Abstract
Resistant hypertension (RHT) is an important disease that causes an increase in cardiovascular risk, yet its etiology remains unclear. The authors aimed to investigate neutrophil/lymphocyte ratio (NLR) as an inflammation marker in patients with RHT. A total of 150 patients were included in the study and grouped according to their office and ambulatory blood pressure measurements. They were classified as having normotension (NT), controlled hypertension (CHT), or RHT. The RHT group had a significantly higher NLR than the CHT group (P=.03), and NLRs of both hypertension groups were significantly higher than those in the NT group (P<.001, for both). NLR and neutrophil count were found to be independent correlates for RHT in multivariate analysis (P<.001). NLR and neutrophil count are increased in RHT patients than both CHT and NT patients. This finding, which is defined for the first time in patients with RHT, may imply the importance of inflammation in blood pressure control.
Resistant hypertension (RHT) is an important contributor to increased cardiovascular risk all over the world and is associated with increased stroke, myocardial infarction, and heart and renal failure.1, 2 It is defined as not achieving target blood pressure (BP) despite using three antihypertensive drugs at their optimal dosages with one being a diuretic or achieving target BP using three or more antihypertensive drugs.3 Its prevalence is between 3% and 30%. While its frequency and consequences are well‐known, there is limited information about its etiology. With some secondary causes, 90% of hypertension (HT) is essential and thought to have multifactorial etiology. There is some literature about the role of inflammation in the pathophysiology of HT. Inflammatory markers such as interleukin (IL) 6, IL‐1b, and tumor necrosis factor α (TNF‐α) are associated with HT.4, 5, 6 Neutrophil/lymphocyte ratio (NLR) is a cost‐effective, easily applicable, and reproducible inflammatory marker used in our clinical practice.7 The relationship between neutrophil count, cardiovascular risk8 and development of HT9 is known. Although the available drug range and invasive methods for the treatment of this entity is improving, it is essential to research more easily detectable and correctable factors that play a role in RHT. In this study, we aimed to investigate NLR and neutrophil count as inflammatory markers in patients with RHT and compare our findings with patients with controlled HT (CHT) and normotension (NT).
Methods
The study group consisted of 150 patients who were recruited from the outpatient clinic of Okmeydanı Training and Research Hospital between September 2013 and September 2014. Patients were classified as having RHT (n=50), CHT (n=50), or NT (n=50) according to their BP measurements. All participants gave written informed consent to participate in the study, which was approved by the institutional ethics committee according to the Declaration of Helsinki (approval number/date: 199/20.05.2013).
The following data were collected: sociodemographic characteristics, medication history (including nonsteroidal anti‐inflammatory drug, steroid, and oral contraceptive use), cardiovascular risk factors, complications of HT, other comorbidities, history of smoking, alcohol consumption, and family history. Height and body weight were measured to calculate body mass index (BMI). Antihypertensive drugs currently used by the patients were recorded according to their classes. Patients' compliance to current treatment was evaluated by patient self‐report, report of the family members, and pill count.
Secondary HT was excluded by means of clinical and biochemical assessment. Additionally, patients with the following characteristics were excluded from the study: heart failure, coronary artery disease, moderate and severe valvular heart disease, diabetes mellitus, peripheral artery disease, stroke, chronic kidney and liver disease, inflammatory disease, thromboembolic and hematological disease,; history of smoking, recent history of infectious disease or clinical evidence of infection, medication that could affect BP and potentially interfere with NLR (oral contraceptive drugs, anti‐inflammatory drugs), abnormality in thyroid function tests, and white blood cell count >12,000 or <4000 cells/μL. In addition, obese patients (BMI ≥30 kg/m²) and pregnant women were not included in the study group.
