Abstract
Although recent guidelines recommend the combination of calcium channel blockers (CCBs) and thiazide (‐like) diuretics, this combination is not widely used in clinical practice. The aim of this meta‐analysis was to assess the efficacy and safety of this combination regarding the following endpoints: all‐cause and cardiovascular mortality, myocardial infarction, and stroke. Four studies with a total of 30,791 of patients met the inclusion criteria. The combination CCB/thiazide (‐like) diuretic was associated with a significant risk reduction for myocardial infarction (risk ratio [RR], 0.83; 95% confidence interval [CI], 0.73–0.95) and stroke (RR, 0.77; CI, 0.64–0.92) compared with other combinations, whereas it was similarly effective compared with other combinations in reducing the risk of all‐cause (RR, 0.89; CI, 0.75–1.06) and cardiovascular (RR, 0.89; CI 0.71–1.10) mortality. Elderly patients with isolated systolic hypertension may particularly benefit from such a combination, since both drug classes have been shown to confer cerebrovascular protection.
Recent guidelines on hypertension treatment emphasize the advantage of combination therapy since the use of two (or more) agents increases the number of patients who achieve target blood pressure (BP) values.1, 2 The synergic effects of different drug classes have been documented to result in greater BP reduction and fewer side effects than monotherapy.3, 4 In clinical trials and in clinical practice the most frequently used drug combinations are renin‐angiotensin‐aldosterone system (RAAS) blockers with either a diuretic or a calcium channel blocker (CCB). Accordingly, RAAS blockers with either diuretics or CCBs are considered preferred combinations in the majority of guidelines on hypertension treatment and are the most frequently used combination in surveys on hypertensive populations.1, 2, 5 Others, such as the combination of CCB and thiazide (‐like) diuretics, have remained in reserve, even if the clinical evidence has shown that this combination is at least as efficient as the above‐mentioned treatment,6, 7, 8, 9, 10 and the new American and European guidelines on hypertension consider the CCB/thiazide (‐like) diuretic combination as one of the preferred drug combinations.1, 2 However, no fixed combinations of a CCB and a thiazide diuretic are available and there seems to have been some reluctance to use this combination. The aim of this meta‐analysis was to investigate the existing published evidence of efficacy and safety of the combination of a CCB with a thiazide (‐like) diuretic.
Methods
Search Strategy
The objective of the current analysis was to evaluate the available studies in which CCBs and thiazide (‐like) diuretics as a combination therapy were compared with any other monotherapy, combination therapy, or placebo for the management of hypertension and reported prespecified endpoints (ie, all‐cause mortality, cardiovascular mortality, myocardial infarction [MI], and stroke).
We limited our search to studies in humans in peer‐reviewed journals with no timeline restriction. No language restriction was applied. The reference lists of bibliographies of identified articles were also reviewed.
We searched PubMed and Embase using the terms calcium channel blocker AND thiazide diuretic OR amlodipine OR aranidipine OR azelnidipine OR barnidipine OR benidipine OR cilnidipine OR clevidipine OR isradipine OR efonidipine OR felodipine OR lacidipine OR lercanidipine OR manidipine OR nicardipine OR nifedipine OR nilvadipine OR nimodipine OR nisoldipine OR nitrendipine OR pranidipine AND chlorothiazide OR chlorthalidone OR hydrochlorothiazide OR hydroflumethiazide OR indapamide OR methyclothizide OR metholazone OR polythiazide.
Selection Criteria and Data Extraction
To be included in the analysis a trial had to fulfill the following criteria: (1) was either prospective or retrospective; (2) documented BP measurements, both baseline and post‐intervention; and (3) reported the following prespecified endpoints: all‐cause and cardiovascular mortality, MI, and stroke.
Data were extracted using standardized protocol and reporting forms. Disagreements were resolved by discussion or by consultation with an additional reviewer. We extracted characteristics of each trial, baseline patient demographics and BP measurements for our analysis.
