stronger in the women than in the men, and the association between 8‐OHdG and UACR was stronger in the men than in the women (females, r=0.168, P=.015; males, r=0.212, P=.007).
We then performed multiple linear regression analyses to assess the factors associated with UACR. In model 1, age and DM were independently associated with log UACR (R 2=0.142) (Table 2). As the regression line of the association between U‐SALT and log UACR was quadratic not linear (Figure), we squared U‐SALT as an independent variable and re‐analyzed the multiple regression models. As shown in Table 3, the addition of U‐SALT (squared) as an independent variable (model 2) improved the goodness‐of‐fit of model 1 (P<.001). The addition of 8‐OHdG as an independent variable (model 3) further improved the goodness‐of‐fit of model 2 (P<.05) (Table 3). In model 3, U‐SALT (squared) and 8‐OHdG were both independently associated with log UACR.
Table 2.
Factors Associated With the Urinary Albumin‐to‐Creatinine Ratio (Model 1)
| β | P Value | |
|---|---|---|
| Age, y | 0.29 | <.001 |
| Sex (male 1, female 0) | −0.003 | .95 |
| Body mass index, kg/m2 | 0.007 | .91 |
| Current smoking (yes 1, no 0) | 0.08 | .16 |
| Mean systolic blood pressure, mm Hg | 0.05 | .40 |
| Diabetes mellitus (yes 1, no 0) | 0.17 | .002 |
| Dyslipidemia (yes 1, no 0) | 0.01 | .87 |
Multiple linear regression analysis.
Table 3.
(Revised) Changes in the Goodness‐of‐Fit
| Urinary Albumin‐to‐Creatinine Ratio | ||
|---|---|---|
| Independent Variables | R 2 | P Value for the Change in Model |
| Model 1: Age, sex, body mass index, current smoking, mean systolic blood pressure, presence of diabetes mellitus, presence of dyslipidemia | 0.142 | |
| Model 2: Model 1 + urinary salt excretion (squared) | 0.174 | .0002 |
| Model 3: Model 2 + urinary 8‐hydroxy‐2′‐deoxyguanosine | 0.188 | .0246 |
When clinic SBP was replaced by hypertension (yes 1 or no 0) in model 3, U‐SALT (squared) remained significantly associated with log UACR (β=0.195, P=.001). When we added the status of antihypertensive medication (yes 1, no 0) to model 3, U‐SALT (squared) remained significantly associated with log UACR (β=0.198, P=.001).
We then divided the patients into two groups by quartiles of the patients' 8‐OHdG values (high 8‐OHdG:fourth quartile [≥14.7 ng/mg‧cre], low 8‐OHdG:first to third quartiles [<14.7 ng/mg‧cre]), as the cutoff value of urinary 8‐OHdG is 15.7 ng/mg‧cre.22 U‐SALT was significantly associated with log UACR in both groups (r=0.26, P=.011 in the group with high 8‐OHdG; r=0.16, P=.001 in the group with low 8‐OHdG). In an analysis of variance, the log UACR in the patients with high U‐SALT and high 8‐OHdG were significantly higher than that in the patients with low U‐SALT and low 8‐OHdG (1.28±0.56 vs 0.65±0.54, P<.001).
To determine whether there is an interaction between U‐SALT and 8‐OHdG in the UACR, we multiplied U‐SALT and 8‐OHdG (continuous parameters, U‐SALT×8‐OHdG) and added it in the model of the multiple regression analysis. U‐SALT×8‐OHdG was not independently correlated with UACR, and thus there was no interaction between U‐SALT and 8‐OHdG (Table S3). We then checked whether the slopes of the regression lines between U‐SALT and UACR differed significantly between the higher and lower values of 8‐OHdG. We multiplied U‐SALT (continuous) and 8‐OHdG (categorical) and added it in the same regression model. As a result, neither U‐SALT nor 8‐OHdG remained significant (Table S4). Thus, no interaction between U‐SALT and 8‐OHdG on UACR was established.
