Abstract
In contrast to middle age, it is unclear whether blood pressure (BP) in older persons is associated with cerebral small vessel disease (cSVD). The authors evaluated the association of BP with signs of cSVD as well as gray and white matter integrity in older persons. In 220 participants aged 75 years and older from the Discontinuation of Antihypertensive Treatment in the Elderly (DANTE) study, cSVD was assessed with conventional magnetic resonance imaging, and microstructural integrity with diffusion tensor and magnetization transfer (MT) imaging. BP measures were not associated with cSVD. However, lower systolic and diastolic BP and mean arterial pressure were associated with decreased gray matter MT ratio peak height and MT ratio in cortical gray matter. Mean arterial pressure was also associated with increased gray matter diffusivity. A lower level of BP was especially associated with worse gray matter integrity. Results suggest that not only upper but preferably lower thresholds of BP values should be observed in older persons.
High blood pressure (BP) is related to increased risk of cerebral small vessel disease in middle age, typically including white matter hyperintensities (WMHs),1, 2, 3, 4, 5, 6, 7, 8, 9, 10 lacunar infarcts,11, 12, 13 and microbleeds14, 15, 16, 17 on magnetic resonance imaging (MRI). Apart from these visual focal changes, more widespread subtle changes in the microstructural integrity of the cerebral white matter have been reported.18, 19, 20, 21
In older persons, the association of BP with MRI findings may be different than in middle‐aged persons, since low BP, rather than high BP, has recently been associated with cerebral damage1, 2, 22 and worse outcomes such as risk of ischemic stroke23, 24 and mortality.25 In these studies it has been suggested that this effect may be caused by hypoperfusion, especially in older people with arteriosclerotic damage or a history of cardiovascular disease. Thus, these persons may be better off with higher BP levels, whereby perfusion is maintained and brain integrity preserved.26, 27 To our knowledge, no studies have assessed the relationship between BP and manifestation of small vessel disease in combination with measurements of gray and white microstructural brain integrity in older persons.
The aim of the present cross‐sectional study was to explore the associations of BP with manifestations of small vessel disease and microstructural brain integrity in both gray and white matter in older persons.
Methods
Participants
Participants for this MRI substudy were included from the Discontinuation of Antihypertensive Treatment in the Elderly (DANTE) trial, a community‐based randomized nonblinded clinical trial assessing the effect of temporary discontinuation of antihypertensive therapy on neuropsychological functioning in older persons with mild cognitive dysfunction. Inclusion criteria were age 75 years and older, use of antihypertensive medication, presence of mild cognitive dysfunction (according to Mini‐Mental State Examination score 21–27), and current systolic blood pressure (SBP) ≤160 mm Hg (or ≤140 mm Hg for persons with diabetes, or myocardial infarction, peripheral artery vascular disease, or coronary reperfusion procedures more than 3 years ago). Current BP was determined based on the last BP measurement obtained from the general practitioners' electronic medical record. Exclusion criteria were a history of stroke or transient ischemic attack, a recent (≤3 years) myocardial infarction or recent coronary reperfusion procedure, current angina pectoris, cardiac arrhythmias, heart failure, use of antihypertensive medication other than for hypertension, a clinical diagnosis of dementia, or a limited life expectancy.
The current study used baseline data of the MRI substudy. From the 430 DANTE participants, 220 nonselected persons underwent MRI of the brain. The Medical Ethical committee of the Leiden University Medical Center approved the study, and written informed consent was obtained from all participants.
Blood Pressure
SBP and diastolic blood pressure (DBP) were measured twice at baseline in a seated position in all participants using a fully automatic electronic sphygmomanometer (Omron M6 Comfort; Omron Healthcare, Inc, Lake Forest, IL). For analyses, the mean of the two measurements was calculated. Mean arterial pressure (MAP) was calculated as 1/3(SBP)+2/3(DBP), and pulse pressure (PP) as SBP–DBP.
MRI Acquisition
All MRI scans were acquired on a whole‐body magnetic resonance system operating at a field strength of 3‐T with a 32‐channel head coil (Philips Medical Systems, Best, The Netherlands). Three‐dimensional (3D) T1‐weighted images were acquired with repetition time (TR)/echo time (TE)=9.7/4.6 ms, flip angle (FA)=8°, and a nominal voxel size of 1.17×1.17×1.4 mm. Fluid‐Attenuated Inversion Recovery (FLAIR) images (TR/TE=11,000/125 ms, FA=90°), T2‐weighted images (TR/TE=4200/80 ms, FA=90°), and T2*‐weighted images (TR/TE=45/31 ms, FA=13°) were acquired. Diffusion tensor images (DTI) (TR/TE=9592/56 ms, FA=90°, 64 slices, 32 measurement directions, b value=1000) and magnetization transfer images (MTI) with and without a saturation pulse (TR/TE=100/11 ms, FA=9°) were acquired. DTI images and MTI images were available for 195 and 216 participants, respectively.