We identified 345 abstracts, of which 45 articles were retrieved and reviewed for possible inclusion (Figure 1). Of these 45 articles, 41 were excluded: 7 because these were duplicate references, 7 because relevant endpoints were not reported, and 27 for other reasons (eg, no reports on BP values, no human studies). Four studies (the European Lacidipine Study on Atherosclerosis [ELSA], the Valsartan Antihypertensive Long‐Term Use Evaluation [VALUE], the Felodipine Event Reduction Study [FEVER], and the Prevention of Cardiovascular Events With Calcium Channel Blocker‐Based Combination Therapies in Patients With Hypertension: A Randomized Controlled Trial From the Combination Therapy of Hypertension to Prevent Cardiovascular Events Trial Group [COPE]) with a total of 30,791 patients reported the prespecified endpoints and were included in the analysis.6, 8, 10, 11
Figure 1.
Flow chart of the study. RCTs includes randomized controlled trials. *Other reasons include no reports on blood pressure values and no human studies.
Statistical Analysis
We performed random‐effects meta‐analyses comparing ischemic outcomes between patients treated with the combination CCB/thiazide diuretic and those treated with other antihypertensive medications at maximum available follow‐up.
We calculated risk ratios (RRs) as measures of treatment effect and used a DerSimonian and Laird random‐effects model to combine estimates across trials. We calculated the I‐squared statistic to measure the heterogeneity between trials with values of 25%, 50%, and 75% showing low, moderate, and high heterogeneity, respectively. Meta‐analyses were performed using the Stata software, version 12.1 for Mac (StataCorp LP, College Station, TX).
Results
Characteristics of the Included Studies
Quality of Included Trials
All four trials were considered to be of high methodological quality. Appropriate methods of allocation concealment were described for three of the four trials. All trials reported blind adjudication of clinical outcomes. Despite one trial that did not perform the primary endpoint analysis according to the intention‐to‐treat principle, we were able to include all randomized patients into the meta‐analysis according to the intention‐to‐treat principle (Table).
Table 1.
Characteristics of the Studies Included in the Analysis
| Variable | ELSA | VALUE | FEVER | COPE |
|---|---|---|---|---|
| Randomized patients, No. | 2334 | 15245 | 9711 | 3501 |
| Mean age, y | 56.1 vs 55.9 | 67.3 vs 67.2 | 61.5 vs 61.5 | 63.1 vs 63.0 vs 63.2 |
| Active treatment | Lacidipine/HCTZ a | Amlodipine/HCTZ | Felodipine/HCTZ | Benidipine/thiazide(‐like) diuretics |
| Comparator | Atenolol/HCTZ a | Valsartan/HCTZ | HCTZ/placebo | Benidipine/ARB and benidipine/β‐blocker |
| BP at entry, mm Hg | 164/101 vs 163/101 | 155/88 vs 154/87 | 154/91 vs 154/91 | 154/89 vs 154/89 and 154/89 |
| Decrease of SBP, mm Hg | 21.6 vs 21.8 | 17.3 vs 15.2 b | 16.9 vs 11.9 b | 20.0 vs 19.3 and 20.1 |
| Decrease of DBP, mm Hg | 15.5 vs 15.6 | 9.9 vs 8.2 b | 8.5 vs 6.3 b | 12.4 vs 11.8 and 12.0 |
| Outcomes | No significant differences | No significant differences (except MI more frequent in the valsartan group) | Felodipine+HCTZ was associated with very substantial reduction of CV events and death |
All treatments were equally effective for CV event prevention. Benidipine+HCTZ was more effective in the prevention of stroke |
| Adverse events, No. | 186 vs 201 c |
Angina pectoris: d 234 vs 335 b Atrial fibrillation: d 151 vs 182 Diarrhea: 515 vs 670 Edema: 462 vs 243 b Hypokalemia: 469 vs 266 b |
Dizziness: 174 vs 203 Flush: 66 vs 9 b Headache: 68 vs 61 Palpitation: 56 vs 49 Fatigue: 31 vs 51 b Ankle edema: 49 vs 18 b |
