Abstract
Imaging of the kidney using blood oxygen level dependent (BOLD) magnetic resonance imaging (MRI) presents a major opportunity to examine differences in tissue oxygenation within the cortex and medulla applicable to human disease. The aim of this study was to evaluate BOLD signals before and after treatment with RAS inhibitors in hypertensive chronic kidney disease (CKD) patients. Ten patients with stable CKD and 5 healthy volunteers were included. Five CKD patients were subjected to BOLD MRI scan before and after chronic treatment with 300 mg/day aliskiren for at least 6 weeks. Five other CKD patients received BOLD MRI before and 1 hour after acute treatment with 50 mg captopril. A group of healthy volunteers (n=5) was scanned before and 1 hour after acute treatment with 50 mg captopril. The 10 patients had a mean age of 61±17 years; eGFR of 30±11 mL/min per 1.73 m2. Office systolic and diastolic blood pressures when on a RAS inhibito, were 130±10 and 86±5 mmHg in CKD patients. Control subjects had normal kidney function and were not on any medication. In untreated condition, systolic and diastolic arterial blood pressure elevated, 145±6 and 95±4 mmHg, respectively. After chronic treatment with aliskiren, arterial blood pressure decreased in all patients in this group, 127±3 mmHg and 77±3 mmHg. After acute treatment with captopril arterial blood pressure reduced to 125±4 and 71±8 mmHg. Tissue intensity signal (T2*) was increased in medulla after chronic treatment from 29±6 to 34±6 and after acute treatment with captopril from 34±9 to 38±11 in CKD patients. In addition, T2* ratio between cortex and medulla decreased in CKD patients after chronic treatment and acute treatment. This ratio remained stable in healthy volunteers before and after treatment with captopril 1.62±0.1 and 1.65±0.1, respectively. This study shows for the first time that RAS inhibitors change BOLD signal in CKD patients. Importantly, in healthy volunteers, a RAS inhibitor had no such effect. Further investigation is required.
Many experimental studies have shown that kidney ischemia is the key finding in the pathogenesis and progression of chronic kidney disease (CKD) and hypertension.1 Inhibitors of the renin‐angiotensin system (RAS) are the cornerstone of the treatment of CKD patients. There are hypotheses that minor kidney damage, not affecting kidney function and therefore difficult to diagnose, might be present, resulting in “essential” hypertension and its subsequent cardiovascular complications.1 Thus far it is a challenge to identify kidney ischemia in its early stage. Imaging of the kidney using blood oxygenation level–dependent (BOLD) magnetic resonance imaging (MRI) presents a major new opportunity to examine differences in kidney tissue oxygenation within the cortex and medulla applicable to human disease. BOLD MRI allows observation of changes in cortical and medullary concentration of deoxyhemoglobin.2 This acquires images sensitive to local tissue oxygen concentration and quantifies changes in tissue oxygenation.2 This technique may bring some novel developments in daily practice. In today's treatment, medication dosage is mainly aimed at its antihypertensive effect. The possible better effect of higher dosages of RAS inhibitors on kidney oxygenation in its early stage is unknown. This can be quantified by applying this new imaging technique. Furthermore, there is no adequate noninvasive method available to detect acute or chronic rejection and kidney ischemia in kidney transplant patients. Clinicians often try to avoid invasive measurements in this vulnerable population as long as possible. With BOLD MRI, however, this problem might be observed earlier. Moreover, different experimental evidence suggests that RAS inhibitors improve kidney oxygenation by improving perfusion.3, 4, 5 However, these findings were restricted to animal models due to lack of decent noninvasive methods. Indeed, in an experimental study in healthy humans, it was shown that intravenous Ang II reduces the BOLD signal in the renal cortex, an effect that rapidly disappears at cessation of the infusion.6 These observations lead to the hypothesis that RAS inhibitors might improve kidney oxygenation in patients with CKD evaluated by this new technique. At the same time, we expect that such an intervention would have no effect in healthy humans. In this pilot study, we addressed this idea by measuring the effects on BOLD signal of an acute RAS inhibitor (before and after 50 mg of captopril) and before and during chronic aliskiren 300 mg/d and compared these results with those in healthy controls before and after acute captopril.