White Matter Hyperintensities, Lacunar Infarcts, and Microbleeds
MRI scans were visualized using Philips DICOM viewer R3.0‐SP03 software (Philips Medical Systems). Cerebral ischemic damage was evaluated as previously reported.28 In short, periventricular and subcortical WMHs were scored semiquantitatively. Periventricular WMHs were present when lateral, posterior, and anterior periventricular regions were scored ≥2. A lacunar infarct, assessed on FLAIR and T2‐ and 3D T1‐weighted images, was defined as a parenchymal defect (signal intensity identical to cerebrospinal fluid on all sequences) of at least 3 mm in diameter, surrounded by a zone of parenchyma with increased signal intensity on T2‐weighted and FLAIR images. Microbleeds were defined as focal areas of signal void (on T2 images), which increased in size on T2*‐weighted images (blooming effect). Symmetric hypointensities in the basal ganglia, likely to represent calcifications or nonhemorrhagic iron deposits, were disregarded. All measurements were obtained blinded to participants' demographic and clinical information.
Image Processing and Analysis
MRI scans were analyzed with FMRIB software version 5.0.1. Library.29 For the automated measurement of WMH volume, 3D T1‐weighted and FLAIR images were skull stripped30 and co‐registered using the FMRIB's Linear Image Registration Tool (FLIRT).31, 32 The FLAIR image was affine‐registered to MNI152 standard space using FLIRT. WMHs were extracted from FLAIR images with a conservative MNI152 white matter mask and a threshold was set to identify which white matter voxels were hyperintense, followed by manually checking and editing for quality control.
Using the FDT (FMRIB's Diffusion Toolbox), individual fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AxD), and radial diffusivity (RD) images were created.33, 34 Using FLIRT to a non–diffusion‐weighted reference volume, original images were corrected for effects of head movement and eddy currents in the gradient coils. A diffusion tensor model was fitted to the corrected images to create individual FA, MD, AxD, and RD images. For global quantification of brain tissue FA, MD, AxD, and RD, 3D T1 images were skull stripped,35 segmented,36 and aligned to MNI152 using FLIRT. Lower FA and higher MD, AxD, and RD indicate poorer integrity.
For MTI data processing nonsaturated (M0) and saturated (M1) images were co‐registered to the 3D T1 image.36 Individual magnetic transfer ratio (MTR) maps were calculated voxel by voxel. 3D T1 cortical gray and white matter masks were corrected for possible partial volume effects.37 Per volume‐of‐interest MTR peak height, normalized for the size for the volume‐of‐interest, were calculated.38 MTR peak height value of one participant exceeded three standard deviations and was excluded. Lower MTR peak height indicates poorer integrity.
For the voxel‐based analysis, MTR gray matter maps were aligned to MNI152 using nonlinear transformation39 and averaged to create a reference template for MTR images. Individual gray matter MTR maps were nonlinearly registered to this template.40 Voxel wise statistics were carried out with FSL randomise using permutation‐based nonparametric testing (5000 permutations). Threshold‐Free Cluster Enhancement was applied with a significance level set at P<.05 Family Wise Error corrected for multiple comparisons. The age and sex of participants were inserted as covariates in the model.
Demographic and Clinical Variables
Demographic and clinical characteristics were obtained using a standardized interview. Education was dichotomized at 6 years of schooling (primary education only) and alcohol use at 14 units per week. Using structured questionnaires, information about medication and medical histories was obtained from the general practitioners.
Statistical Analyses
Characteristics of the study participants are reported as mean (standard deviation), median (interquartile range) for continuous variables when appropriate, and number (percentage) for categorical variables.
For analyses, the SBP and DBP were both grouped into three clinically relevant categories: SBP <140 mm Hg, 140–159 mm Hg and ≥160 mm Hg, and DBP <80 mm Hg, 80–89 mm Hg, and ≥90 mm Hg. Since no clinically relevant cutoff values are known for MAP and PP, these were grouped into tertiles. Accordingly, the associations of clinically relevant groups of SBP, DBP, and tertiles of MAP and PP with parameters of small vessel disease and microstructural integrity were analyzed using logistic or linear regression analysis adjusting for age, sex, and duration of antihypertensive treatment. WMH volume was logarithmically transformed to ensure a normal distribution. Standardized z scores were calculated for the microstructural parameters using the following equation: (=test score−mean)/SD) (Figure 1).
Figure 1.