Total: 522 vs 495 vs 502 e Hyperuricemia: 79 vs 23 vs 22 b Hypokalemia: 29 vs 8 vs 3 b Creatinine increased 19 vs 9 vs 6 b Hyperkalemia: 3 vs 13 vs 7 b Bradycardia: 1 vs 3 vs 48 b |
Abbreviations: ARB, angiotensin receptor blocker; BP, blood pressure; CV, cardiovascular; DBP, diastolic blood pressure; HCTZ, hydrochlorothiazide; MI, myocardial infarction; SBP, systolic blood pressure. a34.4% in the lacidipine group and 35.5% in the atenolol group received HCTZ. b P<.05. cThe following serious adverse events were considered: fatal, life‐threatening, involving prolonged hospitalization, disabling or incapacitating, any laboratory abnormality causing major clinical concern, or relevant signs or symptoms. dHave been reported as serious adverse events. eIn this study, there was no significant difference between the different treatment groups regarding serious adverse events. Only adverse events with a significant difference between the groups are indicated. See text for trial expansions.
The ELSA10 was a randomized, double‐blind trial that included 2334 patients with hypertension and evaluated the impact of CCBs in the setting of arterial hypertension associated with atherosclerosis. It compared the effects of a 4‐year treatment based on either lacidipine or atenolol with open‐labeled hydrochlorothiazide (HCTZ) on BP and carotid intima‐media thickness. Patients underwent a washout period of 4 weeks and were given 4 mg to 6 mg of lacidipine and 50 mg to 100 mg of atenolol, with open‐label HCTZ added (12.5 mg daily starting from month 3 and 25 mg daily starting from month 6). At study end, 34.4% in the lacidipine and 35.5% in the atenolol group received HCTZ. Clinic BP reductions were identical with both treatments, although 24‐hour ambulatory BP changes with lacipidine were slightly lesser when compared with atenolol −7/−5 mm Hg vs −10/−9 mm Hg, respectively. No significant difference was found in terms of cardiovascular events, although the relative risk for stroke, major cardiovascular events, and mortality showed a trend favoring lacidipine. The number of serious adverse events (ie, fatal, life‐threatening, hospitalization, relevant laboratory abnormality, relevant sign or symptoms) was comparable between the two treatment groups (atenolol n=201, vs lacidipine n=186; see also Table).
The VALUE trial6, 12 was an international multicenter, randomized, double‐blind, parallel‐group comparison of therapy based on valsartan or amlodipine. HCTZ was administered to both groups. It proposed that for the same BP control, valsartan would reduce cardiac morbidity and mortality more than amlodipine in hypertensive patients at high cardiovascular risk. It enrolled 15,245 patients, aged 50 years or older, with treated or untreated hypertension and a high risk for cardiac events. Duration of treatment was event‐driven and the trial lasted until at least 1450 patients had reached a primary endpoint, defined as a composite of cardiac mortality and morbidity with a mean follow‐up of 4.2 years. The amlodipine arm included 7596 participants in the intention‐to‐treat group, 1810 of which received combination therapy with amlodipine and HCTZ (amlodipine 5 mg plus HCTZ [n=329; 4.3%] and amlodipine 10 mg plus HCTZ [n=1481; 19.5%]).
BP reduction was 15.2/8.2 mm Hg and 17.3/9.9 mm Hg in the valsartan and amlodipine arms, respectively (P<.0001 between groups). The amlodipine group had a significantly lower incidence of MI and higher rate of new‐onset diabetes than in the valsartan group. The most consistent and statistically significant difference between the groups was in BP control: amlodipine‐based therapy was significantly more effective in reducing BP, especially during the early phases of treatment. In VALUE, MI incidence was lower in the amlodipine group than in the valsartan group (4.1% vs 4.8%; P=.02).