We then performed a parallel analysis with 8‐OHdG as the dependent variable. The U‐SALT and UACR were both significantly associated with 8‐OHdG (Figure S1 and S2, respectively). In the multiple linear regression analyses, age was independently associated with 8‐OHdG (model 1) (Table S1). We also observed an improvement of the goodness‐of‐fit of the linear relationship between clinical parameters and 8‐OHdG (Table S2). The addition of U‐SALT as an independent variable (model 2) improved the goodness‐of‐fit of model 1 (P=.001). Adding UACR as an independent variable (model 3) further improved the goodness‐of‐fit of model 2 (P=.013). In model 3, U‐SALT (β=0.15, P<.01) and UACR (β=0.14, P<.05) were also independently associated with 8‐OHdG. When clinic SBP was replaced by hypertension (yes or no) in models 1 to 3, the results did not essentially change.
Discussion
U‐SALT was associated with the measure of renal TOD independently of the BP level. This finding is important with regard to salt restriction for protecting target organs regardless of BP level. Oxidative stress was a modifying factor in the association between high salt intake and TOD.
Association Between Dietary Salt Intake and the UACR
In this study, estimated U‐SALT was associated with UACR independent of the patients' BP levels. It has been reported that high salt intake increases BP and cardiovascular risk,3, 4 but these reports did not mention the direct association between dietary salt intake and the measures of hypertensive TOD. Others have reported that high salt intake was associated with microalbuminuria in diabetic overweight patients,10 but the clinical implication of UACR in DM is somewhat different from that in nondiabetic patients. To our knowledge, there have been no reports on the direct association between salt intake and UACR in the general population, and it remains unclear whether dietary salt causes TOD directly or through hypertension. In a group of patients consuming a low‐salt diet, arterial stiffness was lower compared with a group consuming a normal salt diet.8 In a randomized controlled trial, a high‐salt diet resulted in reduced brachial artery flow‐mediated dilation in healthy normotensive salt‐resistant individuals.23 In terms of TOD, another study reported an association between salt intake and increased left ventricular mass in normotensive patients.9 High salt intake in treated hypertensive patients has been reported to be associated with enhanced inflammation and renal TOD independent of BP levels.24 High salt intake may thus have some mechanism to directly cause TOD.
Serum Sodium and Endothelial Dysfunction
Endothelial dysfunction is the first step in the development of atherosclerosis and cardiovascular diseases.25 Serum sodium increases endothelial stiffness and impairs endothelial‐dependent vasodilation. Small changes in the serum sodium concentration induce endothelial stiffness within minutes. Sodium promotes cutaneous lymphangiogenesis and increases endothelial cell stiffness. Sodium reduces the size of endothelial cells and its surface area, volume, cytoskeleton, deformability, and pliability. Sodium also reduces endothelial nitric oxide (NO) synthase (eNOS) and NO production26 and increases transforming growth factor‐β (TGF‐β), thus increasing arterial stiffness. Both dietary sodium and dietary potassium promote functional changes in the vasculature and lymphatic system independent of BP changes. Potassium counteracts all of the actions of sodium.
The roles of epithelial sodium channels (ENaC) and the endothelial proteinaceous layer glycocalyx were recently reported to be important in the association between sodium and endothelial dysfunction.27 Endothelial cells are targets for aldosterone, which activates apically located ENaC. The activation of ENaC increases endothelial stiffness and decreases NO release. The glycocalyx of the inner surface of blood vessels contains ENaC and sodium‐potassium adenosine triphosphatase (Na/K‐ATPase), and the glycocalyx binds sodium as a protective barrier. If the glycocalyx is damaged, the sodium permeability of the vascular system will be increased, resulting in increased vascular resistance and disturbed endothelial NO production. Alterations in the sodium/potassium ratio in the glycocalyx shell of the cell membrane control the shear stress–dependent activity of eNOS and thus NO production.27 Aldosterone blockers and ENaC blockers may be effective in reducing salt‐induced endothelial dysfunction.28
Salt and Oxidative Stress
In the present study, salt intake was associated with oxidative stress, and this association was independent of BP. The goodness‐of‐fit of the linear relationship between U‐SALT and UACR was improved by adding 8‐OHdG to the model (Table 3). This means that oxidative stress may be a modifying factor for the association between U‐SALT and UACR.
