Abstract
The association between exaggerated blood pressure (BP) response to exercise (ExBPR) and “masked hypertension” is unclear. Medical records of patients with high‐normal BP who were evaluated in the Chaim Sheba Screening Institute Ramat Gan, Israel, during the years 2002–2007 and referred for 24‐hour ambulatory BP monitoring (ABPM) and exercise test were reviewed. Data on exercise tests performed in the preceding 5 years were retrieved. Reproducible ExBPR was defined when it was recorded at least twice. BP levels on 24‐hour ABPM were compared between patients with a normal BP response and those with an ExBPR (systolic BP ≥200 mm Hg). Sixty‐nine normotensive patients with high normal BP levels were identified. ExBPR was recorded in 43 patients and was reproducible in 28. BP levels on 24‐hour ABPM were similar in patients with and without ExBPR. In patients with high‐normal BP levels, ExBPR is not associated with masked hypertension.
Hypertension is a significant risk factor for the development of cardiovascular (CV) morbidity and mortality. Twenty‐four–hour ambulatory blood pressure (BP) monitoring (ABPM) is used to diagnose hypertension in cases where “white‐coat” or nocturnal hypertension is suspected, in cases where paroxysmal hypertension or episodes of hypotension is a concern, and in the evaluation of response to medical therapy.1 The use of 24‐hour ABPM identified a new group of patients with normal clinic BP levels and elevated 24‐hour ABPM. These patients are said to have “masked hypertension,” and the prevalence of this entity is estimated to be 9% to 20%.2, 3 Several studies have shown that the CV risk in patients with masked hypertension is elevated and similar to the risk in patients with sustained hypertension.4, 5 This condition should be identified and treated adequately to control BP. Yet, it is not practical to perform ABPM in all normotensive patients to reveal masked hypertension. Therefore, ABPM should be performed only in normotensive patients who are likely to have masked hypertension. Patients with evidence of target organ damage or with diabetes mellitus and those with occasional elevated BP readings, a history of peripheral vascular disease, or orthostatic hypertension are at a high risk for masked hypertension.6, 7
Exaggerated BP response to exercise (ExBPR) in normotensive patients has been suggested to predict the development of frank hypertension8, 9, 10, 11 and has been found to be associated with target organ damage, particularly left ventricular hypertrophy.12 However, some studies failed to show an association between ExBPR and the development of hypertension and target organ damage or CV disease.13, 14, 15, 16, 17, 18, 19, 20, 21 Moreover, there is concern regarding the reproducibility of ExBPR, which questioned its prognostic significance.22
The association between ExBPR and 24‐hour ABPM has been reported in several studies.21, 23, 24, 25, 26, 27 Most investigators have reported a high rate of masked hypertension in normotensive patients with ExBPR.21, 24, 25, 26, 27 However, Herkenhoff and colleagues23 failed to show increased 24‐hour ABPM levels in those with ExBPR. The association between repeated ExBPR and masked hypertension has not been studied. This study evaluated the association between ExBPR and masked hypertension in normotensive patients with high normal BP levels, and the influence of reproducibility of ExBPR on this association.
Methods
Study Population
We reviewed the charts of normotensive patients with high normal BP levels (systolic BP [SBP] 130–139 mm Hg and/or diastolic BP [DBP] 85–89 mm Hg) who underwent a routine annual examination at the Chaim Sheba Screening Institute Ramat Gan, Israel, during the years 2002–2007 and were referred for 24‐hour ABPM to exclude hypertension. All patients underwent a comprehensive physical examination including BP measurement, risk profile assessment, laboratory evaluation, and an exercise test. Patients with BP levels >140/90 mm Hg, those who had a history of hypertension, or those taking antihypertensive medications were excluded from the study. Of these, only those who underwent 24‐hour ABPM within 6 months of the exercise test were included.
Exercise Testing
Treadmill exercise testing was conducted in the fasting state using the Bruce protocol.28 SBP and DBP levels were recorded before and during the last minute of each 3‐minute exercise stage and at peak exercise. The patients exercised until an age‐specific target heart rate [220 beats per minute – age] was achieved. Exaggerated BP response was defined as a SBP ≥200 mm Hg at peak exercise.