Methods and Materials
Patients
We selected 10 patients with CKD from our outpatient clinics and 5 healthy volunteers, who were members of the hospital staff. The patients were selected because they had stable CKD and were hypertensive (ie, using antihypertensive drugs and/or blood pressure >145/90 mm Hg when off medication). We excluded patients with a contraindication for MRI and those with polycystic kidney disease because these patients have difficult to interpret MRI images of the kidneys. The kidney diagnoses of the 10 patients with CKD were vascular disease (5), congenital (1), and unknown cause (4).
We consider this a pilot and/or feasibility study. Patients who stopped their medication temporarily did so as part of their participation in another ethics committee–approved study and written informed consent was obtained or medication was stopped because of a medical reason.
The healthy controls were all physicians who worked in the nephrology department and all volunteered to participate.
Protocol MRI
All patients were taken off their RAS inhibitors for at least 3 weeks. Other medications were not changed during the whole study period.
Every participant was scanned twice, ie, before and after the treatment with a RAS inhibitor. In 5 patients, an MRI scan was performed before and after they were put on chronic treatment with 300 mg aliskiren per day for approximately 6 weeks. In the other 5 patients with CKD, the MRI scans were performed before and 1 hour after 50 mg captopril orally. In the healthy volunteers, the MRI studies were performed before and 1 hour after 50 mg captopril orally. Blood pressure was measured with an automated device in all participants in a supine position before and after treatment with the RAS inhibitors before each BOLD MRI session.
BOLD MRI is based on the fact that paramagnetic molecules induce magnetic field perturbations. In the blood, oxyhemoglobin is diamagnetic and changes in its concentration have no effect on T2*‐weighted magnetic resonance images. However, deoxyhemoglobin is paramagnetic and an increase in deoxyhemoglobin concentration results in a decrease in tissue signal in T2*‐weighted images. By acquiring T2*‐weighted images at multiple echo times it is possible to measure the relaxation time T2* by fitting the slope of ln (signal intensity) vs echo time. T2* is inversely proportional to the concentration of deoxyhemoglobin. Here, we used a T2*‐weighted gradient echo sequence with 20 echoes. The first image was acquired at an echo time of 4.6 ms and the echo spacing was 4.6 ms. Further, scan parameters were: 6 slices of 6‐mm thickness (gap 6 mm) with an in‐plane resolution of 1.5×1.5 mm2. The 20 images for each slice were acquired in a single breath hold of 15 seconds. TR was 94 ms and the flip angle was 25°. The quantitative T2* image was automatically calculated on the scanner. T2* values >150 ms were clipped.
For data analysis, regions of interest (ROIs) were manually drawn in the cortex and medulla on the echo time image with best contrast between cortex and medulla. These images are mainly taken for the first 6 echo time images. This image gave the best anatomical details. The ROIs where copied to the quantitative T2* image and average T2* and standard deviation (SD) in T2* were extracted for the ROIs.
For this study, we assume that tissue oxygenation is inversely proportional to T2*. However, T2* is also sensitive to overall inhomogeneities in the magnetic field, which can differ between scan sessions due to differences in patient position and (linear) shim settings. Therefore, besides the changes in T2* values of cortex and medulla, we also used the ratio of T2* in the cortex over T2* in the medulla to evaluate the effect of the RAS inhibitors. Assuming that the background fields are more or less constant over the kidney, the ratios are insensitive to global changes in the field homogeneity.
Laboratory Analyses
Estimated glomerular filtration rate (eGFR) was performed using the plasma creatinine (at the day of MRI scan) by the Modification of Diet in Renal Disease (MDRD) equation.7 Body mass index (BMI), estimated from weight and height, was measured using the metric imperial formula.
Statistical Analysis
Data are given as mean±SD unless otherwise indicated. Baseline parameter analysis was performed with Student unpaired t test between patients and control patients. The changes in T2* ratio (ie, T2* cortex/T2* medulla) before and after treatment were tested by a paired t test. A P value <.05 was considered significant.
Results
The 10 patients (7 men) had a mean age of 61±17 years, BMI of 26±2 kg/m2, and eGFR of 30±11 mL/min per 1.73 m2. BMI and eGFR were stable during the last 3 months before entering the study. Office systolic and diastolic blood pressures at the time of screening, when taking an angiotensin‐converting enzyme (ACE) inhibitor and angiotensin II receptor blocker (ARB) were 130±10 mm Hg and 86±5 mm Hg. Other medications included phosphate binders (n=4), statins (n=9), diuretics (n=9), and vitamine D (n=3). These medications were not changed during the study.