Oxidative stress, an imbalance between ROS production and scavenging, contributes to the development of cardiovascular disease.29 An association between oxidative stress and microalbuminuria has been reported.13 Superoxide levels were elevated in arteries of patients with a high‐salt diet.11, 30, 31 Increased superoxide reacts with NO and forms peroxynitrite, resulting in reduced NO synthesis. Increased stiffness of endothelial cells also results in decreased NO synthesis.32 Increased dietary salt intake was reported to be associated with oxidative stress and hypertensive TOD in salt‐sensitive animal models,20 but there has been no report of human studies showing these relationships.
Oxidative stress may be a modifying factor in the association between U‐SALT and UACR, but it is still unclear whether the overproduction of oxidative stress amplifies the association between high salt intake and UACR or oxidative stress mediates only salt‐induced UACR. Salt intake along with elevated oxidative stress induces microvascular damage,26 and this may be one of the underlying mechanisms in UACR elevation. Salt‐induced factors influence renal damage, and the role of oxidative stress in the progression of renal damage should be investigated in further prospective studies.
Salt Sensitivity
It is estimated that 51% of hypertensive and 26% of normotensive patients are salt‐sensitive.33 High salt intake impairs vasodilation, increases vasoconstriction, and elevates BP in both normotensive and hypertensive patients with or without salt sensitivity. Normotensive patients with salt sensitivity have mortality estimates that are equivalent to those of hypertensive patients.34 In addition, it was reported that salt‐sensitive normotensive patients will have a greater age‐related increase in BP.35 The underlying pathophysiological mechanisms of salt sensitivity are currently unknown, but there is strong evidence that genetic mechanisms may play important roles in the response of BP to salt intake.
Previous studies demonstrated that single nucleotide polymorphisms of the sodium‐bicarbonate cotransporter gene (SLC4A5) are associated with hypertension.36, 37 Salt‐inducible kinase 1 (SIK1) is a sucrose nonfermenting‐like kinase isoform that belongs to the 5′ adenosine monophosphate‐activated protein kinase (AMPK) family. It was reported that SIK1 activity is increased by high salt intake and that its activity plays an essential role in regulating the plasma membrane Na/K‐ATPase in endothelial and vascular smooth muscle cells.38, 39
Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of NO synthase. It was recently reported that in Dahl salt‐sensitive rats, salt affected the protein arginine methyltransferase/ADMA/dimethylarginine dimethylaminohydrolase (PRMT/ADMA/DDAH) and led to endothelial dysfunction independent of BP.40 Angiotensin II receptor type 2 (AT2) induces the constriction of cerebral arterioles. It was reported that high salt intake induced changes in the expression of receptors and loss of AT2‐mediated vasodilation in extracerebral vessels. High salt intake also specifically abolished AT2‐mediated vasodilation via a decreased level of AT2 receptor protein. Such a loss of AT2‐mediated vasodilation increases BP and the risks of stroke and cardiovascular diseases.41
Salt and the Immune System
An adaptive immune response as a result of exposure to mineralocorticoids and high salt intake has been implicated in inflammation and fibrosis.42 Increased salt conditions were recently reported to boost the induction of murine and human helper T (Th) 17 cells. High‐salt conditions activated the p38/mitogen‐activated protein kinase (MAPK) pathway involving nuclear factor of activated T cells 5 (NFAT5) and serum/glucocorticoid‐regulated kinase 1 (SGK1) during cytokine‐induced Th17 polarization. The Th17 cells generated under high‐salt conditions display a highly pathogenic and stable phenotype characterized by the upregulation of proinflammatory cytokines such as granulocyte‐macrophage colony‐stimulating factor, tumor necrosis factor α, and interleukin (IL) 2. Increased salt intake might represent an environmental risk factor for the development of autoimmune diseases through the induction of pathogenic Th17 cells.43 It was also reported that mineralocorticoid receptor activation alters the Th17/regulatory T‐lymphocyte/IL‐17 pathway in mineralocorticoid salt‐induced hypertension as part of an inflammatory mechanism contributing to fibrosis.44
Study Strengths
This study has several strengths. First, we performed this study in a general population, which is generalizable to other populations. Second, we used an established formula to estimate the amount of urinary salt excretion. This formula has been used in previous studies.16, 17 Third, we measured an oxidative stress marker in addition to urinary salt and observed an association between high salt intake and increased oxidative stress in humans. Our findings will contribute to explore the mechanism underlying the relationship between U‐SALT and the UACR.