BP Measurements
Office BP was measured after 5 minutes of rest in a quiet environment with a mercury sphygmomanometer with the patient in a sitting position. Systolic BP (SBP) and diastolic BP (DBP) (Korotkoff phase I and phase V, respectively) represented the mean of three different readings measured at 5‐minute intervals. According to inclusion criteria, since patients were not diabetic and had normal renal function, normal office BP was defined as an SBP <140 mm Hg and a DBP <90 mm Hg. Patients were categorized into RHT, CHT, and NT groups. RHT was defined as having suboptimal BP despite using three antihypertensive agents inclusive of a diuretic, and patients who needed four or more drugs to control BP.3 Patients using up to three antihypertensive drugs with normal BP measurements were included in the CHT group. After office BP measurements, patients also underwent ambulatory BP measurement (ABPM) in order to detect white‐coat and masked HT. A commercially available monitor (CONTEC PM50; Contec Medical Systems, Hebei, China) was used. BP measurements were performed at 15‐minute intervals between 7 am and 11 pm and at 30‐minute intervals between 11 pm and 7 am. A mean ambulatory daytime BP of 135/85 mm Hg was considered elevated.10 Patients not using any antihypertensive medication and with normal office and ABPM constituted the NT group.
Laboratory Evaluation
Blood samples were withdrawn from an antecubital vein, with atraumatic venipuncture, in the morning after a 12‐hour fasting period. NLR was calculated by absolute neutrophil count divided by absolute lymphocyte count. In addition, serum total cholesterol, low‐density lipoprotein‐cholesterol, high‐density lipoprotein‐cholesterol, triglycerides, glucose, creatinine, and C‐reactive protein (CRP) were measured.
Statistical Analysis
SPSS 17.0 for Windows software package (SPSS Inc, Chicago, IL) was used in all analyses. Analysis of normality was performed with the Kolmogorov‐Smirnov test. Because CRP levels were not normally distributed, logarithmic transformation was performed. The continuous variables were expressed as mean±standard deviation and median (interquartile range) as appropriate and categorical variables were expressed as frequencies and percentages. Chi‐square test was used to compare categorical variables between groups. Analysis of variance tests were used for the comparison of continuous variables between the three groups followed by Tukey test. The relationship between NLR and other continuous variables were assessed by Pearson's correlation coefficients. Multivariate logistic regression analysis was performed to determine independent predictors of RHT. Variables with a P value <.1 in univariate analyses and those considered to be of clinical importance were included in the model. A two‐sided P value <.05 was considered significant within a 95% confidence interval (CI).
Results
A total of 150 patients (56.6% women, 61.3±8 years) were included in the study. Each group (NT, CHT, and RHT) consisted of 50 patients. Clinical and biochemical characteristics of the study population by groups are outlined in Table 1 and Table 2. There was no significant difference found among groups in terms of age, sex, or BMI (Table 1).
Table 1.
General Characteristics of Normotensive, Controlled Hypertensive, and Resistant Hypertensive Patients
| NT (n=50) | CHT (n=50) | RHT (n=50) | P Value | |
|---|---|---|---|---|
| Women, % | 27 (54) | 30 (60) | 28 (56) | .827 |
| Age, y | 61.2±7 | 62.06±8 | 60.8±8 | .744 |
| BMI, kg/m2 | 26.8±2.8 | 27.4±2.6 | 27.01±2.9 | .524 |
| SBP (office), mm Hg | 125.8±6.2 | 126.5±6.8 | 137.7±10.8 | <.001 |
| DBP (office), mm Hg | 71.6±8.2 | 72.5±9.5 | 88.5±14.1 | <.001 |
| SBP (ABPM), mm Hg | 118.2±5.4 | 124.3±5.2 | 132.3±11.4 | .021 |
| DBP (ABPM), mm Hg | 70.8±6.3 | 74.3±8.1 | 83.6±12.1 | .013 |
| ACE inhibitor, % | 34 (68) | 39 (78) | .184 | |
| ARB, % | 16 (32) | 28 (56) | .013 | |
| β‐Blocker, % | 18 (36) | 30 (60) | .014 | |
| CCB, % | 20 (40) | 29 (58) | .191 | |
| α‐Blocker, % | 6 (12) | 13 (26) | .062 | |
| Diuretic, % | 17 (34) | 50 (100) | <.001 | |
| Statin, % | 9 (18) | 7 (14) | .393 |
Abbreviations: ABPM, ambulatory blood pressure measurement; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; CHT, controlled hypertension; DBP, diastolic blood pressure; NT, normotension; RHT, resistant hypertension; SBP, systolic blood pressure. Data are expressed in frequencies (percentages) or mean±standard deviation. Bold values indicate significance.