Control patients (5 men) had a mean age of 40±13 years, BMI of 22±2 kg/m2, normal kidney function, and were not taking any medication.
Both captopril and aliskiren lowered blood pressure in patients with CKD, whereas captopril had no effect in controls (Table 1). Both in patients and in controls, T2* of the medulla was lower than T2* of the cortex (although not significant) (Table 1). In patients with CKD, T2* showed variable changes after captopril 50 mg. After chronic treatment with aliskiren, T2* of the cortex remained unchanged and T2* of the medulla increased (Table 1). However, none of the changes were significant.
Table 1.
Change in Blood Pressure and BOLD MRI Assessed by Quantitative T2* Values in Patients With CKD a
| Group | Patients With CKD Acute Captopril | Patients With CKD Chronic Aliskiren | Healthy Volunteers Acute Captopril | |||
|---|---|---|---|---|---|---|
| Untreated (n=5) | Treated (n=5) | Untreated (n=5) | Treated (n=5) | Untreated (n=5) | Treated (n=5) | |
| Systolic arterial blood pressure, mm Hg | 143±5 | 125±4 | 145±6 | 127±3 | 120±5 | 122±5 |
| Diastolic arterial blood pressure, mm Hg | 92±4 | 71±8 | 95±4 | 77±3 | 70±4 | 69±5 |
| T2* cortex, ms | 51±10 | 45±10 | 46±5 | 45±3 | 53±9 | 56±2 |
| T2* medulla, ms | 34±9 | 38±11 | 29±6 | 34±6 | 32±5 | 33±4 |
| T2* cortex/medulla | 1.52±0.2 | 1.21±0.1 | 1.60±0.32 | 1.35±0.3 | 1.62±0.1 | 1.65±0.1 |
Abbreviations: BOLD MRI, blood oxygenation level–dependent magnetic resonance imaging; CKD, chronic kidney disease. aBefore and after acute captopril 50 mg and before and after chronic treatment with aliskiren 300 mg per day and in healthy volunteers before and after captopril 50 mg. P<.05 is considered statistically significant. Comparison of results within groups showed no statistically significant differences (see Results section).
The T2* ratio between the cortex and medulla decreased in patients with CKD after treatment with both RAS inhibitors (Figure 1a and 1b) (P=.23 in the group on chronic treatment and P=.18 in the group on acute treatment). When combining the results of all 10 patients, the change in the ratio was significant (Figure 2a) (P<.05). In the healthy volunteers, there was no change in any variable (P=.80).
Figure 1.
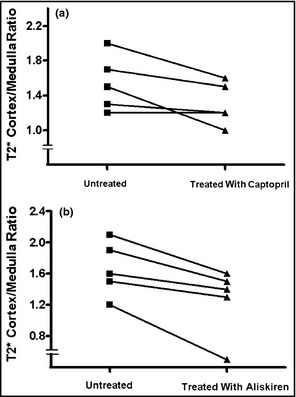
(a) Individual results of the ratios of T2* before and after captopril 50 mg in patients with chronic kidney disease (CKD). (b) Individual results of the ratios of T2* before and after aliskiren 300 mg/d in CKD patients.
Figure 2.
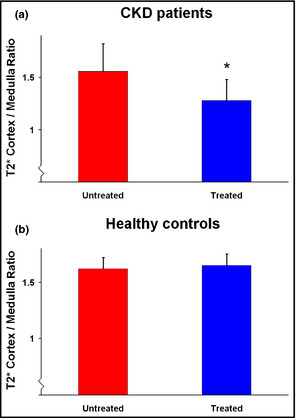
(a) T2* cortex/medulla ratio in patients before and after renin‐angiotensin system (RAS) inhibitors (n=10) (*P<.05). (b) T2* cortex/medulla ratio in healthy volunteers before and after RAS inhibitors (n=5).
Discussion
To the best of our knowledge, we are the first to show that in patients with CKD both acute and chronic treatment with a RAS inhibitor result in changes in BOLD signal. Importantly, in healthy volunteers, a RAS inhibitor had no such effect. The observations are compatible with the idea that RAS inhibitors improve kidney oxygenation in patients with CKD, especially of the medulla.