Study Limitations
Our study has some limitations. First, the design of the study was cross‐sectional with a relatively small sample size. However, the present population is suitable for a population study because it represents the Japanese population. Second, we did not obtain data of home BP and ambulatory BP measurements for all patients. However, in a partial analysis of data (n=106) replacing these ambulatory BP variables with clinic BP, the results did not essentially change. In addition, when the BP level was replaced with hypertension (yes or no) in the models of the regression analysis, the results did not change. Third, we estimated the UACR and 8‐OHdG by only a single measurement of the spot urine sample, and intra‐individual variability may have influenced the data. However, as we did this study at the time of the patients' annual health check‐up, it was difficult to obtain multiple measurements. Fourth, we estimated the urinary salt excretion once as a single morning urine sample, not as a 24‐hour urine sample. Spot urine sodium and creatinine levels can be used as a surrogate of 24‐hour urinary salt excretion for individual analyses. These approximations, which have a 0.86 correlation between actual and predicted values at best,45 are used for comparisons between populations or individuals with multiple spot urine samples. Originally, we chose spot urine samples because it is useful to know the trend regarding the amount of urinary salt excretion for an entire population both cross‐sectionally and over the years. In addition, the use of spot urine samples makes it possible to investigate the association between urinary salt excretion and the markers of TOD. The validity of this method has been confirmed.16, 17, 18
Conclusions
We found a significant association between urinary salt excretion and the UACR independent of BP levels. Oxidative stress may be a modifying factor in the association between U‐SALT and the UACR, and the elevation of 8‐OHdG may be involved in the pathophysiology of TOD induced by salt intake.
Disclosures
None.
Sources of Funding
This study was supported by grants from the Foundation for the Development of the Community, Tochigi, Japan.
Supporting information
Figure S1. Association between urinary salt excretion (U‐SALT) and urinary 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) in all patients (N=369). U‐SALT was significantly correlated with the oxidative stress marker 8‐OHdG (r=0.13, P<.05).
Figure S2. Association between urinary albumin creatinine ratio (UACR) and 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) in all patients (N=369). UACR was significantly correlated with the oxidative stress marker 8‐OHdG (r=0.19, P<.001).
Table S1. Factors associated with urinary 8‐hydroxy‐2′‐deoxyguanosine (model 1).
Table S2. Changes in goodness‐of‐fit.
Table S3. Factors associated with urinary albumin‐to‐creatinine ratio.
Table S4. Factors associated with urinary albumin‐to‐creatinine ratio.
Acknowledgments
We thank all of the patients, physicians, and medical staff involved for their participation in this study. We thank Professor Joe E. Schwartz for his advice on the data analyses. We thank Ms Tomoko Endo, Ms Ayumi Endo, Ms Naomi Kido, Ms Michi Funakoshi, Ms Haruka Kawano, Ms Konomi Shiraishi, Ms Shiori Sato, Dr Minako Sakaki, Ms Yuka Mihara, and Ms. Yuki Nakamura for the study coordination and data management, and Ms Ayako Okura for her editorial assistance. Dr Imaizumi designed the study and planned and performed the research. Drs Imaizumi and Eguchi equally contributed to the writing process of the manuscript.