In this study, the following serious adverse events were more frequent in the valsartan‐treated patients: angina pectoris (valsartan vs amlodipine: 335 [4.4%] vs 234 [3.1%], P<.0001), syncope (129 [1.7%] vs 75 [1.0%], P<.0001), and atrial fibrillation (182 [2.4%] vs 151 [2.0%], P=.12]. Interestingly, however, specific analysis revealed that valsartan‐based treatment reduced the development of new‐onset AF, particularly sustained, compared with amlodipine‐based therapy.13 Moreover, amlodipine‐treated patients reported edema (amlodipine vs valsartan: 462 [6.1%] vs 243 [3.2%]; P<.0001) and hypokalemia (469 [6.2%] vs 266 [3.5%], P<.0001) more frequently. Finally, diarrhea was more frequent in the valsartan‐treated group (670 [8.8%] vs 515 [6.8%], P<.0001; Table).
The FEVER study11 was a prospective, multicenter, double‐blind, randomized, placebo‐controlled, parallel group trial. It enrolled 9711 Chinese patients. From these, at least 4841 patients were included in the intention‐to‐treat group. In the felodipine group, BP decreased (from randomization to study end) from 154.2/91.0 mm Hg to 137.3/82.5 mm Hg, and in the placebo group from 154.4/91.3 mm Hg to 142.5/85.0 mm Hg, with an average difference throughout the trial of 4.2/2.1 mm Hg.
In the felodipine group, the primary endpoint (fatal and nonfatal stroke) was reduced by 27% (P<.001). Among secondary endpoints in the felodipine group, all cardiovascular events were reduced by 27% (P<.001), all cardiac events by 35% (P<.012), death by any cause by 31% (P<.006), coronary events by 32% (P<.024), heart failure by 30% (P<.239), and cardiovascular death by 33% (P<.019). In this study, both treatments were well tolerated. Flushing and edema occurred significantly more frequently in the felodipine group (felodipine vs placebo: 66 [1.4%] vs 9 [0.2%], P<.001 and 49 [1.0%] vs 18 [0.4%], P<.001, respectively) whereas fatigue was significantly more frequent in the placebo group (51 [1.0%] vs 31 [0.6%], P=.037).
The COPE trial8 was a prospective, randomized, open‐label, blinded‐endpoint study. It enrolled individuals from the outpatient setting who did not achieve target BP (<140/90 mm Hg). All patients received benidipine 4 mg/d and were randomly assigned to receive in addition to benidipine an angiotensin receptor blocker (ARB), a β‐blocker, or a thiazide(‐like) diuretic. It included a total of 3501 participants, 1094 of whom were included in the benidipine/diuretic combination therapy arm. At least 66% of the patients in the CCB‐diuretic treatment group achieved target BP and 2.9% had cardiovascular events, which represented a lower percentage when compared with the other groups. In addition, the hazard ratios for cardiovascular events were higher for the two other combination treatment groups when compared with the CCB/diuretic group. The benidipine and diuretic combination significantly reduced the incidence of fatal and nonfatal strokes. The total duration of the trial was 7 years with a 3‐year follow‐up after study termination. The dose was escalated according to periodic BP measurements. The participants in the CCB‐diuretic combination treatment group received trichlormethiazide and indapamide (72.8% and 16.3%, respectively, and others 10.9%). In terms of BP control, the percentage of patients who achieved target BP did not differ among the three groups. The initial BP of the CCB‐diuretic participants was 154.1/88.7 (12/9.8) mm Hg and the end BP was 134.0/76.6 (14.4/10.6) mm Hg.