We reviewed previous exercise tests performed during previous annual health examinations for each patient. For those who had at least one additional previous exercise test, we defined reproducible response when ExBPR was recorded at least twice.
24‐Hour ABPM
Twenty‐four–hour ABPM was performed by the Oscar 2 oscillometric 24‐hour ABPM system (SunTech Medical Inc, Morrisville, NC). The monitor was mounted on the left arm. A mercury sphygmomanometer was initially attached to the monitor through a Y connector to ensure conformity between the two modes of measurements. BP was measured every 20 minutes during the day and evening (from 6 am to 10 pm) and every 30 minutes at night (from 10 pm to 6 am).
Measurements were not acceptable when pulse pressure was <20 mm Hg, DBP was <40 mm Hg, or SBP was <80 mm Hg or when a single value differed greatly from the preceding and subsequent values. An acceptable 24‐hour ABPM recording for our study should have had at least 50 acceptable measurements. The average SBP and DBP levels were calculated for 24 hours and separately for day and night. BP load was calculated as the percentage of measurements >140 mm Hg for SBP and >90 mm Hg for DBP during the day and >125 mm Hg for SBP and >70 mm Hg for DBP during the night.
Masked hypertension was defined according to the 24‐hour ABPM when average BP was ≥135/85 mm Hg during waking hours and/or 120/70 mm Hg during sleep hours.
Data Collection
Age, sex, height, and weight were measured with participants wearing light clothing without shoes. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). BP was measured twice in the seated position after 3 minutes of rest and the average was recorded. Laboratory evaluation included blood creatinine, fasting glucose, total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and triglycerides. Diabetes mellitus (DM) was defined when fasting plasma glucose was >126 mg/dL (7.0 mmol/L) on two separate readings, or a history of DM was reported, or when the patient used insulin or oral hypoglycemic medications.29 Hypercholesterolemia was defined when measured total cholesterol was >250 mg/dL or when the patient reported using cholesterol‐lowering medications.30 Positive family history was considered when a first‐degree relative had early CV disease (younger than 55 for men and/or younger than 65 for women). Smoking status was determined according to the questionnaire, and participants were divided into current smokers, past smokers, or nonsmokers. The definition of physical activity was a slight modification of the American Heart Association recommendation.31 Physical activity status was divided into two categories: >3 or <3 times a week (45 minutes each time).
Statistical Analysis
Statistical analysis was performed using SPSS 21.0 (SPSS, Inc, Chicago, IL). Statistical significance was set at .05. Data are presented as mean±standard deviation for continuous variables and as frequency and percentage for categorical variables. Homogeneity for continuous variables was performed using Kolmogorov‐Smirnov nonparametric test. Comparison between groups was performed using an independent t test for continuous variables and using chi‐square test for categorical variables.
Results
Patient Characteristics
Of 277 patients, we identified 69 normotensive patients with high normal BP levels (60 men, 9 women) with a mean age of 54±9 years who underwent 24‐hour ABPM within 6 months of the index exercise test. Baseline characteristics are depicted in Table 1. Forty‐three patients had ExBPR. Patients with ExBPR were more likely to be males and had lower DBP and higher serum fasting glucose levels than those with normotensive response (Table 1).
Table 1.
