Abstract
The largest percentage of mortality from tobacco smoking is cardiovascular‐related. It is not known whether regular participation in exercise mitigates the adverse influence of smoking on vasculature. Accordingly, the authors determined whether regular aerobic exercise is associated with reduced arterial stiffness in men who smoke cigarettes. Using a cross‐sectional study design, 78 young men were studied, including sedentary nonsmokers (n=20), sedentary smokers (n=12), physically active nonsmokers (n=21), and physically active smokers (n=25). Arterial stiffness was assessed by brachial‐ankle pulse wave velocity (baPWV). There were no group differences in height, body fat, and systolic and diastolic blood pressure. As expected, both physically active groups demonstrated greater maximal oxygen consumption and lower heart rate at rest than their sedentary peers. The sedentary smokers demonstrated greater baPWV than the sedentary nonsmokers (11.8±1 m/s vs 10.6±1 m/s, P=.036). baPWV values were not different between the physically active nonsmokers and the physically active smokers (10.8±1 m/s vs 10.7±1 m/s). Chronic smoking is associated with arterial stiffening in sedentary men but a significant smoking‐induced increase in arterial stiffness was not observed in physically active adults. These results are consistent with the idea that regular participation in physical activity may mitigate the adverse effects of smoking on the vasculature.
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity and accounts for 40% of all‐cause mortality in developed countries. CVD is caused by nonmodifiable risk factors (eg, genetic predisposition, age, and sex) as well as those that can be modified by lifestyle intervention such as diet, exercise, and smoking cessation.1 Cigarette smoking is one of the most preventable risk factors for CVD.2 Despite recent success in reducing the prevalence of cigarette smoking, tobacco remains one of the most important public health burdens.3 The effect of smoking on CVD appears to be mediated, at least in part, by arterial stiffening.2 Arterial stiffness is increased significantly after acute smoking,2, 4, 5 and chronic smoking is associated with increasing arterial stiffness.6, 7
Increasing physical activity is a potential strategy that can reduce harmful effects of chronic smoking.8 However, it is unknown whether regular exercise mitigates the adverse effects of smoking on large artery distensibility. The primary aim of this study was to determine whether smokers who perform regular aerobic exercise demonstrate lower arterial stiffness than their sedentary peers. To do so, we measured pulse wave velocity (PWV), a measure of arterial stiffness, in nonsmoking control groups and groups of smokers who were either physically active or not. Even though the majority of smokers remain sedentary,9, 10 the proportion of chronic smokers who perform regular physical activity is surprisingly high, comprising almost one quarter of the entire smoking population.10 We reasoned that the potentially important message would arise from this study for chronic smokers who remain physically active in spite of repeated attempts to quit smoking.
Materials and Methods
Participants
A total of 78 men (22±4 years) participated in the study. The patients were recruited through flyers, advertisements, word of mouth, and patient database. Participants were assigned to groups based on their smoking habit and reported physical activity: sedentary nonsmokers (n=20), sedentary smokers (n=12), physically active nonsmokers (n=21), and physically active smokers (n=25). Smokers had been smoking at least 8 to 10 cigarettes per day for at least 2 years preceding the study. The minimum smoking dose was implemented in order to establish the minimum amount of smoking exposure that is consistent with the smoking dose threshold for light smokers (≤10 cigarettes per day). For at least the previous 6 months, the patients had been either sedentary (no regular physically activity) or physically active (≥2 h/wk of aerobic or endurance exercise). Exclusion criteria included established obesity (body mass index ≥30 kg/m2), hypertension (systolic blood pressure [BP] >140 mm Hg and/or diastolic BP >90 mm Hg), and apparent CVD or other chronic diseases as assessed by the medical history questionnaire.
All experimental procedures were explained to the individuals, and patients were informed of the potential risks of the study and given written informed consent to participate. All procedures were approved by the institutional review board at the University of Texas at Austin. The present study was part of a larger‐scale study to examine the modulatory influences of lifestyle factors on vascular function.