The primary cardiovascular composite endpoint occurred in 41 (3.7%), 48 (4.4%), and 32 (2.9%) patients in the benidipine‐ARB, benidipine–β‐blocker, and benidipine‐diuretic groups, respectively. The incidence of hard cardiovascular composite endpoints was significantly higher in the benidipine–β‐blocker group than in the benidipine‐diuretic group, although all‐cause mortality was no different among the three groups. The percentage of adverse events was comparable between the different treatment groups: ARB 505 (45.5%), β‐blocker 495 (45.5%), and diuretic 522 (47.7%; Table).
Results of the Meta‐Analysis
In the meta‐analysis including these four studies with a total of 30,791 patients, the combination of a CCB with a thiazide (‐like) diuretic exhibited similar risks of all‐cause mortality (RR, 0.89; 95% CI, 0.75–1.06) and cardiovascular mortality (RR, 0.89; CI, 0.71–1.10) compared with other hypertensive medications (Figure 2, panel A and B). Moreover, the combination CCB/thiazide (‐like) diuretic was associated with a significant risk reduction for MI (RR, 0.83; CI, 0.73–0.95) and stroke (RR, 0.77; CI, 0.64–0.92) compared with other hypertensive medications (Figure 2, panel C and D).
Figure 2.
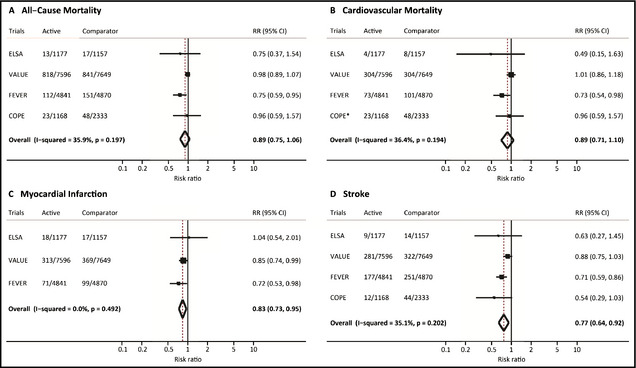
Ischemic outcomes with combinations of calcium antagonists and thiazide (‐like) diuretics as compared with other antihypertensive medical strategies. Random‐effects meta‐analyses for all‐cause mortality (A), cardiovascular mortality (B), myocardial infarction (C), and stroke (D) at maximum available follow‐up. Boxes represent the risk ratio (RR) and lines the 95% confidence interval (CI) for individual studies, and diamonds represent pooled RRs with respective 95% Cis. *All‐cause mortality was used as a proxy for cardiovascular mortality for the COPE trial since cardiovascular mortality was not reported. See text for trial expansions.
Discussion
CCBs and thiazide(‐like) diuretics are widely used as monotherapy for the treatment of hypertension because they are effective in reducing cardiovascular morbidity and mortality and are well tolerated, causing relatively few side effects.3, 4 Surprisingly, the combination of the two has been rarely studied. The present meta‐analysis comprising data on 30,791 patients documents efficacy and safety of the CCB/thiazide (‐like) diuretic combination in reducing cardiovascular morbidity and mortality. Moreover, the present data suggest that CCB/thiazide (‐like) diuretic combinations might be more effective in reducing MI and stroke than other combinations.