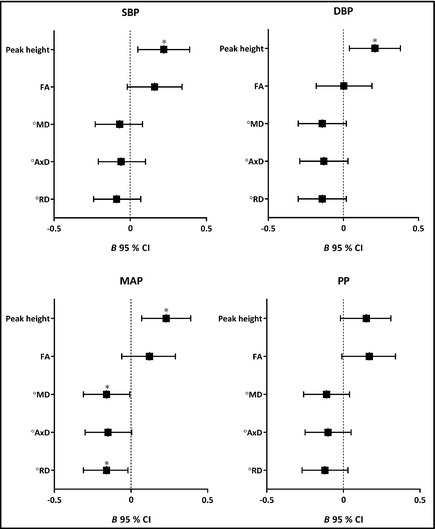
β (95% confidence interval [CI]) of the associations of systolic blood pressure (SBP) and diastolic blood pressure (DBP) and mean arterial pressure (MAP) or pulse pressure (PP) tertiles, with z scores of magnetization transfer ratio peak height (peak height), fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AxD), and radial diffusivity (RD) in gray matter adjusted for age, sex, and duration of antihypertensive treatment. *P<.05. ° Higher diffusivity indicates poorer microstructural integrity.
As SBP <140 mm Hg, 140–159 mm Hg, and ≥160 mm Hg groups included different percentages of persons with diabetes (27.3%, 18.7% and 13.0%, respectively), we performed additional analyses with further adjustment for diabetes.
To assess whether associations found were nonlinear, additional analyses were performed for which the lowest SBP group was grouped into three clinically relevant low BP categories: SBP <120 mm Hg, 120–129 mm Hg, and 130–140 mm Hg. In addition, we evaluated whether J‐shaped relationships were present by adding quadratic terms of continuous BP measures to the model.
A P value of <.05 was considered statistically significant. Data were analyzed using an exploratory approach, therefore no formal adjustments for multiple comparisons were used. Statistical analysis was performed with SPSS software (version 20.0; SPSS, Chicago, IL).
Results
Table 1 summarizes the characteristics of the study participants. Mean age was 80.7 (SD 4.1) years; median Mini‐Mental State Examination score was 26 (IQR 25–27), reflecting mild cognitive dysfunction; median WMH volume was 22 (IQR 9–56) mL; and lacunar infarcts or microbleeds were present in 59 (27%) and 55 (25%) of participants, respectively.
Table 1.
Characteristics of Participants
| Characteristic | (N=220) |
|---|---|
| Demographics | |
| Age, y | 80.7 (4.1) |
| Women | 125 (56.8) |
| Lower education (≤6 y) | 64 (29.1) |
| Clinical characteristics | |
| Current smoking | 17 (7.7) |
| Alcohol ≥14 units per wk | 24 (10.9) |
| History of CVDa | 20 (9.1) |
| Presence of chronic diseasesb | 135 (61.4) |
| MMSE (points) | 26 (25–27) |
| Duration of antihypertensive treatment, y | |
| <1 | 5 (2.3) |
| 1–5 | 57 (25.9) |
| >5 | 149 (67.7) |
| Blood pressure, mm Hg | |
| Systolic | 146 (21) |
| Diastolic | 81 (11) |
| Mean arterial pressure | 102 (13) |
| Pulse pressure | 65 (15) |
| MRI characteristics | |
| WMH volume, mL | 22 (9–56) |
| Periventricular WMH | 132 (60.0) |
| Subcortical WMH | 113 (51.4) |
| Lacunar infarcts | 59 (26.8) |
| Microbleeds | 55 (25.0) |
Abbreviations: MMSE, Mini‐Mental State Examination; MRI, magnetic resonance imaging; WMH, white matter hyperintensity. Data are presented as mean (standard deviation), median (interquartile range), or number (percentage) when appropriate. aCardiovascular disease (CVD) includes myocardial infarction, percutaneous coronary intervention, or coronary artery bypass graft. bChronic diseases include diabetes mellitus, Parkinson's disease, chronic obstructive pulmonary disease, malignancy, and osteoarthritis.
No significant associations were found between SBP or DBP with volumes of WMH, presence of periventricular or subcortical WMH, lacunar infarcts, or microbleeds. There was also no significant association of MAP or PP with any of these parameters (Table 2).
Table 2.