Experimental Procedures
Patients reported to the laboratory in the morning having refrained from alcoholic beverages, caffeine (including coffee, tea, and carbonated beverages), food, and smoking for at least 4 hours prior to the experiments. All testing was conducted at the same time of day among the groups. Physically active smokers were studied at least 24 hours after their last exercise session to avoid any acute effects of exercise while still being representative of their normal physiological state (ie, habitually exercising).11 Patients were studied under quiet, comfortable, and ambient laboratory (~24°C) conditions. In an attempt to eliminate investigator bias, all of vascular measurements were performed using automated devices.
Body Composition
Body composition was assessed by dual‐energy X‐ray absorptiometry (Lunar DPX; GE Medical Systems, Fairfield, CT). During the body composition test, participants laid in the supine position on a table for the scan.
Blood Pressure
BP was measured with an automatic device (VP‐1000; Omron Healthcare, Lake Forest, IL) in duplicate on the right arm after 5 minutes in the upright seated position with the arm at heart level. Carotid (central) BP was measured using applanation tonometry noninvasively placed on the carotid artery as previously described.11
Arterial Stiffness
A measure of arterial stiffness, by PWV, was determined using the automated vascular screening device (VP‐1000; Omron Healthcare) as previously described.12 Bilateral brachial and post‐tibial arterial pressure waveforms were stored for 10 seconds by extremities cuffs connected to a plethysmographic sensor and an oscillometric pressure sensor wrapped on both arms and ankles. PWV was calculated from the distance between two arterial recording sites divided by transit time. Transit time was determined from the time delay between the proximal and distal “foot” waveforms. The foot of the wave was identified as the commencement of the sharp systolic upstroke, which was automatically detected by a band‐path filter (5–30 Hz). The arterial path length was automatically determined by the machine based on the patients' height.13
Maximal Oxygen Consumption
All participants performed exercise testing on a treadmill in order to determine the maximal aerobic capacity via a modified Bruce protocol. Expired gases were sampled continuously via a mixing chamber and analyzed for the concentration of oxygen and carbon dioxide (Physio‐Dyne Instrument Corp, Quogue, NY).
Statistical Analyses
Group differences for variables were performed using one‐way analysis of variance. If a significant F value was shown, post‐hoc test (least significant difference) was used to determine significant differences among groups. All data are represented as mean±standard deviation unless stated otherwise. A significance level of α<0.05 was used to determine statistical difference. SPSS version 22 (SPSS Inc; IBM, Armonk, NY) was used for all statistical analyses.
Results
Select physical characteristics of the patients are shown in Table 1. The smokers were slightly older and had greater body mass and body mass index than the nonsmokers. As expected, both physically active groups had significantly greater maximal oxygen consumption than their sedentary peers (P<.05). Most of the physically active patients engaged in endurance exercises including running and/or cycling. The pack‐years smoking history was not different between sedentary and active smokers.
Table 1.
Select Patient Characteristics in Four Groups
| Sedentary Nonsmokers (20) | Sedentary Smokers (12) | Active Nonsmokers (21) | Active Smokers (25) | |
|---|---|---|---|---|
| Age, y | 22±3 | 26±7a | 21±2b | 23±5c |
| Height, cm | 170±5 | 173±5 | 172±5 | 172±7 |
| Body mass, kg | 64±13 | 73±10a | 65±6b | 70±9a |
| BMI, kg/m2 | 22±3 | 24±3a | 22±2b | 24±3 |
| Body fat, % | 23±9 | 25±9 | 18±4 | 22±8 |
| vo 2max, mL/kg/min | 39±10 | 35±6 | 52±4a , b | 46±8a , b , c |
| Smoking history, pack‐years | 0±0 | 9±14 | 0±0 | 5±2 |
Abbreivations: BMI, body mass index; vo 2max, maximal oxygen consumption. Data are expressed as mean±standard deviation. a P<.05 vs sedentary nonsmokers. b P<.05 vs sedentary smokers. c P<.05 vs active nonsmokers.
As shown in Table 2, heart rate at rest was significantly lower in both physically active groups than their sedentary counterparts (P<.05). There were no significant differences in brachial or carotid BP among groups.
Table 2.