The combination of CCB/thiazide (‐like) diuretics makes sense from a (patho)physiological point of view. In particular, patients with isolated systolic hypertension,14 African Americans,15 and East Asians16 are salt‐sensitive and display lower RAAS activity, both factors that favor the use of CCBs and diuretics. Furthermore, CCBs and diuretics have favorable effects on target organ damage and on cardiovascular hemodynamics, two important predictors of future cardiovascular risk that are particularly relevant in hypertensive patients.10, 17, 18, 19
In the United States and Europe, the number of persons 65 years and older is rapidly increasing and in 20 years approximately 1 of 5 persons will be in this age category.14
Aging induces progressive stiffening of the large arteries and increases wave reflections with a consequent rise in systolic BP and a decrease in diastolic BP.20 As a consequence, the proportion of patients with isolated systolic hypertension increases with age21 from about 47% in the decade 50 to 59 years to >75% a decade later. An important observation in this context is that pulse pressure (ie, systolic BP – diastolic BP) represents a valid and widely available parameter for estimating the degree of age‐related vascular stiffening.22 Arterial stiffness is an independent predictor of cardiovascular morbidity and mortality22, 23 and has been identified as a key factor associated with isolated hypertension,24 secondary hypertension,25 and therapy‐resistant hypertension.26 Accordingly, in many clinical trials, sustained systolic BP elevation is the main component responsible for poor BP control.14, 27 In general, a decrease of central BP can be obtained by de‐stiffening of the vasculature or by modification of the reflected wave in order to “resynchronize” the forward with the backward wave. While changes of the arterial wall elastic components to decrease arterial stiffness are difficult to obtain, particularly in older patients, and take place only after long‐term drug therapy, modification of wave reflection is a rapid and an efficient mechanism.28 Antihypertensive agents with vasodilation proprieties, and/or with the capacity to reduce pressure wave reflections, are particularly effective in reducing the pressure augmentation in the aorta. In line with this concept, in patients with isolated hypertension, several studies assessed the effect on (central) pulse pressure of different antihypertensive agents.28, 29, 30 Most of them identified CCBs and thiazide (‐like) diuretics as effective agents in decreasing central systolic BP and pulse pressure.30
While the present meta‐analysis showed only a trend for the endpoints all‐cause and cardiovascular mortality in favor of the combination CCB/diuretic, this combination was significantly better than the comparators in reducing MI and stroke. Increased stiffness and consequent increased central BP are associated with an increased risk for MI31 and stroke.32 In line with this concept, a recent study comparing a CCB‐based regimen with a β‐blocker–based regimen, showed that despite similar reduction in systolic BP and PP at the brachial level, the CCB‐based regimen significantly lowered the central systolic BP and PP. This was accompanied by a lower incidence of cardiovascular events (ie, stroke and coronary events).33
We speculate that in our meta‐analysis a similar mechanism is responsible for the lower risk for MI and stroke with the CCB/diuretic combination.
In line with these observations, the new European guidelines on hypertension identify thiazide (‐like) diuretics and CCBs as preferred antihypertensive agents in the elderly (and African Americans) and consider the combination of a CCB with diuretics a good choice.2
The CCB/diuretic combination is in general well tolerated. The most frequently reported adverse events with the combination CCB/diuretic were hypokalemia and ankle edema. The major concern about hypokalemia is the increased risk for cardiac arrhythmias.34 On the other side, controlled studies have not shown an increased incidence of ventricular tachycardia during therapy with high‐dose (ie, 100 mg/d) HCTZ in hypertensive patients.35 In line with this observation, in the studies included in our meta‐analysis, cardiac arrhythmias, palpitations, dizziness, and syncope were equal or even less frequent in patients treated with the a CCB/diuretic combination than in patients treated with other combinations.
Ankle edema is a quite frequent adverse event in patients treated with CCBs.36 The combination of a CCB with a diuretic may reduce the incidence of edema. In agreement with this concept, a very recent study that included more than 190 patients treated with a single‐pill combination of CCB/thiazide‐like diuretic (ie, indapamide),37 the authors reported a very low incidence of pedal edema.
Limitations
In this meta‐analysis we did not include unpublished data and limited our information sources to two databases (PubMed and Embase) without including the Cochrane Library. However, the current practice for meta‐analysis search strategies consider two databases as appropriate.38 While we could identify only four studies reporting hard endpoints (ie, MI, stroke, and mortality) for this meta‐analysis, these studies included more than 30,000 participants and were of good quality, allowing to conclude that the combination of CCB/thiazide (‐like) diuretics is a valid treatment option. In the VALUE trial, the CCB/diuretic combination had a significantly faster and greater BP‐lowering effect than the comparator. This might have resulted in an imbalance of events in favor of the CCB/diuretic combination. However, in a post‐hoc analysis, investigating the BP‐dependent and BP‐independent effects of antihypertensive treatment on clinical events in the VALUE trial showed that in patients matched for systolic BP, sex, age, presence of diabetes, and previous CV events (number of patients: 5006; mean systolic BP, 139.9 mm Hg) the following endpoints were almost identical in the two cohorts: combined cardiac events, MI, stroke, and mortality.39
Finally, because we had no access to the raw data of the four included studies in the meta‐analysis, we were unable to perform an analysis of the effect of age on the different endpoints.