Demographic and Clinical Variables of the Study Population
| Parameter | All Patients (n=69) | ExBPR (n=43) | Normal BP Response (n=26) | P Value |
|---|---|---|---|---|
| Sex, male/female | 60/9 | 40/3 | 20/6 | .05 |
| Age, y | 54±9 | 53±9 | 54±9 | .69 |
| BMI, kg/m2 | 27.2±3.1 | 27.3±3.4 | 26.9±2.5 | .575 |
| SBP, mm Hg | 131±8 | 131±7 | 131±8 | .833 |
| DBP, mm Hg | 82±5 | 80±5 | 83±4 | .013 |
| Heart rate, beats per min | 75±11 | 74±12 | 76±11 | .608 |
| Risk factors | ||||
| Diabetes mellitus, No. (%) | 5 (7) | 3 (7.0) | 2 (7.7) | .91 |
| Hypercholesterolemia, No. (%) | 31 (45) | 18 (41.9) | 13 (50) | .51 |
| Positive family history, No. (%) | 15 (22) | 11 (25.6) | 4 (15.4) | .32 |
| Physical activity, No. (%) | 51 (74) | 31 (72.1) | 20 (76.9) | .66 |
| Current smoking, No. (%) | 6 (9) | 4 (9) | 2 (8) | |
| Past smokers, No. (%) | 32 (46) | 20 (47) | 12 (46) | |
| Nonsmokers, No. (%) | 31 (45) | 19 (44) | 12 (46) | .97 |
| Laboratory results | ||||
| Plasma glucose, mg/dL | 92±16 | 96±19 | 87±8 | .04 |
| Total cholesterol, mg/dL | 197±35 | 197±37 | 197±32 | .94 |
| HDL cholesterol, mg/dL | 49±12 | 48±13 | 51±12 | .29 |
| LDL cholesterol, mg/dL | 122±30 | 123±32 | 122±29 | .84 |
| Triglycerides, mg/dL | 128±57 | 128±59 | 128±55 | .97 |
| Serum creatinine, mg/dL | 1.06±0.14 | 1.08±0.14 | 1.04±0.14 | .29 |
Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; ExBPR, exaggerated blood pressure response to exercise (SBP ≥200 mm Hg); HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
Ambulatory BP Measurements
Fifty patients (72.5%) had masked hypertension according to 24‐hour ABPM; 13 patients (18.8%) had elevated BP levels only during the day, 2 patients (2.9%) had elevated BP levels only during the night, and 35 patients (50.7%) had elevated BP during the day and the night (Table 2). Average BP levels and the rate of masked hypertension were similar in those with ExBPR and those with normal BP response to exercise (Table 2). Patients with masked hypertension had the same characteristics as those with normal BP levels (Table 3). There was no correlation between demographic and laboratory parameters and 24‐hour ABPM values.
Table 2.
24‐Hour Ambulatory Blood Pressure Monitoring Results in Patients With and Without ExBPR
| Parameter | All Patients (n=69) | ExBPR (n=43) | Normal BP Response (n=26) | P Valuea |
|---|---|---|---|---|
| Day SBP, mm Hg | 138.7±11.5 | 139±11 | 137±12 | .72 |
| Day DBP, mm Hg | 84.6±7.9 | 84±7 | 86±9 | .68 |
| Day HR, beats per min | 72.8±10.0 | 72±10 | 74±11 | .54 |
| Day SBP load, % | 45.6±29.0 | 47±29 | 44±29 | .86 |
| Day DBP load, % | 29.2±24.7 | 26±23 | 34±27 | .31 |
| Night SBP, mm Hg | 121.1±12.8 | 122±11 | 120±15 | .69 |
| Night DBP, mm Hg | 70.4±8.4 | 69±7 | 72±10 | .24 |
| Night HR, beats per min | 61.6±7.9 | 61±8 | 62±8 | .52 |
| Night SBP load, % | 49.7±32.9 | 52±31 | 46±35 | .53 |
| Night DBP load, % | 19.6±23.7 | 16±20 | 25±28 | .14 |
| Masked HTN, No. (%) | 50 (72.5) | 29 (67) | 21 (80) | .27 |
| Day only HTN, No. (%) | 13 (18.8) | 7 (16.3) | 6 (23.1) | .346 |
| Night only HTN, No. (%) | 2 (2.9) | 1 (2.3) | 1 (3.8) | .615 |
| Day and night HTN, No. (%) | 35 (50.7) | 21 (48.8) | 14 (53.8) | .439 |
| Nondippers (%) | 18 (26) | 8 (18.6) | 10 (38.5) | .069 |
Abbreviation: BP, blood pressure; DBP, diastolic blood pressure; ExBPR, exaggerated blood pressure response to exercise (systolic BP ≥200 mm Hg); HR, heart rate; HTN, Hypertension; SBP, systolic blood pressure. Day SBP load, percentage of measurements >140 mm Hg; day DBP load, percentage of measurements >90 mm Hg; night SBP load, percentage of measurements >125 mm Hg; night DBP load, percentage of measurements >70 mm Hg; masked HTN, average BP was ≥135/85 mm Hg during waking hours and/or 120/70 mm Hg during sleep hours; nondippers, <10% reduction in BP during nighttime. aBetween patients with ExSBP and those with normal response.