Parameters of Small Vessel Disease in Groups of Systolic and Diastolic Blood Pressure and Tertiles of Mean Arterial Pressure and Pulse Pressure
| Parameters of Small Vessel Disease | Systolic Blood Pressure, mm Hg | β/OR (95% CI) | P Trend | Diastolic Blood Pressure, mm Hg | β/OR (95% CI) | P Trend | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| <140 (n=88) | 140–159 (n=75) | ≥160 (n=57) | <80 (n=99) | 80–89 (n=78) | ≥90 (n=43) | |||||
| Volume WMH, mL | 34.8 (4.1) | 38.7 (5.1) | 38.1 (37.9) | 0.02 (−0.19, 0.24) | .84 | 33.2 (3.3) | 40.4 (5.1) | 46.2 (7.1) | 0.09 (−0.14, 0.31) | .45 |
| Periventricular WMH, No. (%) | 54 (61.4) | 45 (60.0) | 33 (57.9) | 0.93 (0.65, 1.32) | .68 | 54 (54.5) | 51 (61.4) | 27 (62.8) | 1.34 (0.92, 1.95) | .13 |
| Subcortical WMH, No. (%) | 46 (52.3) | 38 (50.7) | 29 (50.9) | 1.04 (0.73, 1.48) | .83 | 52 (52.2) | 39 (50.0) | 22 (51.2) | 0.98 (0.68, 1.41) | .90 |
| Lacunar infarcts, No. (%) | 21 (24.1) | 21 (28.0) | 17 (29.8) | 1.18 (0.79, 1.76) | .41 | 27 (27.6) | 20 (26.6) | 12 (27.9) | 1.04 (0.69, 1.57) | .86 |
| Microbleeds, No. (%) | 19 (22.1) | 18 (25.0) | 18 (32.1) | 1.38 (0.92, 2.08) | .12 | 20 (20.6) | 24 (32.0) | 11 (26.2) | 1.45 (0.95, 2.21) | .09 |
| Basal, No. (%) | 11 (12.8) | 12 (16.7) | 10 (17.9) | 1.34 (0.82, 2.19) | .25 | 12 (12.4) | 13 (17.3) | 8 (19.0) | 1.61 (0.96, 2.70) | .07 |
| Lobar, No. (%) | 14 (16.3) | 13 (18.1) | 14 (25.0) | 1.33 (0.84, 2.10) | .23 | 15 (15.5) | 18 (24.0) | 8 (19.0) | 1.40 (0.87, 2.27) | .17 |
| Mean Arterial Pressure, mm Hg | β/OR (95% CI) | P Trend | Pulse Pressure, mm Hg | β/OR (95% CI) | P Trend | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| <97 (n=73) | 97–107 (n=73) | >107 (n=74) | <58 (n=74) | 58–69 (n=72) | >69 (n=74) | |||||
| Volume WMH, mL | 32.8 (3.8) | 41.0 (5.1) | 38.1 (5.1) | 0.02 (−0.19, 0.23) | .85 | 33.5 (4.4) | 37.1 (4.9) | 40.3 (4.9) | 0.06 (−0.15, 0.26) | .59 |
| Periventricular WMH, No. (%) | 43 (58.9) | 47 (64.4) | 42 (56.8) | 0.95 (0.46, 1.43) | .78 | 46 (62.2) | 46 (63.9) | 40 (54.1) | 0.81 (0.58, 1.15) | .81 |
| Subcortical WMH, No. (%) | 37 (50.7) | 42 (57.5) | 34 (45.9) | 1.43 (0.67, 1.33) | .74 | 38 (51.4) | 36 (50.0) | 39 (52.3) | 1.11 (0.79, 1.56) | .53 |
| Lacunar infarcts, No. (%) | 18 (25.0) | 22 (30.1) | 19 (25.7) | 1.04 (0.71, 1.52) | .86 | 18 (24.7) | 19 (26.4) | 22 (29.7) | 1.16 (0.79, 1.71) | .46 |
| Microbleeds, No. (%) | 15 (21.1) | 18 (25.7) | 22 (30.1) | 1.40 (0.94, 2.10) | .10 | 17 (23.9) | 19 (27.1) | 19 (26.0) | 1.03 (0.69, 1.53) | .89 |
| Basal, No. (%) | 9 (12.7) | 15 (12.9) | 9 (20.5) | 1.59 (0.97, 2.62) | .07 | 10 (14.1) | 11 (15.7) | 12 (16.4) | 1.06 (0.68, 1.76) | .71 |
| Lobar, No. (%) | 11 (15.5) | 13 (18.6) | 17 (23.3) | 1.39 (0.88, 2.20) | .16 | 12 (16.9) | 14 (20.0) | 15 (20.5) | 1.05 (0.68, 1.63) | .83 |
Abbreviation: WMH, white matter hyperintensity. Missing values: n=3 for volume WMH, n=1 for lacunar infarcts, and n=6 for microbleeds. Data are presented as mean (standard error) or number (percentage), and β or odds ratios (ORs) (per increase in blood pressure group or tertile) (95% confidence interval [CI]), adjusted for age, sex, and duration of antihypertensive treatment.
The associations of BP measures with microstructural parameters (MTI and DTI) are shown in Table 3. Our data show that both lower SBP and DBP were significantly associated (P<.05) with decreased gray MTR peak height. Accordingly, lower MAP was also significantly associated with decreased gray matter MTR peak height (P=.01). Moreover, lower MAP was significantly associated with decreased white matter MTR peak height and with increased MD and RD in the gray matter (all P<.05). There were no significant associations between PP and any of the parameters of microstructural integrity.
Table 3.