Conclusions
Our meta‐analysis demonstrates that in patients with arterial hypertension the CCB/thiazide (‐like) diuretic combination reduces cardiovascular morbidity and mortality and that it might be more effective than other combinations in reducing MI and stroke. Elderly patients with isolated systolic hypertension may particularly benefit from this combination, since both drug classes have been shown to confer better cerebrovascular protection than other antihypertensive drug classes.10, 36, 40, 41
Conflict of Interest
None.
Source of Support
None.
J Clin Hypertens (Greenwich). 2015;17:193–199. DOI: 10.1111/jch.12462. © 2015 Wiley Periodicals, Inc.
References
- 1. James PA, Oparil S, Carter BL, et al. 2014 Evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 3. Mahmud A, Feely J. Low‐dose quadruple antihypertensive combination: more efficacious than individual agents–a preliminary report. Hypertension. 2007;49:272–275. [DOI] [PubMed] [Google Scholar]
- 4. Makani H, Bangalore S, Romero J, et al. Effect of renin‐angiotensin system blockade on calcium channel blocker‐associated peripheral edema. Am J Med. 2011;124:128–135. [DOI] [PubMed] [Google Scholar]
- 5. Krause T, Lovibond K, Caulfield M, et al. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. [DOI] [PubMed] [Google Scholar]
- 6. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 7. Lu F, Zhao Y, Liu Z, et al. A 48‐week study of amlodipine plus amiloride/hydrochlorothiazide vs amlodipine plus telmisartan in the treatment of hypertension. Int J Clin Pract. 2012;66:792–799. [DOI] [PubMed] [Google Scholar]
- 8. Matsuzaki M, Ogihara T, Umemoto S, et al. Prevention of cardiovascular events with calcium channel blocker‐based combination therapies in patients with hypertension: a randomized controlled trial. J Hypertens. 2011;29:1649–1659. [DOI] [PubMed] [Google Scholar]
- 9. Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive‐drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta‐analysis. Lancet. 2010;375:906–915. [DOI] [PubMed] [Google Scholar]
- 10. Zanchetti A, Bond MG, Hennig M, et al. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double‐blind, long‐term trial. Circulation. 2002;106:2422–2427. [DOI] [PubMed] [Google Scholar]
- 11. Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) Study: a randomized long‐term placebo‐controlled trial in Chinese hypertensive patients. J Hypertens. 2005;23:2157–2172. [DOI] [PubMed] [Google Scholar]
- 12. Julius S, Weber MA, Kjeldsen SE, et al. The Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) trial: outcomes in patients receiving monotherapy. Hypertension. 2006;48:385–391. [DOI] [PubMed] [Google Scholar]
- 13. Schmieder RE, Kjeldsen SE, Julius S, et al. Reduced incidence of new‐onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trial. J Hypertens. 2008;26:403–411. [DOI] [PubMed] [Google Scholar]
- 14. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011;123:2434–2506. [DOI] [PubMed] [Google Scholar]
- 15. Brewster LM, van Montfrans GA, Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med. 2004;141:614–627. [DOI] [PubMed] [Google Scholar]
- 16. Katsuya T, Ishikawa K, Sugimoto K, et al. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521–525. [DOI] [PubMed] [Google Scholar]
- 17. Ghiadoni L, Bruno RM, Stea F, et al. Central blood pressure, arterial stiffness, and wave reflection: new targets of treatment in essential hypertension. Curr Hypertens Rep. 2009;11:190–196. [DOI] [PubMed] [Google Scholar]
- 18. Taddei S, Virdis A, Ghiadoni L, et al. Lacidipine restores endothelium‐dependent vasodilation in essential hypertensive patients. Hypertension. 1997;30:1606–1612. [DOI] [PubMed] [Google Scholar]