Table 2.
Biochemical Parameters of Normotensive, Controlled Hypertensive, and Resistant Hypertensive Patients
| NT (n=50) | CHT (n=50) | RHT (n=50) | P Value | |
|---|---|---|---|---|
| Glucose, mg/dL | 82.2±11.3 | 83.1±11.4 | 80.3±10 | .456 |
| Creatinine, mg/dL | 0.85±0.11 | 0.85±0.12 | 0.84±0.09 | .777 |
| eGFR (MDRD), mL/min/1.73 m2 | 87.5±7.2 | 85.6±5.7 | 89.3±6.6 | .951 |
| Phosphate, mg/dL | 3.5±0.6 | 3.3±0.7 | 3.4±0.6 | .412 |
| Calcium, mg/dL | 8.7±0.7 | 8.4±0.6 | 8.6±0.7 | .096 |
| CRP, mg/L | 3.7 (1.23) | 4.9 (3.28) | 4.95 (3.05) | <.001 |
| Triglycerides, mg/dL | 142±34 | 151±41 | 139±38 | .432 |
| HDL‐C, mg/dL | 46.5±9 | 45.3±11.6 | 49.7±10.5 | .387 |
| LDL‐C, mg/dL | 120±29 | 127±28 | 122±24 | .491 |
| Cholesterol, mg/dL | 203.7±50.3 | 207.8±43.4 | 193.8±31.2 | .673 |
| Neutrophils, mm3 | 5297±978 | 6075±993 | 6670±872 | <.001 |
| Lymphocytes, mm3 | 2154±435 | 2024±407 | 1920±376 | .017 |
| Leukocytes, mm3 | 6098±504 | 6528±646 | 7978±1014 | .001 |
| Monocytes, mm3 | 508.7±51 | 596±107 | 601.5±122 | .131 |
| NLR | 1.87±0.35 | 2.11±0.39 | 3.15±0.63 | <.001 |
| RDW | 15.76±0.91 | 15.82±0.81 | 16.01±0.83 | .54 |
Abbreviations: CHT, controlled hypertension; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MDRD, Modification of Diet in Renal Disease; NLR, neutrophil/lymphocyte ratio; NT, normotension; RDW, red cell distribution width; RHT, resistant hypertension. Variables are expressed as mean±standard deviation and median (interquartile range) as appropriate. Bold values indicate significance.
Office and ambulatory systolic and diastolic BP measurements showed significant differences between groups (P<.05, for all) (Table 1). RHT patients were taking β‐blocker, angiotensin receptor blocker, and diuretic classes of antihypertensive drugs more than the CHT group (P<.05, for all). No significant difference was observed in other classes of antihypertensive drugs between groups (Table 1). Mean antihypertensive drug use was 1.72±0.7 in the CHT group and 3.9±0.4 in the RHT group.
As shown in Table 2, glucose, creatinine, estimated glomerular filtration rate, and electrolyte and lipid levels were similar between groups. CRP levels in patients with RHT and CHT (4.95 mg/L and 4.9 mg/L, respectively) were significantly higher than patients with NT (3.7 mg/L) (P<.001). Patients in the RHT group had significantly higher NLR than that in the CHT group (P=.03), and NLRs of both hypertensive groups were significantly higher than the NT group (P<.001, for both) (Figure). Similarly, neutrophil count was found to be significantly higher in the RHT group (6670±872 mm3) than in the CHT group (6075±993 mm3) (P=.006), and both hypertensive groups had significantly higher neutrophil counts than the NT group (5297±978 mm3) (P<.001, for both). Leukocyte count was higher in the RHT group (7978±1014 mm3) than the other groups (P=.001, for both), while no significant difference was observed between the CHT (6528±646 mm3) and NT (6098±504 mm3) groups. Besides being significantly lower than in the NT group (2154±435 mm3) (P=.017), the lymphocyte count in the RHT group (1920±376 mm3) showed no difference from that in CHT group (2024±407 mm3). Red cell distribution width did not significantly differ between groups (Table 2).
Figure 1.