Table 3.
Demographic and Clinical Variables by Masked Hypertension
| Masked Hypertensiona (n=50) | Normotensiona (n=19) | P Valueb | |
|---|---|---|---|
| Age, y | 54±9 | 52±8 | .272 |
| Sex, male/female | 44/6 | 16/3 | .676 |
| BMI, kg/m2 | 27.3±3.3 | 26.8±2.4 | .544 |
| SBP, mm Hg | 132±8 | 129±7 | .277 |
| DBP, mm Hg | 82±5 | 81±6 | .326 |
| HR, beats per min | 73±11 | 78±12 | .176 |
| Risk factors | |||
| Diabetes mellitus, No. (%) | 3 (6) | 2 (10.5) | .517 |
| Hypercholesterolemia, No. (%) | 21 (42) | 10 (52.6) | .428 |
| Positive family history, No. (%) | 9 (18) | 6 (31.6) | .222 |
| Physical activity, No. (%) | 36 (72) | 15 (78.9) | .557 |
| Current smoking, No. (%) | 4 (8) | 2 (10) | |
| Past smokers, No. (%) | 25 (50) | 7 (37) | |
| Nonsmokers, No. (%) | 21 (42) | 10 (53) | .618 |
| Laboratory results | |||
| Serum glucose, mg/dL | 93±15 | 92±21 | .952 |
| Total cholesterol, mg/dL | 193±31 | 206±43 | .180 |
| HDL cholesterol, mg/dL | 48±13 | 52±12 | .365 |
| LDL cholesterol, mg/dL | 120±26 | 128±41 | .381 |
| Triglycerides, mg/dL | 122±58 | 143±54 | .190 |
| Serum creatinine, mg/dL | 1.06±0.13 | 1.08±0.17 | .620 |
| ExBPR, No. (%) | 29 (58) | 14 (73.7) | .276 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; ExBPR, exaggerated blood pressure response to exercise (SBP ≥200 mm Hg); HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure. Normotension, average 24‐hour ambulatory BP <135/85 mm Hg during waking hours and/or 120/70 mm Hg during sleep hours. aMasked hypertension, average 24‐hour ambulatory BP ≥135/85 mm Hg during waking hours and/or 120/70 mm Hg during sleep hours. bBetween patients with masked hypertension and those with normotension.
Reproducibility of ExBPR
Reviewing previous exercise tests revealed that 5 patients had only the index exercise tests and 64 patients had at least one additional previous exercise test in their records. The time interval between the exercise tests was 1 year. ExBPR at least once was recorded in 51 patients (80%). Of these, 30 patients (59%) exhibited ExBPR on ≤50% of the tests and 21 patients (41%) exhibited ExBPR on >50% of the tests (Figure). Only 11 patients (21.5%) exhibited ExBPR on all tests. Twenty‐eight patients exhibited ExBPR at least twice (reproducible response). Baseline DBP levels were lower and serum fasting glucose levels were higher in those with reproducible ExBPR than in those without reproducible ExBPR (Table 4). Average BP levels and the rate of masked hypertension were similar in those with and those without reproducible ExBPR (Table 4).
Figure .
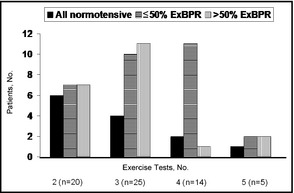
Blood pressure response to repeated exercise tests. ExBPR indicates exaggerated blood pressure response to exercise (systolic blood pressure ≥200 mm Hg).