- 19. Versari D, Virdis A, Ghiadoni L, et al. Effect of verapamil, trandolapril and their combination on vascular function and structure in essential hypertensive patients. Atherosclerosis. 2009;205:214–220. [DOI] [PubMed] [Google Scholar]
- 20. O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. [DOI] [PubMed] [Google Scholar]
- 21. Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. [DOI] [PubMed] [Google Scholar]
- 22. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 23. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 24. O'Rourke MF. Isolated systolic hypertension, pulse pressure, and arterial stiffness as risk factors for cardiovascular disease. Curr Hypertens Rep. 1999;1:204–211. [DOI] [PubMed] [Google Scholar]
- 25. Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245–1254. [DOI] [PubMed] [Google Scholar]
- 26. von Arx R, Rexhaj E, Allemann Y, et al. Lack of blood pressure‐lowering effect of renal denervation in a drug‐naive patient with pronounced arterial stiffening. Am J Med. 2014;127:e3–e4. [DOI] [PubMed] [Google Scholar]
- 27. Tocci G, Rosei EA, Ambrosioni E, et al. Blood pressure control in Italy: analysis of clinical data from 2005‐2011 surveys on hypertension. J Hypertens. 2012;30:1065–1074. [DOI] [PubMed] [Google Scholar]
- 28. Agnoletti D, Zhang Y, Borghi C, et al. Effects of antihypertensive drugs on central blood pressure in humans: a preliminary observation. Am J Hypertens. 2013;26:1045–1052. [DOI] [PubMed] [Google Scholar]
- 29. Mackenzie IS, McEniery CM, Dhakam Z, et al. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54:409–413. [DOI] [PubMed] [Google Scholar]
- 30. Morgan T, Lauri J, Bertram D, et al. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17:118–123. [DOI] [PubMed] [Google Scholar]
- 31. Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. [DOI] [PubMed] [Google Scholar]
- 32. Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. [DOI] [PubMed] [Google Scholar]
- 33. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 34. Sica DA, Struthers AD, Cushman WC, et al. Importance of potassium in cardiovascular disease. J Clin Hypertens (Greenwich). 2002;4:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Papademetriou V, Burris JF, Notargiacomo A, et al. Thiazide therapy is not a cause of arrhythmia in patients with systemic hypertension. Arch Intern Med. 1988;148:1272–1276. [PubMed] [Google Scholar]
- 36. Messerli FH, Grossman E, Lever AF. Do thiazide diuretics confer specific protection against strokes? Arch Intern Med. 2003;163:2557–2560. [DOI] [PubMed] [Google Scholar]
- 37. Jadhav U, Hiremath J, Namjoshi DJ, et al. Blood pressure control with a single‐pill combination of indapamide sustained‐release and amlodipine in patients with hypertension: the EFFICIENT study. PLoS ONE. 2014;9:e92955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Egger M, Smith GD, Altman DG. Systemic Reviews in Health Care; Meta‐Analysis in Context. London: BMJ Publishing Group; 2001. [Google Scholar]
- 39. Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. [DOI] [PubMed] [Google Scholar]
- 40. Messerli FH, Staessen JA. Amlodipine better than lisinopril? How one randomized clinical trial ended fallacies from observational studies Hypertension. 2006;48:359–361. [DOI] [PubMed] [Google Scholar]
- 41. Wang JG, Li Y, Franklin SS, et al. Prevention of stroke and myocardial infarction by amlodipine and angiotensin receptor blockers: a quantitative overview. Hypertension. 2007;50:181–188. [DOI] [PubMed] [Google Scholar]