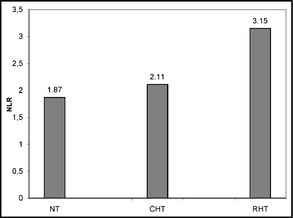
Neutrophil lymphocyte ratios (NLR) of normotension (NT), controlled hypertension (CHT) and resistant hypertension (RHT) groups.
NLR correlated positively with office SBP, office DBP, and ambulatory DBP measurements (Table 3).
Table 3.
Correlates of the NLR
| Variable | r | P Value |
|---|---|---|
| Age, y | 0.04 | .628 |
| BMI, kg/m2 | 0.007 | .936 |
| CRP, mg/L | 0.126 | .123 |
| SBP (office), mm Hg | 0.422 | <.001 |
| DBP (office), mm Hg | 0.497 | <.001 |
| SBP (ABPM), mm Hg | 0.566 | .112 |
| DBP (ABPM), mm Hg | 0.706 | .01 |
| eGFR, mL/min/1.73 m2 | −0.123 | .626 |
| Glucose, mg/dL | −0.094 | .253 |
| Creatinin, mg/dL | −0.01 | .9 |
| Calcium, mg/dL | −0.035 | .669 |
| Phosphate, mg/dL | 0.017 | .839 |
Abbreviations: ABPM, ambulatory blood pressure measurement; BMI, body mass index; CRP, C‐reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; NLR, neutrophil/lymphocyte ratio; SBP, systolic blood pressure. Bold values indicate significance.
In multivariate analysis, NLR and neutrophil count were found to be independent predictors of RHT (Table 4).
Table 4.
Independent Predictors of Resistant Hypertension in Multivariate Logistic Regression Analysis
| Variable | β | 95% CI | P Value |
|---|---|---|---|
| Age, y | 0.960 | 0.875–1.053 | .385 |
| Sex | 0.618 | 0.163–2.333 | .478 |
| BMI, kg/m2 | 1.001 | 0.826–1.243 | .991 |
| CRP, mg/L | 1.137 | 0.800–1.614 | .475 |
| Neutrophils, mm3 | 1.002 | 0.993–1.007 | <.001 |
| Lymphocytes, mm3 | 1 | 0.998–1.002 | .878 |
| NLR | 11.91 | 11.68–12.15 | <.001 |
| eGFR, mL/min/1.73 m2 | 0.980 | 0.834–1.151 | .803 |
Abbreviations: BMI, body mass index; CI, confidence interval; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; NLR, neutrophil/lymphocyte ratio. Bold values indicate significance.
Discussion
The analysis of our data demonstrated, for the first time, that neutrophil count and NLR were significantly higher in the RHT group than in the CHT group and that hypertensive patients had higher levels than NT patients. Lymphocyte count in the RHT group was found to be significantly lower than that in the NT group.
It is known that high white blood cell count11, 12 and NLR13 are associated with increased cardiovascular risk. However, the mechanism of increased leukocyte activation in patients with HT14 is not clear. Autologous and heterologous plasma incubation studies pointed out factors such as angiotensin and some hereditary metabolic components.15 Enhanced leukocyte activation and cytokine production causes endothelial dysfunction by aggravating oxidative stress and inflammation. Direct endothelial damage as a result of inflammation was also defined regardless of vascular damage caused by HT.16
The relationship between inflammatory markers and HT was observed in epidemiological studies.17 Cross‐sectional studies showed that there is a positive correlation between BP, CRP, and IL‐6 levels.18, 19 CRP and TNF‐α levels and leukocyte count were found to be higher even in patients with slightly elevated BP and pre‐HT than normotensive patients.20 In addition, in a study of 508 apparently healthy people, there was a significant relationship between BP and intercellular adhesion molecule 1 and IL‐6, taking into account the effect of age and other cardiovascular risk factors.21 Abramson and colleagues22 demonstrated that in healthy normotensive people, a 10‐mm Hg increase in pulse pressure was associated with a 15% increase in the odds of having an elevated CRP level, meaning inflammation and BP had a positive correlation even within normal limits. Cohort studies revealed similar results. In the Women's Health Study, with a 7.8 years of mean follow‐up, HT developed in 5365 of 20,525 patients and it was shown that CRP levels played an important role in this process.23 Tatsukawa and colleagues9 published a 40‐year follow‐up study of 9383 patients and concluded that high white blood cell count, especially neutrophil count, is an important risk factor in the development of HT. Natalia and colleagues studied IL‐1β and IL‐10 levels in patients with RHT (n=32), CHT (n=20), and NT (n=20). Levels of both were found to be higher in the RHT group than in the CHT and NT groups.24 In a study of African Americans, a significant correlation was observed between BP regulation, HT development, and high neutrophil and low lymphocyte counts.25 Similarly, Kawada and colleagues26 defined a relationship between neutrophil count and HT independent of other factors. In this study, consistent with the aforementioned studies, NLR and neutrophil count were shown to be higher in the RHT group than in the CHT and NT groups with similar characteristics. In addition, CRP levels were higher in the RHT and CHT groups than in the NT group. These results, defined in patients with RHT for the first time, may imply increased progression to RHT, use of more antihypertensive drugs, and employment of invasive procedures in the treatment of HT caused by inflammation.