Table 4.
Comparison Between Patients With and Without Reproducible ExBPR
| Reproducible EBPR to Exercise (≥2 ExBPR) | Nonreproducible EBPR to Exercise (<2 ExBPR) | P Valuea | |
|---|---|---|---|
| No. | 28 | 41 | |
| Age, y | 54±8 | 53±9 | .579 |
| Sex, male/female | 25/3 | 35/6 | .635 |
| BMI, kg/m2 | 27.3±3.3 | 27.1±2.9 | .828 |
| SBP, mm Hg | 133±7 | 130±8 | .120 |
| DBP, mm Hg | 80±5 | 83±5 | .053 |
| HR, beats per min | 74±11 | 76±12 | .436 |
| Risk factors | |||
| Diabetes mellitus, No. (%) | 2 (7) | 3 (7) | .978 |
| Hypercholesterolemia, No. (%) | 14 (50) | 17 (42) | .484 |
| Positive family history, No. (%) | 5 (18) | 10 (24) | .518 |
| Physical activity, No. (%) | 18 (64) | 33 (81) | .132 |
| Current smoking, No. (%) | 1 (4) | 5 (12) | |
| Past smokers, No. (%) | 16 (57) | 16 (39) | |
| Nonsmokers, No. (%) | 11 (39) | 20 (49) | .231 |
| Laboratory results | |||
| Plasma glucose, mg/dL | 98±20 | 89±12 | .025 |
| Total cholesterol, mg/dL | 192±39 | 200±32 | .362 |
| HDL cholesterol, mg/dL | 48±11 | 51±13 | .343 |
| LDL cholesterol, mg/dL | 118±35 | 126±27 | .342 |
| Triglycerides, mg/dL | 131±69 | 126±48 | .683 |
| Serum creatinine, mg/dL | 1.07±0.16 | 1.06±0.13 | .764 |
| 24‐h ABPM results | |||
| ABPM day SBP, mm Hg | 141±13 | 137±10 | .186 |
| ABPM day DBP, mm Hg | 84±7 | 85±8 | .396 |
| ABPM day HR, beats per min | 72±10 | 73±10 | .864 |
| ABPM day SBP load, % | 50±30 | 42±28 | .275 |
| ABPM day DBP load, % | 25±23 | 32±26 | .240 |
| ABPM night SBP, mm Hg | 124±13 | 119±12 | .138 |
| ABPM night DBP, mm Hg | 70±8 | 71±9 | .768 |
| ABPM night HR, beats per min | 62±6 | 62±9 | .923 |
| ABPM night SBP load, % | 56±33 | 45±32 | .160 |
| ABPM night DBP load, % | 20±23 | 20±24 | .970 |
| Masked HTN, No. (%) | 21 (75) | 29 (71) | .781 |
| Day only HTN, No. (%) | 4 (14) | 9 (22) | .372 |
| Night only HTN, No. (%) | 1 (3.6) | 1 (2.4) | .563 |
| Day and night HTN, No. (%) | 16 (57) | 19 (46) | .337 |
| Nondippers | 7 (25) | 11 (27) | .865 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BMI, body mass index; DBP, diastolic blood pressure; ExBPR, exaggerated blood pressure response to exercise (systolic BP ≥200 mm Hg); HDL, high‐density lipoprotein; HR, heart rate; HTN, hypertension; LDL, low‐density lipoprotein; SBP, systolic blood pressure; TG, triglycerides. Day SBP load, percentage of measurements >140 mm Hg; day DBP load, percentage of measurements >90 mm Hg; night SBP load, percentage of measurements >125 mm Hg; night DBP load, percentage of measurements >70 mm Hg; masked HTN, average BP ≥135/85 mm Hg during waking hours and/or 120/70 mm Hg during sleep hours; nondippers, <10% reduction in BP during nighttime. aBetween patients with reproducible ExSBP and those without reproducible response.