Gray and White Matter Microstructural Parameters in Groups of Systolic and Diastolic Blood Pressure and Tertiles of Mean Arterial Pressure and Pulse Pressure
| Microstructural Parameters | Systolic Blood Pressure, mm Hg | β (95% CI) | P Trend | Diastolic Blood Pressure, mm Hg | β (95% CI) | P Trend | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| <140 (n=88) | 140–159 (n=75) | ≥160 (n=57) | <80 (n=99) | 80–89 (n=78) | ≥90 (n=43) | |||||
| Gray matter | ||||||||||
| Peak height | 65 (1.1) | 66 (1.2) | 70 (1.6) | 2.4 (0.6, 4.2) | .01 | 65 (1.1) | 67 (1.2) | 70 (1.70 | 2.3 (0.4, 4.1) | .02 |
| FA | 166 (0.9) | 170 (1.0) | 168 (1.2) | 1.4 (−0.2, 2.9) | .08 | 168 (0.8) | 169 (1.1) | 168 (1.3) | 0.0 (−1.6, 1.6) | .97 |
| MD | 1151 (7.7) | 1149 (7.5) | 1146 (10.9) | −5.1 (−15.8, 5.9) | .35 | 1156 (7.3) | 1155 (7.6) | 1122 (11.8) | −9.2 (−20.2, 1.7) | .10 |
| AxD | 1339 (8.3) | 1341 (8.1) | 1335 (11.8) | −4.2 (−15.7, 7.4) | .48 | 1346 (7.9) | 1345 (8.0) | 1310 (12.9) | −9.4 (−21.2, 2.4) | .12 |
| RD | 1057 (7.5) | 1054 (7.3) | 1051 (10.5) | −5.6 (−16.0, 4.7) | .28 | 1061 (7.1) | 1059 (7.4) | 1028 (11.3) | −9.1 (−19.7, 1.4) | .09 |
| White matter | ||||||||||
| Peak height | 103 (1.9) | 107 (2.0) | 109 (2.7) | 2.8 (−0.4, 5.9) | .08 | 104 (1.7) | 107 (2.1) | 110 (3.0) | 2.3 (−0.9, 5.5) | .16 |
| FA | 243 (2.0) | 248 (2.3) | 242 (2.6) | 0.4 (−2.9, 3.7) | .83 | 244 (1.9) | 245 (2.4) | 244 (2.9) | −1.5 (−4.8, 1.9) | .40 |
| MD | 1009 (6.5) | 1006 (6.7) | 1011 (9.0) | −1.3 (−10.7, 8.1) | .78 | 1011 (6.4) | 1013 (6.9) | 994 (9.2) | −1.6 (−11.3, 8.1) | .75 |
| AxD | 1243 (6.2) | 1245 (6.2) | 1245 (8.5) | −0.5 (−9.2, 8.3) | .91 | 1246 (6.0) | 1250 (6.2) | 1228 (8.9) | −2.1 (−11.1, 6.8) | .64 |
| RD | 893 (6.8) | 887 (7.1) | 894 (9.4) | −1.7 (−11.7, 8.2) | .73 | 894 (6.6) | 895 (7.3) | 877 (9.5) | −1.3 (−11.5, 9.0) | .81 |
| Mean Arterial Pressure, mm Hg | β (95% CI) | P Trend | Pulse Pressure, mm Hg | β (95% CI) | P Trend | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| <97 (n=73) | 97–107 (n=73) | >107 (n=74) | <58 (n=74) | 58–69 (n=72) | >69 (n=74) | |||||
| Gray matter | ||||||||||
| Peak height | 64 (1.2) | 67 (1.3) | 69 (1.3) | 2.5 (0.8, 4.2) | .01 | 65 (1.3) | 67 (1.2) | 67 (1.4) | 1.6 (−0.2, 3.3) | .08 |
| FA | 167 (1.0) | 169 (1.0) | 168 (1.1) | 1.0 (−0.5, 2.5) | .18 | 167 (1.0) | 169 (1.1) | 169 (1.0) | 1.4 (−0.0, 2.9) | .06 |
| MD | 1159 (8.4) | 1150 (8.4) | 1139 (8.5) | −10.7 (‐20.9, −0.6) | .04 | 1149 (7.9) | 1148 (8.7) | 1149 (8.7) | −7.8 (−17.9, 2.4) | .13 |
| AxD | 1348 (9.0) | 1342 (9.1) | 1327 (9.2) | −10.7 (−21.7, 0.3) | .06 | 1338 (8.5) | 1339 (9.5) | 1339 (9.3) | −7.2 (−18.2, 3.7) | .20 |
| RD | 1065 (7.8) | 1055 (8.1) | 1044 (8.3) | −10.8 (−20.6, −1.0) | .03 | 1155 (7.7) | 1153 (8.4) | 1154 (8.5) | −8.1 (−17.9, 1.7) | .10 |
| White matter | ||||||||||
| Peak height | 102 (1.9) | 106 (2.3) | 109 (2.2) | 3.3 (0.3, 6.3) | .03 | 103 (2.3) | 107 (1.9) | 107 (2.2) | 2.3 (−0.7, 5.3) | .13 |
| FA | 243 (2.3) | 245 (2.1) | 245 (2.4) | 0.9 (−2.3, 4.0) | .59 | 242 (2.2) | 246 (2.4) | 245 (2.2) | 2.1 (−1.1, 5.2) | .19 |
| MD | 1014 (7.4) | 1011 (6.8) | 1002 (7.4) | −6.0 (−15.0, 3.0) | .19 | 1011 (7.1) | 1006 (7.2) | 1008 (7.3) | −7.3 (−16.2, 1.6) | .11 |
| AxD | 1247 (6.9) | 1248 (6.5) | 1237 (6.9) | −5.1 (−13.4, 3.3) | .24 | 1246 (6.7) | 1242 (6.7) | 1244 (6.9) | −6.4 (−14.6, 1.9) | .13 |
| RD | 897 (7.8) | 893 (7.1) | 884 (7.8) | −6.4 (−15.9, 3.1) | .19 | 894 (7.4) | 888 (7.6) | 890 (7.6) | −7.8 (−17.2, 1.6) | .10 |
Abbreviations: AxD, axial diffusivity mm2/s x106; FA, fractional anisotropy value x103; MD, mean diffusivity mm2/s x106; peak height, normalized magnetization transfer ratio peak height value x103; RD, radial diffusivity mm2/s x106. Missing values: n=5 for MTR peak height, n=15 for FA, MD, AxD, and RD. Data are presented as mean (standard error) and β (per increase in blood pressure group or tertile) (95% confidence interval [CI]), adjusted for age, sex, and duration of antihypertensive treatment. Bold values indicate significance.