The first studies about the subject defined the relationship between HT and leukocyte count independent of BMI, smoking, and triglyceride level.27 Contrary to these findings, the PIUMA study found that leukocyte count was related to smoking and BMI.28 Current data reveal a relationship between NLR and RHT in a nonsmoking study group with similar BMI and lipid levels. Antihypertensive drug classes have different anti‐inflammatory effects shown by their power to decrease CRP levels. Angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, and (less pronounced) calcium channel blockers also have some anti‐inflammatory effects.29 Fulop and colleagues30 showed that renin‐angiotensin‐aldosterone system inhibitors were more effective in reducing CRP levels than other classes in a study of 662 hypertensive patients taking single‐agent antihypertensive treatment. Despite use of these classes of antihypertensives in the RHT group in our study, NLR and CRP levels were found to be higher than in the NT group. These findings may indicate the clinical importance of inflammation in the pathophysiology of HT. Clinical benefits of these known effects of antihypertensive drugs on inflammation is not clear. Therefore, beyond medication, modification of traditional risk factors that affect inflammation such as BMI, smoking, and sedentary lifestyle becomes more of an issue in not only the control of HT and inflammation but also total cardiovascular risk.
Red cell distribution width was also defined as a marker of inflammation shown to return to normal with the use of some antihypertensive drugs irrespective of BP levels.31 It has not been studied in RHT patients but our analysis revealed no significant difference between groups. It is known that obesity and sedentary lifestyle have adverse effects on BP. Obese people even if they do not have metabolic syndrome have increased leukocyte count, with physical activity showing a lowering effect.32 Additional inflammation is not only related to BP but is also involved in the development of target organ damage caused by HT.33, 34 A decrease in inflammation assessed by CRP levels predicts decrease in target organ damage.35 While high‐sensitivity CRP level measurements can lead to increased costs, NLR can be easily calculated from complete blood cell count performed in nearly every patient. With this knowledge, NLR may lead physicians to put emphasis on lifestyle changes and identify patients who require closer follow‐up because of increased risks of hypertensive complication. In addition, studies examining the effects of antihypertensive drug classes on NLR can be planned to specify the treatment plans of these patients. There is evidence revealing that some anticytokine molecules targeting the immune system have favorable effects on BP and cardiovascular complications, but these results are still experimental.36
Study Limitations and Strengths
There were several limitations of the study. Although we used pill count and family member reports in addition to patient self‐report, true assessment of medication adherence is a problem that can be encountered in every RHT study. Specific inflammation markers in addition to NLR was not measured. Plasma cortisol levels were measured in the study group to exclude patients with secondary HT, but the 24‐hour urinary cortisol excretion test was not routinely performed to identify subclinical cases or to examine the effect of increased urinary glucocorticoid excretion within normal limits on NLR. There are some studies that investigated this subject but further research is needed in this area.37, 38 On the other hand, choosing nonsmoking and similar groups regarding factors that can affect leukocyte count such as age, BMI, and lipid levels strengthened our study. ABPM to eliminate white‐coat and masked HT ensured correct classification of patients between groups.