Discussion
As masked hypertension is associated with cardiovascular morbidity and mortality,32 it seems that identification of these patients is essential. Yet, because it is implausible to perform routine 24‐hour ABPM in the general population, identification of individuals at high risk for masked hypertension may enable performance of selective 24‐hour ABPM in order to identify this population. Various studies have tried to characterize patients at risk for masked hypertension and factors associated with this phenomenon include age, SBP, and markers of atherosclerosis such as the presence of peripheral arterial disease, diabetes, left ventricular hypertrophy, and smoking.6, 33
The association between ExBPR and masked hypertension has been reported in several studies. Miyai and colleagues34 compared 24‐hour ABPM results of 27 men with ExBPR and 27 men with a normal BP response to exercise and found that BP values on 24‐hour ABPM were significantly higher in those with ExBPR. Nazar and colleagues25 reported similar findings, and Kayrak and colleagues24 reported that the prevalence of masked hypertension in patients with ExBPR was 41%, which is significantly higher than reported in the general population. We failed to show an association between ExBPR and masked hypertension. Our results are in accordance with the results of Herkenhoff who also showed no association between ExBPR and masked hypertension.23 One can argue that ExBPR is not reproducible22 and therefore a single ExBPR is not associated with masked hypertension. Indeed, in the present study, the reproducibility of ExBPR was relatively low, as only 41% of the patients exhibited ExBPR in >50% of the tests and only 21% of the patients exhibited ExBPR in all the exercise tests. We therefore evaluated previous exercise tests and showed that even when ExBPR was reproducible, it was not associated with masked hypertension. This observation further substantiates the lack of association between ExBPR and masked hypertension. As far as we know, this is the only study that evaluates the association between repeated ExBPR and masked hypertension. We defined ExBPR when SBP during exercise was ≥200 mm Hg, while other investigators used a threshold of 190 mm Hg for women and 210 mm Hg for men.35 However, there are varying definitions for ExSBP and we prefered to use an SBP value of 200 mm Hg as a cutpoint for both sexes as we used in our previous studies.10, 22 We also analyzed the data using an SBP of 210 mm Hg as a cutpoint for the definition of ExBPR and we received the same results (data not shown). Our population is unique since the patients underwent a routine annual examination at our screening institute and therefore precise data on all CV risk factors are available. Patients with ExBPR had similar CV risk factors as those with normal BP response to exercise. We indeed observed higher fasting serum glucose levels in those with ExBPR than in those with normal BP response to exercise, but the rate of diabetes was the same in both groups.
The rate of masked hypertension was very high in our study group because we referred for 24‐hour ABPM only normotensive patients with high normal resting BP levels to exclude hypertension. In this subgroup, ExBPR is not useful to identify masked hypertension. However, it is still possible that in young patients with low BP levels, ExBPR is associated with masked hypertension, as has been previously described.24, 25, 34 In our population, we could not identify risk factors for masked hypertension. Thus, it seems that in patients with high‐normal BP levels, neither ExBPR nor known CV risk factors contribute to the identification of patients with masked hypertension. Since in this group the rate of masked hypertension is high, it may be justified to perform 24‐hour ABPM in all patients with high‐normal BP levels.
Study Limitations
The study has several limitations. The retrospective nature of the study limited the ability to recruit patients who underwent both exercise testing and 24‐hour ABPM at the same time. In addition, we accepted the results of the 24‐hour ABPM if >50% of the measurements were satisfactory, whereas the European Society of Hypertension guidelines require that at least 70% of BP readings should be satisfactory. The study population was a selected group of normotensive patients with high‐normal BP levels who were referred for 24‐hour ABPM to exclude hypertension. The retrospective nature of the study also resulted in a different number of exercise tests for patients included in the study. In addition, the high prevalence of smokers or past smokers in this cohort may have influenced the results; these being inapplicable for populations with lower rates of smoking. Nevertheless, our results may suggest that ExBPR is not useful to identify masked hypertension, particularly in patients with high‐normal BP levels.
Conclusions
The reproducibility of ExBPR is low and unpredictable. ExBPR in normotensive patients with high‐normal BP levels has not been found to be associated with masked hypertension even in patients in whom this response was reproducible. These findings question the clinical significance of ExBPR for detecting masked hypertension in normotensive patients.