Heart Rate and BP at Rest in Four Groups
| Sedentary Nonsmokers (20) | Sedentary Smokers (12) | Active Nonsmokers (21) | Active Smokers (25) | |
|---|---|---|---|---|
| Heart rate, beats per min | 68±11 | 65±9 | 55±11a , b | 53±6a , b |
| Brachial systolic BP, mm Hg | 115±10 | 118±10 | 118±9 | 117±8 |
| Brachial diastolic BP, mm Hg | 66±7 | 67±7 | 66±10 | 67±7 |
| Brachial mean BP, mm Hg | 84±7 | 84±7 | 83±10 | 86±7 |
| Carotid systolic BP, mm Hg | 98±4 | 103±11 | 104±11 | 105±12 |
| Carotid pulse pressure, mm Hg | 36±7 | 36±10 | 35±8 | 38±10 |
Abbreviation: BP, blood pressure. Data are expressed as mean±standard deviation. a P<.05 vs sedentary nonsmokers. b P<.05 vs sedentary smokers.
The Figure illustrates group differences in brachial‐ankle PWV (baPWV) among the 4 groups. baPWV was significantly elevated in sedentary smokers compared with patients in the other groups. There was no significant group difference between active nonsmokers and active smokers.
Figure 1.
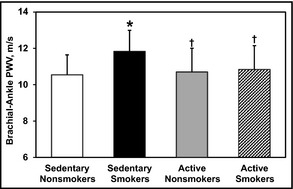
Brachial‐ankle pulse wave velocity (PWV) at rest compared among sedentary nonsmokers, sedentary smokers, active nonsmokers, and active smokers by analysis of variance. Data are expressed as mean±standard deviation. *P<.05 vs sedentary nonsmokers. † P<.05 vs sedentary smokers.
Discussion
The present cross‐sectional study was conducted to investigate the potential influence of habitual aerobic exercise on mitigating smoking‐induced arterial stiffening. Our findings indicate that chronic smoking elevates arterial stiffness in sedentary individuals but that smoking‐induced arterial stiffening is absent in chronic smokers who engage in regular physical activity. These results are consistent with the idea that regular participation in physical activity may mitigate the adverse effect of smoking on the vasculature.
Smoking leads to the initiation and progression of atherosclerosis and other vascular diseases.14 Arterial stiffness may be one of the mechanisms underlying smoking‐related vascular disease. In the present study, arterial stiffness as measured by baPWV was significantly elevated in sedentary smokers compared with their nonsmoking peers. This was observed even in young otherwise healthy adult samples. The present findings are consistent with previous studies showing the arterial stiffening effects of cigarette smoking.2, 4, 7
There is no question that the most important strategy for improving cardiovascular health in chronic smokers should be smoking cessation.15, 16 However, sustained smoking cessation is difficult to achieve primarily because of the addictive nature of nicotine.17 Thus, interventions targeted at reducing or minimizing smoking‐induced cardiovascular harm are needed. In this context, physical activity may be a cost‐effective harm reduction strategy for those who cannot use or afford medications or who cannot access traditional therapeutic choices (eg, pregnant smokers).18 Considering the multitude of benefits induced by regular exercise, it seems logical that the detrimental effects of cigarette smoking on vascular function could be antagonized through participation in regular physical activity. We found that the PWV of smokers who are physically active is not different than that of sedentary and active nonsmokers. The epidemiological report that smokers who habitually exercise exhibit a lower relative risk for CVD than their sedentary peers19 is consistent with these findings. Taken together, these results suggest that regular aerobic exercise may be an important lifestyle modification for chronic smokers who attempt to reduce cardiovascular harm while trying to quit smoking.