RAS inhibitors are the cornerstone of treatment of CKD. They are effective antihypertensive agents. Importantly, these agents seem particularly effective in reducing progression to kidney failure, suggesting increased Ang II activity as one of the important factors contributing to the progression of CKD. Although the beneficial effects of RAS inhibitors on glomerular hemodynamics have been widely accepted, other effects may be operational and relevant as well, including changes in kidney oxygenation. Several lines of experimental evidence suggest that kidney hypoxia is an important feature in CKD and that it may be of relevance in determining kidney failure progression.1 Indeed, Ang II has been implicated in the regulation of oxygen utilization in experimental CKD.8 As a consequence, the logical next question seems to be whether inhibition of Ang II improves oxygen availability in CKD. Indeed, several lines of experimental evidence show that a RAS inhibitor can improve kidney oxygenation. For instance, Ang II receptor blockade can improve renal oxygenation in rats by decreasing tubular re‐absorptive work.3 Moreover, Manotham and colleagues9 showed that ARB treatment prevented vascular changes and ameliorated tubular hypoxia. They suggested that the initial tubulo‐interstitial hypoxia in a remnant kidney model consequently results in development of tubulo‐interstitial damage.9 Izuhara and colleagues10 compared renoprotective effects of calcium channel antagonists, β‐blockers, and ARBs. Interestingly, only ARBs corrected chronic hypoxia. In addition, both an ACE inhibitor and an ARB improved directly measured partial pressure of oxygen in the interstitial microvascular compartment of the normal rat kidney.11 Our findings that RAS inhibitors especially affect the medulla are consistent with these experimental findings.
Schachinger and colleagues studied the idea that increased levels of Ang II in humans may affect the BOLD signal. Schachinger and colleagues6 showed that intravenously administered Ang II reduces the renal cortex BOLD signal. This BOLD response starts to occur almost instantaneously (within seconds after peripheral intravenous Ang II administration). Authors suggest that this response is a consequence of altered perfusion rather than altered kidney oxygen consumption. The present results fit well within this existing knowledge. The results show that in kidneys in conditions commonly associated with activated RAS (ie, CKD), acute or chronic inhibition of the Ang II is associated with a change in BOLD signal, indicating improved kidney oxygenation, especially during chronic use of RAS inhibitors. This effect rapidly occurs as it is already seen approximately 1 hour after oral administration of captopril. An important feature of this study is that the RAS inhibitor had no effect in healthy volunteers. This study confirms the existing experimental evidence of the contribution of Ang II to kidney oxygenation. However, the study by Stein and colleagues12 measured an alteration in renal blood oxygenation in healthy volunteers after oral administration of RAS inhibitors.
The considerable limitation of this study is the small study population and absence of a placebo group. These controversial results have to be studied in larger study populations.
Kidney oxygenation is difficult to quantify in humans. Measuring the BOLD signal by MRI offers the possibility to learn more about kidney oxygenation. Indeed, it was shown that patients with renal vascular disease have various degrees of hypoxic areas evidenced by BOLD MRI.13 Moreover, Sadowski and colleagues14 demonstrated that BOLD MRI can noninvasively assess changes in oxygen bioavailability in the cortex and medulla of transplanted kidneys. They showed an increase in medullary T2* (reduced deoxyhemoglobin concentration) on BOLD MRI in allografts with acute rejection. Furthermore, using BOLD MRI, it has been shown that renal tissue oxygenation is influenced by carbogen or oxygen breathing. The changes are assessed by detecting BOLD MRI signal (T2*) at high field strengths in patients with CKD.15
Given the present findings, it is attractive to speculate on the possible indications of BOLD MRI. Firstly, it is necessary to study whether this effect is indeed specific for RAS inhibitors, for instance, by studying the effect of other antihypertensive agents. Given the earlier studies on the effects of Ang II and the present findings, our hypothesis would be that the effects are indeed RAS‐inhibitor specific.6 The effect of furosemide has been tested.2 This agent improves kidney oxygenation presumably by its effect on energy‐dependent natrium reabsorption, ie, not by improving oxygen delivery but by reducing oxygen consumption.2 This illustrates a limitation of the technique that it cannot differentiate between these two mechanisms. A second important question is the effect of various dosages. As mentioned earlier, in today's treatment, medication dosage is mainly aimed at its antihypertensive effect. It might be interesting to study the effect of higher dosages of RAS inhibitors on kidney oxygenation. It is possible that the dosages beyond the therapeutic range with respect to blood pressure might have a greater effect on kidney oxygenation. It is tempting to speculate that this will result in a better clinical outcome. Thus, this technique is an interesting new tool, which requires further investigation.