Acknoweledgments
Cohen Noa performed this work in partial fulfillment of the M.D. thesis requirements of the Sackler Faculty of Medicine, Tel Aviv University.
Disclosure
None.
J Clin Hypertens(Greenwich). 2014:277–282. DOI: 10.1111/jch.12303. ©2014. Wiley Periodicals, Inc.
References
- 1. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 2. Bobrie G, Clerson P, Menard J, et al. Masked hypertension: a systematic review. J Hypertens. 2008;26:1715–1725. [DOI] [PubMed] [Google Scholar]
- 3. Kawano Y, Horio T, Matayoshi T, Kamide K. Masked hypertension: subtypes and target organ damage. Clin Exp Hypertens. 2008;30:289–296. [DOI] [PubMed] [Google Scholar]
- 4. Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white‐coat, masked and sustained hypertension versus true normotension: a meta‐analysis. J Hypertens. 2007;25:2193–2198. [DOI] [PubMed] [Google Scholar]
- 5. Pierdomenico SD, Cuccurullo F. Prognostic value of white‐coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens. 2011;24:52–58. [DOI] [PubMed] [Google Scholar]
- 6. Barochiner J, Cuffaro PE, Aparicio LS, et al. Predictors of masked hypertension among treated hypertensive patients: an interesting association with orthostatic hypertension. Am J Hypertens. 2013;26:872–878. [DOI] [PubMed] [Google Scholar]
- 7. Grossman E. Ambulatory blood pressure monitoring in the diagnosis and management of hypertension. Diabetes Care. 2013;36(suppl 2):S307–S311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benbassat J, Froom P. Blood pressure response to exercise as a predictor of hypertension. Arch Intern Med. 1986;146:2053–2055. [PubMed] [Google Scholar]
- 9. Farah R, Shurtz‐Swirski R, Nicola M. High blood pressure response to stress ergometry could predict future hypertension. Eur J Intern Med. 2009;20:366–368. [DOI] [PubMed] [Google Scholar]
- 10. Sharabi Y, Ben‐Cnaan R, Hanin A, et al. The significance of hypertensive response to exercise as a predictor of hypertension and cardiovascular disease. J Hum Hypertens. 2001;15:353–356. [DOI] [PubMed] [Google Scholar]
- 11. Singh JP, Larson MG, Manolio TA, et al. Blood pressure response during treadmill testing as a risk factor for new‐onset hypertension. The Framingham heart study. Circulation. 1999;99:1831–1836. [DOI] [PubMed] [Google Scholar]
- 12. Gottdiener JS, Brown J, Zoltick J, Fletcher RD. Left ventricular hypertrophy in men with normal blood pressure: relation to exaggerated blood pressure response to exercise. Ann Intern Med. 1990;112:161–166. [DOI] [PubMed] [Google Scholar]
- 13. Drory Y, Pines A, Fisman EZ, Kellermann JJ. Exercise response in young women with borderline hypertension. Chest. 1990;97:298–301. [DOI] [PubMed] [Google Scholar]
- 14. Hansen HS, Hyldebrandt N, Nielsen JR, Froberg K. Exercise testing in children as a diagnostic tool of future hypertension: the Odense Schoolchild Study. J Hypertens Suppl. 1989;7:S41–S42. [DOI] [PubMed] [Google Scholar]
- 15. Hedberg P, Ohrvik J, Lonnberg I, Nilsson G. Augmented blood pressure response to exercise is associated with improved long‐term survival in older people. Heart. 2009;95:1072–1078. [DOI] [PubMed] [Google Scholar]
- 16. Khanna CM, Dubey YS, Khanna G, Kaur G. Response to exercise and ambulatory blood pressure monitoring in essential hypertension. J Assoc Physicians India. 1999;47:393–396. [PubMed] [Google Scholar]
- 17. Lauer MS, Pashkow FJ, Harvey SA, et al. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol. 1995;26:1630–1636. [DOI] [PubMed] [Google Scholar]