Conclusions
NLR and neutrophil count were significantly higher in RHT patients than both CHT and NT patients. Current practice against uncontrolled HT is to employ more medication and invasive procedures. With the increase of data in the literature about the effect of inflammation in uncontrolled HT, it is possible for inflammation to be targeted in the follow‐up and treatment of these patients. For this reason, more studies are needed to determine the significance of the NLR to define cardiovascular risk, especially in patients with uncontrolled HT and those in need of aggressive treatment and closer follow‐up. In addition, the effects of antihypertensive drug classes on NLRs should be clarified with larger‐scale studies.
Acknowledgment
The authors declare that there are no financial or any other factors that may have led to a conflict of interest.
J Clin Hypertens (Greenwich). 2015;17:532–537. DOI: 10.1111/jch.12533. © 2015 Wiley Periodicals, Inc.
References
- 1. Lawes CMM, Hoorn SV, Rodgers A. Global burden of blood pressure related disease. Lancet. 2001;371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 2. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 3. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:510–526. [DOI] [PubMed] [Google Scholar]
- 4. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46:1118–1122. [DOI] [PubMed] [Google Scholar]
- 5. Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C‐reactive protein, interleukin‐6, and TNF‐alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. [DOI] [PubMed] [Google Scholar]
- 6. Mauno V, Hannu K, Esko K. Proinflammation and hypertension: a population‐based study. Mediators Inflamm. 2008;2008:619–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azab B, Chainani V, Shah N, McGinn JT. Neutrophil‐lymphocyte ratio as a predictor of major adverse cardiac events among diabetic population: a 4‐year follow‐up study. Angiology. 2013;64:456–465. [DOI] [PubMed] [Google Scholar]
- 8. Sabatine MS, Morrow DA, Cannon CP, et al. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICSTIMI 18 (treat angina with aggrastat and determine cost of therapy with an invasive or conservative strategy‐trombolysis myocardial infarction 18) substudy. J Am Coll Cardiol. 2002;40:1761–1768. [DOI] [PubMed] [Google Scholar]
- 9. Tatsukawa Y, Hsu WL, Yamada M, et al. White blood cell count, especially neutrophil count, as a predictor of hypertension in a Japanese population. Hypertens Res. 2008;31:1391–1397. [DOI] [PubMed] [Google Scholar]
- 10. Pickering TG, Hall JE, Appel LJ, et al. Recommendations of blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans. A Statement for Professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 11. Gillum RF, Mussolino ME. White blood cell count and hypertension incidence. The NHANES I Epidemiologic Follow‐up Study. J Clin Epidemiol. 1994;47:911–919. [DOI] [PubMed] [Google Scholar]
- 12. Margolis KL, Manson JE, Greenland P, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women's Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. [DOI] [PubMed] [Google Scholar]
- 13. Shen XH, Chen Q, Shi Y, Li HW. Association of neutrophil/lymphocyte ratio with long‐term mortality after ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Chin Med J. 2010;123:3438–3443. [PubMed] [Google Scholar]
- 14. Schmid‐Schonbein GW, Seiffge D, DeLano FA, et al. Leukocyte counts and activation in spontaneously hypertensive and normotensive rats. Hypertension. 1991;17:323–330. [DOI] [PubMed] [Google Scholar]
- 15. Kristal B, Shurtz‐Swirski R, Chezar J, et al. Participation of peripheral polymorphonuclear leukocytes in the oxidative stress and inflammation in patients with essential hypertension. Am J Hypertens. 1998;11:921–928. [DOI] [PubMed] [Google Scholar]
- 16. Rizzoni D, Porteri E, Castellano M, et al. Endothelial dysfunction in hypertension is independent from the etiology and from vascular structure. Hypertension. 1998;31:335–341. [DOI] [PubMed] [Google Scholar]
- 17. Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrol Dial Transplant. 2006;21:850–853. [DOI] [PubMed] [Google Scholar]