J Clin Hypertens (Greenwich). 2016;18:315–321. DOI: 10.1111/jch.12668. © 2015. Wiley Periodicals, Inc.
References
- 1. Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. [DOI] [PubMed] [Google Scholar]
- 2. Intersalt Cooperative Research Group . Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1988;297:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He J, Ogden LG, Vupputuri S, et al. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282:2027–2034. [DOI] [PubMed] [Google Scholar]
- 4. Tuomilehto J, Jousilahti P, Rastenyte D, et al. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet. 2001;357:848–851. [DOI] [PubMed] [Google Scholar]
- 5. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH‐Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 6. Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farquhar WB, Edwards DG, Jurkovitz CT, Weintraub WS. Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol. 2015;65:1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avolio AP, Clyde KM, Beard TC, et al. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis. 1986;6:166–169. [DOI] [PubMed] [Google Scholar]
- 9. Seta H, Ishimitsu T, Tamano K, et al. Increase in left ventricular mass is influenced by blood pressure and obesity, whereas reduction in left ventricular diastolic function is affected by greater age and salt intake. J Cardiol. 2001;37:249–256. [PubMed] [Google Scholar]
- 10. Engelen L, Soedamah‐Muthu SS, Geleijnse JM, et al. Higher dietary salt intake is associated with microalbuminuria, but not with retinopathy in individuals with type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetologia. 2014;57:2315–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu J, Huang T, Lombard JH. Effect of high‐salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res. 2007;44:382–390. [DOI] [PubMed] [Google Scholar]
- 12. Dobrian AD, Schriver SD, Lynch T, Prewitt RL. Effect of salt on hypertension and oxidative stress in a rat model of diet‐induced obesity. Am J Physiol Renal Physiol. 2003;285:F619–F628. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki K, Honjo H, Ichino N, et al. Association of serum carotenoid levels with urinary albumin excretion in a general Japanese population: The Yakumo Study. J Epidemiol. 2013;23:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. http://www.dableducational.org/sphygmomanometers/devices_2_sbpm.html.
- 15. Ogihara T, Kikuchi K, Matsuoka H, et al. Japanese Society of Hypertension Committee. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res. 2009;32:3–107. [PubMed] [Google Scholar]
- 16. Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24‐h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. [DOI] [PubMed] [Google Scholar]
- 17. Mente A, O'Donnell MJ, Dagenais G, et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24‐h measures in 11 countries. J Hypertens. 2014;32:1005–1014. [DOI] [PubMed] [Google Scholar]
- 18. Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol 1993;20:7–14. [DOI] [PubMed] [Google Scholar]
- 19. Floyd RA, Watson JJ, Wong PK, et al. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radical Res. 1986;1:163–172. [DOI] [PubMed] [Google Scholar]
- 20. Kushiro T, Fujita H, Hisaki R, et al. Oxidative stress in the Dahl salt‐sensitive hypertensive rat. Clin Exp Hypertens. 2005;27:9–15. [DOI] [PubMed] [Google Scholar]
- 21. Matsumoto Y, Ogawa Y, Yoshida R, et al. The stability of the oxidative stress marker, urinary 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG), when stored at room temperature. J Occup Health. 2008;50:366–372. [DOI] [PubMed] [Google Scholar]
- 22. http://www.jaica.com/guidance_8ohdg/.