- 18. Majahalme S, Turjanmaa V, Tuomisto M, et al. Blood pressure responses to exercise as predictors of blood pressure level after 5 years. Am J Hypertens. 1997;10:106–116. [DOI] [PubMed] [Google Scholar]
- 19. Tanaka H, Bassett DR Jr, Turner MJ. Exaggerated blood pressure response to maximal exercise in endurance‐trained individuals. Am J Hypertens. 1996;9:1099–1103. [DOI] [PubMed] [Google Scholar]
- 20. Lauer MS, Levy D, Anderson KM, Plehn JF. Is there a relationship between exercise systolic blood pressure response and left ventricular mass? The Framingham Heart Study Ann Intern Med. 1992;116:203–210. [DOI] [PubMed] [Google Scholar]
- 21. Lima EG, Spritzer N, Herkenhoff FL, et al. Noninvasive ambulatory 24‐hour blood pressure in patients with high normal blood pressure and exaggerated systolic pressure response to exercise. Hypertension. 1995;26:1121–1124. [DOI] [PubMed] [Google Scholar]
- 22. Sharabi Y, Almer Z, Hanin A, et al. Reproducibility of exaggerated blood pressure response to exercise in healthy patients. Am Heart J. 2001;141:1014–1017. [DOI] [PubMed] [Google Scholar]
- 23. Herkenhoff FL, Lima EG, Goncalves RA, et al. Doppler echocardiographic indexes and 24‐h ambulatory blood pressure data in sedentary middle‐aged men presenting exaggerated blood pressure response during dynamical exercise test. Clin Exp Hypertens. 1997;19:1101–1116. [DOI] [PubMed] [Google Scholar]
- 24. Kayrak M, Bacaksiz A, Vatankulu MA, et al. Exaggerated blood pressure response to exercise–a new portent of masked hypertension. Clin Exp Hypertens. 2010;32:560–568. [DOI] [PubMed] [Google Scholar]
- 25. Nazar K, Kaciuba‐Uscilko H, Ziemba W, et al. Physiological characteristics and hormonal profile of young normotensive men with exaggerated blood pressure response to exercise. Clin Physiol. 1997;17:1–18. [DOI] [PubMed] [Google Scholar]
- 26. Schultz MG, Hare JL, Marwick TH, et al. Masked hypertension is “unmasked” by low‐intensity exercise blood pressure. Blood Press. 2011;20:284–289. [DOI] [PubMed] [Google Scholar]
- 27. Sharman JE, Hare JL, Thomas S, et al. Association of masked hypertension and left ventricular remodeling with the hypertensive response to exercise. Am J Hypertens. 2011;24:898–903. [DOI] [PubMed] [Google Scholar]
- 28. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. [DOI] [PubMed] [Google Scholar]
- 29. American Diabetes Association . Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(suppl 1):S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double‐blind treatment with a long‐acting calcium‐channel blocker or diuretic in the International Nifedipine GITS study: intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet. 2000;356:366–372. [DOI] [PubMed] [Google Scholar]
- 31. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. [DOI] [PubMed] [Google Scholar]
- 32. Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension”. Am J Hypertens. 2008;21:393–399. [DOI] [PubMed] [Google Scholar]
- 33. Hanninen MR, Niiranen TJ, Puukka PJ, et al. Determinants of masked hypertension in the general population: the Finn‐Home study. J Hypertens. 2011;29:1880–1888. [DOI] [PubMed] [Google Scholar]
- 34. Miyai N, Arita M, Morioka I, et al. Ambulatory blood pressure, sympathetic activity, and left ventricular structure and function in middle‐aged normotensive men with exaggerated blood pressure response to exercise. Med Sci Monit. 2005;11:CR478–CR484. [PubMed] [Google Scholar]
- 35. Le VV, Mitiku T, Sungar G, et al. The blood pressure response to dynamic exercise testing: a systematic review. Prog Cardiovasc Dis. 2008;51:135–160. [DOI] [PubMed] [Google Scholar]