The additional adjustments for diabetes, to explore whether associations could be affected by higher percentage of persons with diabetes in the subgroup having lower SBP, did not alter associations. SBP and DBP were still significantly associated with gray matter MTR peak height (β=2.4, [95% CI, 0.6–4.2] P=.01 and B=2.3 [95% CI, 0.4–4.2] P=.02, respectively). Whereas, MAP was still significantly associated with gray matter MTR peak height, MD, and RD in the gray matter (β=2.5 [95% CI, 0.7–4.3] P=.01, β=−10.7 [95% CI, −21.0 to −0.4] P=.04, and β=−10.8 [95% CI, −20.8 to −0.9] P=.03, respectively) and with white matter MTR peak height (β=3.4 [95% CI, 0.4–6.5] P=.03).
Modeling SBP in low BP groups and the analyses performed with quadratic terms of BP measures confirmed a linear relation. The effect size of the low clinical SBP categories and gray matter MTR peak height was β=2.2 (95% CI, −0.6 to 5.0), which was comparable to the associations found in the entire group; however, because of the small numbers in the low SBP groups (<120 mm Hg: n=23; 120–129 mm Hg: n=24; and 130–140 mm Hg: n=42), this association was not statistically significant. Quadratic terms of continuous SBP, DBP, MAP, and PP were all not statistically significant.
Figure 1 shows forest plots of all BP measures and MTR peak height, FA, MD, AxD, and RD in gray matter. The direction of all of the associations is similar and indicates that SBP, DBP, and MAP are significantly associated with lower gray matter MTR peak height and lower MAP with higher MD and RD in the gray matter.
To investigate potential preferential focal associations between BP measures and MTR in cortical gray matter, VBM analysis was performed. Results of these voxel‐based analyses are shown in Figure 2, indicating that mainly lower SBP, DBP, and MAP were statistically significantly associated with a decrease of MTR in cortical gray matter with a symmetrical diffuse pattern in both hemispheres, with a slight preference for the frontal lobe. For PP, no cortical areas that demonstrated a significant association with MTR was found.
Figure 2.
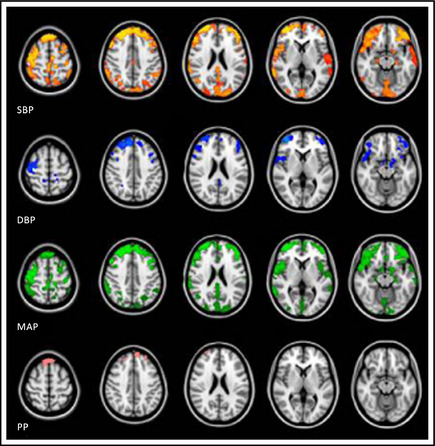
Voxel‐based analyses of blood pressure measures associated with cortical gray matter magnetization transfer ratio (MTR). Results are projected on the MNI152 standard space image. Areas show statistically significant associations (P<.05) of lower blood pressure with a decrease in gray matter MTR (adjusted for age and sex). SBP indicates systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure.
Discussion
In older persons with mild cognitive impairment using antihypertensive medication, BP measures were not associated with WMHs, lacunar infarcts, or microbleeds. However, significant associations were present for SBP, DBP, and MAP with MTR peak height, mean and/or radial diffusivity, and MTR in cortical gray matter, indicating that lower BP was associated with poorer gray matter microstructural integrity.