- 18. Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. [DOI] [PubMed] [Google Scholar]
- 19. Yamada S, Gotoh T, Nakashima Y, et al. Distribution of serum C‐reactive protein and its association with atherosclerotic risk factors in a Japanese population: Jichi Medical School Cohort Study. Am J Epidemiol. 2001;153:1183–1190. [DOI] [PubMed] [Google Scholar]
- 20. Chrysohoou C, Pitsavos C, Panagiotakos DB, et al. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: the ATTICA Study. Am J Hypertens. 2004;17:568–573. [DOI] [PubMed] [Google Scholar]
- 21. Sung KC, Suh JY, Kim BS, et al. High sensitivity C‐reactive protein as an independent risk factor for essential hypertension. Am J Hypertens. 2003;16:429–433. [DOI] [PubMed] [Google Scholar]
- 22. Abramson JL, Weintraub WS, Vaccarino V. Association between pulse pressure and C‐reactive protein among apparently healthy US adults. Hypertension. 2002;39:197–202. [DOI] [PubMed] [Google Scholar]
- 23. Lakoski SG, Cushman M, Palmas W, et al. The relationship between blood pressure and C‐reactive protein in the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2005;46:1869–1874. [DOI] [PubMed] [Google Scholar]
- 24. Barbaro NR, Fontana V, Modolo R, et al. Increased arterial stiffness in resistant hypertension is associated with inflammatory biomarkers. Blood Press. 2014;25:1–7. [DOI] [PubMed] [Google Scholar]
- 25. Tian N, Penman AD, Mawson AR, et al. Association between circulating specific leukocyte types and blood pressure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Hypertens. 2010;4:272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawada T, Morihashi M, Ueda H, Sirato T. Neutrophil cell count is related to hypertension in workers: a cross‐sectional study. Vasc Dis Prev. 2007;4:225–228. [Google Scholar]
- 27. Friedman GD, Selby JY, Quesenberry CP Jr. The leukocyte count: a predictor of hypertension. J Clin Epidemiol. 1990;43:907–911. [DOI] [PubMed] [Google Scholar]
- 28. Schillaci G, Pirro M, Pucci G, et al. Prognostic value of elevated white blood cell count in hypertension. Am J Hypertens. 2007;20:364–369. [DOI] [PubMed] [Google Scholar]
- 29. Prasad K. C‐reactive protein (CRP)‐lowering agents. Cardiovasc Drug Rev. 2006;24:33–50. [DOI] [PubMed] [Google Scholar]
- 30. Fulop T, Rule AD, Schmidt DW, et al. C‐reactive protein among community‐dwelling hypertensives on single‐agent antihypertensive treatment. J Am Soc Hypertens. 2009;3:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fici F, Celik T, Balta S, et al. Comparative effects of nebivolol and metoprolol on red cell distribution width and neutrophil/lymphocyte ratio in patients with newly diagnosed essential hypertension. J Cardiovasc Pharmacol. 2013;62:388–393. [DOI] [PubMed] [Google Scholar]
- 32. Ryder E, Diez‐Ewald M, Mosquera J, et al. Association of obesity with leukocyte count in obese individuals without metabolic syndrome. Diabetes Metab Syndr. 2014;8:197–204. [DOI] [PubMed] [Google Scholar]
- 33. Salles GF, Fiszman R, Cardoso CR, Muxfeldt ES. Relation of left ventricular hypertrophy with systemic inflammation and endothelial damage in resistant hypertension. Hypertension. 2007;50:723–728. [DOI] [PubMed] [Google Scholar]
- 34. Magen E, Mishal J, Paskin J, et al. Resistant arterial hypertension is associated with higher blood levels of complement C3 and C‐reactive protein. J Clin Hypertens (Greenwich). 2008;10:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 36. Liu W, Wang X, Feng W, et al. Lentivirus mediated IL‐17R blockade improves diastolic cardiac function in spontaneously hypertensive rats. Exp Mol Pathol. 2011;91:362–367. [DOI] [PubMed] [Google Scholar]
- 37. Litchfield WR, Hunt SC, Jeunemaitre X, et al. Increased urinary free cortisol: a potential intermediate phenotype of essential hypertension. Hypertension. 1998;31:569–574. [DOI] [PubMed] [Google Scholar]
- 38. Martins LC, Conceição FL, Muxfeldt ES, Salles GF. Prevalence and associated factors of subclinical hypercortisolism in patients with resistant hypertension. J Hypertens. 2012;30:967–973. [DOI] [PubMed] [Google Scholar]