Although lower arterial stiffness was observed in physically active smokers compared with their sedentary peers, a similar exercise‐associated reduction in arterial stiffness was not seen in nonsmoking groups. This is probably because young sedentary smokers have such low arterial stiffness that chronic exercise would not further reduce arterial stiffness. We have observed similar trends in relation to aging. In older patients, physically active adults demonstrate lower arterial stiffness than their sedentary peers, but, in young adults, there was no group difference between sedentary and physically active adults.20
Skepticism over whether smokers, who are considered unhealthy, will actually adopt healthy behavior such as regular physical activity is justifiable. In contrast to the prevalent thought, however, the proportion of chronic smokers who perform regular physical activity is fairly high. In the Canadian Community Health Survey, physically active smokers represented almost one quarter of the entire smoking population.10 These data suggest that a large proportion of the daily‐smoking population possess the motivation and willingness to make a deliberate effort to engage in physical activity and that any concerns regarding the practicality of prescribing physical activity as a smoking harm reduction therapy are unwarranted. Indeed, smoking has not been shown to be a barrier to participating in regular physical activity,21 and chronic smokers are capable of becoming more physically active.22 Exercise adherence of smokers with exercise intervention are comparable to that of nonsmokers.23 Interestingly, compared with their inactive peers, a greater proportion of physically active smokers had tried to quit smoking but failed.10 Collectively, these observations make the results of our present study clinically relevant.
Arterial stiffness is determined by the intrinsic properties of the arterial wall and may be improved by alterations in the contractile states of the vascular smooth muscle tone.8 In apparently healthy older adults, a reduction in α‐adrenergic receptor‐mediated vascular tone contributes to the decrease in arterial stiffness with endurance training.24 It is feasible to hypothesize that the similar physiological mechanism may act on the arterial wall of chronic smokers. Indeed, we have previously reported that smokers who habitually perform physical activity demonstrate greater levels of peripheral blood flow and peripheral vascular conductance.25
Carotid‐femoral PWV has been considered by some to be the gold standard measure of arterial stiffness.26 baPWV is a measure of arterial stiffness that is widely used in east Asian countries, including Japan and Korea. Carotid‐femoral PWV and baPWV are significantly associated.27 However, typical values of baPWV are substantially higher than other PWV measures, including carotid‐femoral PWV. In baPWV, the distance between the brachial and ankle sites is computed automatically using the height‐based algorithm. The greater values in baPWV are attributed, in part, to the incorrect estimation of the arterial wave travel distance based on height as we have previously shown.13
Study Limitations
There are a number of limitations in the present study that should be noted. First, as we used a cross‐sectional study design to address the stated aim, the cause‐and effect relationship cannot be determined. Second, the sample size was relatively small. Third, groups were not well matched for some characteristics as there were significant differences in age and body mass and a seemingly large, albeit nonsignificant, difference in smoking history. However, these differences were fairly small. Fourth, it is possible that potential differences in unmeasured factors (eg, dietary intake) may have influenced the results.
Conclusions
The present study demonstrated that chronic smokers who habitually engage in aerobic exercise had lower arterial stiffness compared with sedentary smokers. These results suggest that regular participation in physical activity may mitigate smoking‐induced arterial stiffening. Interventional studies are needed to confirm the present findings obtained using the cross‐sectional approach.
Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
J Clin Hypertens (Greenwich). 2014;16:640–644. © 2014 Wiley Periodicals, Inc.25135246
References
- 1. Campbell SC, Moffatt RJ, Stamford BA. Smoking and smoking cessation – the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis. 2008;201:225–235. [DOI] [PubMed] [Google Scholar]
- 2. Rhee MY, Na SH, Kim YK, et al. Acute effects of cigarette smoking on arterial stiffness and blood pressure in male smokers with hypertension. Am J Hypertens. 2007;20:637–641. [DOI] [PubMed] [Google Scholar]
- 3. Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980‐2012. JAMA. 2014;311:183–192. [DOI] [PubMed] [Google Scholar]
- 4. Mahmud A, Feely JF. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension. 2003;41:183–187. [DOI] [PubMed] [Google Scholar]
- 5. Scallan C, Doonan RJ, Daskalopoulou SS. The combined effect of hypertension and smoking on arterial stiffness. Clin Exp Hypertens. 2010;32:319–328. [DOI] [PubMed] [Google Scholar]
- 6. Xing GD, Wang YL. Regular aerobic exercise training improves endothelium‐dependent arterial dilation in patients with impaired fasting glucose. Diabetes Care. 2004;27:801–802. [DOI] [PubMed] [Google Scholar]
- 7. Kim JW, Park CG, Hong SJ, et al. Acute and chronic effects of cigarette smoking on arterial stiffness. Blood Press. 2005;14:80–85. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka H, Safar ME. Influence of lifestyle modification on arterial stiffness and wave reflections. Am J Hypertens. 2005;18:137–144. [DOI] [PubMed] [Google Scholar]
- 9. Conway TL, Cronan TA. Smoking, exercise, and physical fitness. Prev Med. 1992;21:723–734. [DOI] [PubMed] [Google Scholar]
- 10. Deruiter WK, Faulkner G, Cairney J, Veldhuizen S. Chacteristics of physically active smokers and implications for harm reduction. Am J Public Health. 2008;98:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeVan AE, Anton MM, Cook JN, et al. Acute effects of resistance exercise on arterial compliance. J Appl Physiol. 2005;98:2287–2291. [DOI] [PubMed] [Google Scholar]
- 12. Sugawara J, Hayashi K, Yokoi T, et al. Brachial‐ankle pulse wave velocity: an index of central arterial stiffness? J Human Hypertens. 2005;19:401–406. [DOI] [PubMed] [Google Scholar]
- 13. Sugawara J, Hayashi K, Tanaka H. Arterial path length estimation on brachial‐ankle pulse wave velocity: validity of height‐based formulas. J Hypertens. 2014;32:881–889. [DOI] [PubMed] [Google Scholar]
- 14. Doonan RJ, Scheffler P, Yu A, et al. Altered arterial stiffness and subendocardial viability ratio in young healthy light smokers after acute exercise. PLoS One. 2011;6:e26151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. US Department of Health and Human Services . The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Rockville, MD: 2014. [Google Scholar]
- 16. Jatoi NA, Jerrard‐Dunne P, Feely J, Mahmud A. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension. 2007;49:981–985. [DOI] [PubMed] [Google Scholar]
- 17. Ussher MH, Taylor A, Faulkner G. Exercise for smoking cessation. Mental Health Phys Act. 2012;5:99–100. [Google Scholar]
- 18. Deruiter W, Faulkner G. Tobacco harm reduction strategies: the case for physical activity. Nicotine Tob Res. 2006;8:1–57. [DOI] [PubMed] [Google Scholar]
- 19. Ferrucci L, Izmirlian G, Leveille S, et al. Smoking, physical activity, and active life expectancy. Am J Epidemiol. 1999;149:645–653. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka H, DeSouza CA, Seals DR. Absence of age‐related increase in physically active women. Arteriosclerosis Thromb Vasc Biol. 1998;18:127–132. [DOI] [PubMed] [Google Scholar]
- 21. Crombie IK, Irvine L, Williams B, et al. Why older people do not participate in leisure time physical activity: a survey of activity levels, beliefs and deterrents. Age Ageing. 2004;33:287–292. [DOI] [PubMed] [Google Scholar]
- 22. Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2008;8:CD002295. [DOI] [PubMed] [Google Scholar]
- 23. Ussher MH, Taylor A, West R, McEwen A. Does exercise aid smoking cessation? A systematic review. Addiction. 2000;95:199–208. [DOI] [PubMed] [Google Scholar]
- 24. Sugawara J, Komine H, Hayashi K, et al. Reduction in α‐adrenergic receptor‐mediated vascular tone contributes to improved arterial compliance with endurance training. Int J Cardiol. 2009;135:346–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anton MM, Cortez‐Cooper MY, DeVan AE, et al. Cigarette smoking, regular exercise, and peripheral blood flow. Atherosclerosis. 2006;185:201–205. [DOI] [PubMed] [Google Scholar]
- 26. O'Rourke MF, Staessen JA, Vlachopoulos C, et al. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–444. [DOI] [PubMed] [Google Scholar]
- 27. Tanaka H, Masanori M, Yuhei K, et al. Comparison between carotid‐femoral and brachial‐ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–2027. [DOI] [PubMed] [Google Scholar]