In line with our results, results of the Rotterdam Scan Study showed no association of increasing SBP or DBP with increasing WMH in persons older than 74 years, whereas in persons up to 74 years such associations existed.1, 22 Even though data from the Framingham Heart Study showed an association of increasing SBP and DBP with accumulating white matter microstructural damage in healthy young adults,21 and it has been shown that in middle‐aged persons with signs of small vessel disease, a linear relationship between SBP and DBP levels (per 10 mm Hg) and white matter microstructural damage was present.18 Our data indicate that, besides no associations of BP with manifest vascular white matter damage, more sensitive techniques to pick up ischemic changes, DTI and MTI, did not reveal any association of BP (SBP, DBP, MAP, or PP) with white matter damage.
In contrast, our data show that BP measures were mainly associated with gray matter integrity. Rather than higher BP, lower SBP, DBP, and MAP were all associated with gray matter tissue damage, reflected by the lower MTR peak height values, higher MD and RD in gray matter, and lower MTR in cortical gray matter. No associations were found between BP measures and FA and AxD, as these predominantly represent axonal and myelin integrity in the white matter related to preferred diffusion directionality. The MD and RD parameters are indicators for both white and gray matter microstructure, and it has been suggested that these are higher in damaged gray matter tissue as a result of increased free diffusion.41, 42, 43
The findings of lower BP with subtle gray matter damage fit the observation of the diminished or even reversed detrimental effect of elevated BP in older persons. It is plausible that in our study population of older persons with hypertension, cerebral autoregulation has become impaired by arteriosclerotic damage in such a way that it no longer compensates for reduced cerebral blood flow. While previous studies have shown that white matter is more sensitive than gray matter for hypoperfusion,44 our data in a sample of older persons with hypertension show that gray rather than white matter integrity is associated with low BP, indicating that there may be a difference in hemodynamic physiology between gray and white matter. On the other hand, a second explanation for our findings cannot exclude the possibility that lower gray matter microstructural integrity may have been the cause rather than the consequence of low BP and cerebrovascular homeostasis.
Study Limitations
The stringent selection criteria resulted in a group of relatively healthy older people, which limits the generalizability of our findings. In addition, due to the cross‐sectional design of our study, no causal inference can be made; therefore, future studies are necessary to elucidate whether low BP precedes gray matter integrity in older persons or vice versa.
Conclusions
Our data show that in our population of older persons, lower BP is associated with subtle cerebral ischemic changes specifically in the gray matter. These findings imply that in older persons with mild cognitive dysfunction using antihypertensive medication, not only upper thresholds of BP values, but preferably lower thresholds, should be observed.
Funding
The DANTE Study Leiden was funded by a grant from the ZonMW, the Netherlands Organization for Health Research and Development, Program Priority Medicines for the Elderly, grant number 40‐41600‐98‐9014.
Disclosures
The authors declare no conflicts of interest.
Acknowledgments
We thank all of the DANTE Study Leiden co‐investigators.
J Clin Hypertens (Greenwich). 2015;17:630–637. DOI: 10.1111/jch.12550. © 2015 Wiley Periodicals, Inc.
References
- 1. Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population‐based study: the Rotterdam Study. Neurology. 1994;44:1246–1252. [DOI] [PubMed] [Google Scholar]
- 2. van Dijk EJ, Breteler MM, Schmidt R, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension. 2004;44:625–630. [DOI] [PubMed] [Google Scholar]
- 3. Dufouil C, de Kersaint‐Gilly A, Besancon V, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56:921–926. [DOI] [PubMed] [Google Scholar]
- 4. Verhaaren BF, Vernooij MW, de Boer R, et al. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61:1354–1359. [DOI] [PubMed] [Google Scholar]
- 5. de Leeuw FE, De Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. [DOI] [PubMed] [Google Scholar]
- 6. Gouw AA, van der Flier WM, Fazekas F, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3‐year period: the Leukoaraiosis and Disability study. Stroke. 2008;39:1414–1420. [DOI] [PubMed] [Google Scholar]
- 7. Hiroki M, Miyashita K. Linear hyperintensity objects on magnetic resonance imaging related to hypertension. Cerebrovasc Dis. 2001;11:164–168. [DOI] [PubMed] [Google Scholar]
- 8. Liao D, Cooper L, Cai J, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke. 1996;27:2262–2270. [DOI] [PubMed] [Google Scholar]
- 9. van Swieten JC, Geyskes GG, Derix MM, et al. Hypertension in the elderly is associated with white matter lesions and cognitive decline. Ann Neurol. 1991;30:825–830. [DOI] [PubMed] [Google Scholar]
- 10. Vlek AL, Visseren FL, Kappelle LJ, et al. Blood pressure and white matter lesions in patients with vascular disease: the SMART‐MR study. Curr Neurovasc Res. 2009;6:155–162. [DOI] [PubMed] [Google Scholar]