- 23. DuPont JJ, Greaney JL, Wenner MM, et al. High dietary sodium intake impairs endothelium‐dependent dilation in healthy salt‐resistant humans. J Hypertens. 2013;31:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yilmaz R, Akoglu H, Altun B, et al. Dietary salt intake is related to inflammation and albuminuria in primary hypertensive patients. Eur J Clin Nutr. 2012;66:1214–1218. [DOI] [PubMed] [Google Scholar]
- 25. Lüscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395–418. [DOI] [PubMed] [Google Scholar]
- 26. Boegehold MA. The effect of high salt intake on endothelial function: reduced vascular nitric oxide in the absence of hypertension. J Vasc Res. 2013;50:458–467. [DOI] [PubMed] [Google Scholar]
- 27. Nijst P, Verbrugge FH, Grieten L, et al. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol. 2015;65:378–388. [DOI] [PubMed] [Google Scholar]
- 28. Bhagatwala J, Harris RA, Parikh SJ, et al. Epithelial sodium channel inhibition by amiloride on blood pressure and cardiovascular disease risk in young prehypertensives. J Clin Hypertens (Greenwich). 2014;16:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edwards DG, Farquhar WB. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens. 2015;1:8–13. doi: 10.1097/MNH.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium‐dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol. 2000;279:7–17. [DOI] [PubMed] [Google Scholar]
- 31. Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res. 2002;39:41–50. [DOI] [PubMed] [Google Scholar]
- 32. Oberleithner H, Riethmüller C, Schillers H, et al. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA. 2007;104:16281–16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weinberger MH, Miller JZ, Luft FC, et al. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:127–134. [DOI] [PubMed] [Google Scholar]
- 34. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. [DOI] [PubMed] [Google Scholar]
- 35. Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18:67–71. [DOI] [PubMed] [Google Scholar]
- 36. Hunt SC, Xin Y, Wu LL, et al. Sodium bicarbonate cotransporter polymorphisms are associated with baseline and 10‐year follow‐up blood pressures. Hypertension. 2006;47:532–536. [DOI] [PubMed] [Google Scholar]
- 37. Carey RM, Schoeffel CD, Gildea JJ, et al. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium‐bicarbonate cotransporter. Hypertension. 2012;60:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bertorello AM, Pires N, Igreja B, et al. Increased arterial blood pressure and vascular remodeling in mice lacking salt‐inducible kinase 1 (SIK1). Circ Res. 2015;116:642–652. [DOI] [PubMed] [Google Scholar]
- 39. Popov S, Silveira A, Wågsäter D, et al. Salt‐inducible kinase 1 influences Na(+), K(+)‐ATPase activity in vascular smooth muscle cells and associates with variations in blood pressure. J Hypertens. 2011;29:2395–2403. [DOI] [PubMed] [Google Scholar]
- 40. Cao Y, Mu JJ, Fang Y, et al. Impact of high salt independent of blood pressure on PRMT/ADMA/DDAH pathway in the aorta of Dahl salt‐sensitive rats. Int J Mol Sci. 2013;14:8062–8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Foulquier S, Dupuis F, Perrin‐Sarrado C, et al. High salt intake abolishes AT(2)‐mediated vasodilation of pial arterioles in rats. J Hypertens 2011;29:1392–1399. [DOI] [PubMed] [Google Scholar]
- 42. Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kleinewietfeld M, Manzel A, Titze J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013;496:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amador CA, Barrientos V, Peña J, et al. Spironolactone decreases DOCA‐salt‐induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension. 2014;63:797–803. [DOI] [PubMed] [Google Scholar]
- 45. Mann SJ, Gerber LM. Estimation of 24‐hour sodium excretion from spot urine samples. J Clin Hypertens (Greenwich). 2010;12:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Association between urinary salt excretion (U‐SALT) and urinary 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) in all patients (N=369). U‐SALT was significantly correlated with the oxidative stress marker 8‐OHdG (r=0.13, P<.05).
Figure S2. Association between urinary albumin creatinine ratio (UACR) and 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) in all patients (N=369). UACR was significantly correlated with the oxidative stress marker 8‐OHdG (r=0.19, P<.001).
Table S1. Factors associated with urinary 8‐hydroxy‐2′‐deoxyguanosine (model 1).
Table S2. Changes in goodness‐of‐fit.
Table S3. Factors associated with urinary albumin‐to‐creatinine ratio.
Table S4. Factors associated with urinary albumin‐to‐creatinine ratio.