A significant feature of our study is that 9 of the 10 patients were also taking diuretics. Diuretics increase the activity of the RAS. It is important to realize that patients were on both occasions clinically normovolemic and diuretic dosages were not changed. Body weight remained unchanged, making it unlikely that fluid status showed any relevant changes during the course of the study in patients taking chronic RAS inhibitors. Therefore, it seems safe to exclude the major effects of diuretics on our results. In addition, in patients with CKD, RAS inhibitors, often combined with diuretics, are the preferred agents to obtain the antihypertensive goals according to the guidelines committees.16 The present study, in which medication dosage is based on office arterial blood pressure readings and eGFR, most likely reflects every day practice in most clinics.
Conclusions
The present pilot study demonstrates that a detectable change in BOLD MRI signal after acute and chronic RAS inhibition in patients with CKD can be found, while no change in signal is detected in healthy volunteers. The change in T2* in patients is consistent with the idea that the RAS inhibitor especially affects the medulla. Importantly, the present data seem to indicate that the BOLD MRI technique offers a method to study a physiological variable thus far impossible to study in humans.
Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
J Clin Hypertens (Greenwich). 2014;16:214–218. ©2014 Wiley Periodicals, Inc.24708383
References
- 1. Siddiqi L, Joles JA, Grassi G, Blankestijn PJ. Is kidney ischemia the central mechanism in parallel activation of the renin and sympathetic system? J Hypertens. 2009;27:1341–1349. [DOI] [PubMed] [Google Scholar]
- 2. Gomez SI, Warner L, Haas JA, et al. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol. 2009;297:F981–F986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cervenka L, Wang CT, Navar LG. Effects of acute AT1 receptor blockade by candesartan on arterial pressure and renal function in rats. Am J Physiol. 1998;274:F940–F945. [DOI] [PubMed] [Google Scholar]
- 4. Gardiner SM, Kemp PA, March JE, Bennett T. Temporal differences between the involvement of angiotensin II and endothelin in the cardiovascular responses to endotoxaemia in conscious rats. Br J Pharmacol. 1996;119:1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardiner SM, March JE, Kemp PA, Bennett T. Influence of CGRP (8‐37), but not adrenomedullin (22‐52), on the haemodynamic responses to lipopolysaccharide in conscious rats. Br J Pharmacol. 1999;127:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schachinger H, Klarhofer M, Linder L, et al. Angiotensin II decreases the renalMRI blood oxygenation level‐dependent signal. Hypertension. 2006;47:1062–1066. [DOI] [PubMed] [Google Scholar]
- 7. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 8. Deng A, Tang T, Singh P, et al. Regulation of oxygen utilization by angiotensin II in chronic kidney disease. Kidney Int. 2009;75:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manotham K, Tanaka T, Matsumoto M, et al. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol. 2004;15:1277–1288. [DOI] [PubMed] [Google Scholar]
- 10. Izuhara Y, Nangaku M, Inagi R, et al. Renoprotective properties of angiotensin receptor blockers beyond blood pressure lowering. J Am Soc Nephrol. 2005;16:3631–3641. [DOI] [PubMed] [Google Scholar]
- 11. Norman JT, Stidwill R, Singer M, Fine LG. Angiotensin II blockade augments renal cortical microvascular pO2 indicating a novel, potentially renoprotective action. Nephron Physiol. 2003;94:p39–p46. [DOI] [PubMed] [Google Scholar]
- 12. Stein A, Goldmeier S, Voltolini S, et al. Renal oxygen content is increased in healthy subjects after angiotensin‐converting enzyme inhibition. Clinics (Sao Paulo). 2012;67:761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Textor SC, Glockner JF, Lerman LO, et al. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008;19:780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sadowski EA, Djamali A, Wentland AL, et al. Blood oxygen level‐dependent and perfusion magnetic resonance imaging: detecting differences in oxygen bioavailability and blood flow in transplanted kidneys. Magn Reson Imaging. 2010;28:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boss A, Martirosian P, Jehs MC, et al. Influence of oxygen and carbogen breathing on renal oxygenation measured by T2*‐weighted imaging at 3.0 T. NMR Biomed. 2009;22:638–645. [DOI] [PubMed] [Google Scholar]
- 16. ESH/ESC Task Force for the Management of Arterial Hypertension . 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–1938. [DOI] [PubMed] [Google Scholar]