- 11. Dozono K, Ishii N, Nishihara Y, Horie A. An autopsy study of the incidence of lacunes in relation to age, hypertension, and arteriosclerosis. Stroke. 1991;22:993–996. [DOI] [PubMed] [Google Scholar]
- 12. Longstreth WT Jr, Bernick C, Manolio TA, et al. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. [DOI] [PubMed] [Google Scholar]
- 13. Mast H, Thompson JL, Lee SH, et al. Hypertension and diabetes mellitus as determinants of multiple lacunar infarcts. Stroke. 1995;26:30–33. [DOI] [PubMed] [Google Scholar]
- 14. Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, et al. Higher ambulatory blood pressure relates to new cerebral microbleeds: 2‐year follow‐up study in lacunar stroke patients. Stroke. 2013;44:978–983. [DOI] [PubMed] [Google Scholar]
- 15. Henskens LH, van Oostenbrugge RJ, Kroon AA, et al. Brain microbleeds are associated with ambulatory blood pressure levels in a hypertensive population. Hypertension. 2008;51:62–68. [DOI] [PubMed] [Google Scholar]
- 16. van Es AC, van der Grond J, de Craen AJ, et al. Risk factors for cerebral microbleeds in the elderly. Cerebrovasc Dis. 2008;26:397–403. [DOI] [PubMed] [Google Scholar]
- 17. van Dooren M, Staals J, de Leeuw PW, et al. Progression of brain microbleeds in essential hypertensive patients: a 2‐year follow‐up study. Am J Hypertens. 2014;27:1045–1051. [DOI] [PubMed] [Google Scholar]
- 18. Gons RA, de Laat KF, van Norden AG, et al. Hypertension and cerebral diffusion tensor imaging in small vessel disease. Stroke. 2010;41:2801–2806. [DOI] [PubMed] [Google Scholar]
- 19. Gons RA, van Oudheusden LJ, de Laat KF, et al. Hypertension is related to the microstructure of the corpus callosum: the RUN DMC study. J Alzheimers Dis. 2012;32:623–631. [DOI] [PubMed] [Google Scholar]
- 20. Maclullich AM, Ferguson KJ, Reid LM, et al. Higher systolic blood pressure is associated with increased water diffusivity in normal‐appearing white matter. Stroke. 2009;40:3869–3871. [DOI] [PubMed] [Google Scholar]
- 21. Maillard P, Seshadri S, Beiser A, et al. Effects of systolic blood pressure on white‐matter integrity in young adults in the Framingham Heart Study: a cross‐sectional study. Lancet Neurol. 2012;11:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Dijk EJ, Prins ND, Vrooman HA, et al. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39:2712–2719. [DOI] [PubMed] [Google Scholar]
- 23. Leonardi‐Bee J, Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–1320. [DOI] [PubMed] [Google Scholar]
- 24. Reshef S, Fried L, Beauchamp N, et al. Diastolic blood pressure levels and ischemic stroke incidence in older adults with white matter lesions. J Gerontol A Biol Sci Med Sci. 2011;66:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molander L, Lovheim H, Norman T, et al. Lower systolic blood pressure is associated with greater mortality in people aged 85 and older. J Am Geriatr Soc. 2008;56:1853–1859. [DOI] [PubMed] [Google Scholar]
- 26. Birns J, Markus H, Kalra L. Blood pressure reduction for vascular risk – is there a price to be paid? Stroke. 2005;36:1308–1313. [DOI] [PubMed] [Google Scholar]
- 27. Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev. 2009;CD000028. [DOI] [PubMed] [Google Scholar]
- 28. Altmann‐Schneider I, van der Grond J, Slagboom PE, et al. Lower susceptibility to cerebral small vessel disease in human familial longevity: the Leiden Longevity Study. Stroke. 2013;44:9–14. [DOI] [PubMed] [Google Scholar]
- 29. Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–S186. [DOI] [PubMed] [Google Scholar]
- 30. Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross‐sectional brain change analysis. NeuroImage. 2002;17:479–489. [DOI] [PubMed] [Google Scholar]
- 31. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. [DOI] [PubMed] [Google Scholar]
- 32. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 33. Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. [DOI] [PubMed] [Google Scholar]
- 34. Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage. 2007;34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. [DOI] [PubMed] [Google Scholar]
- 37. van den Bogaard SJ, Dumas EM, Milles J, et al. Magnetization transfer imaging in premanifest and manifest Huntington disease. AJNR Am J Neuroradiol. 2012;33:884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Buchem MA, McGowan JC, Kolson DL, et al. Quantitative volumetric magnetization transfer analysis in multiple sclerosis: estimation of macroscopic and microscopic disease burden. Magn Reson Med. 1996;36:632–636. [DOI] [PubMed] [Google Scholar]
- 39. Andersson M, Jenkinson M, Smith S. Non‐linear registration FMRIB technical report TR07JA2. 2007.
- 40. Cosottini M, Pesaresi I, Piazza S, et al. Magnetization transfer imaging demonstrates a distributed pattern of microstructural changes of the cerebral cortex in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol. 2011;32:704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soares JM, Marques P, Alves V, Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front Neurosci. 2013;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jeon T, Mishra V, Uh J, et al. Regional changes of cortical mean diffusivities with aging after correction of partial volume effects. NeuroImage. 2012;62:1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. [DOI] [PubMed] [Google Scholar]


