Abstract
The authors sought to retrospectively analyze the real‐world evidence on aliskiren in diabetic patients with or without concomitant renin‐angiotensin system (RAS) blocker use based on the Registry for Ambulant Therapy With RAS Inhibitors in Hypertension Patients in Germany (3A). Of 14,986 patients included, 3772 patients had diabetes and 28.5% received aliskiren, 14.3% received angiotensin‐converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs), 35.4% received aliskiren plus an ACE inhibitor/ARB, and 10.5% received other drugs. Ambulatory blood pressure (BP) monitoring (baseline BP 148±15.8/84.0±10.9 mm Hg) revealed stronger diastolic BP reduction for aliskiren plus ACE inhibitor/ARB than aliskiren alone in the low (2.8±0.5 vs 0.6±0.6; P=.004) and intermediate (5.9±0.5 vs 4.5±0.5; P=.04) baseline BP groups. There was a lesser ambulatory BP reduction observed for patients receiving non‐RAS in the high baseline category for both systolic (12.5±1.8 vs 17.1±1.0; P=.02) and diastolic (6.9±1.0 vs 9.8±0.6; P=.01) BP. In patients with hypertension and type 2 diabetes, aliskiren was beneficial in lowering BP, with no observed increases in major adverse effects compared with RAS‐blocking therapy alone.
Hypertension is a major cause of premature death around the world.1, 2 Among those with hypertension, patients with comorbid diabetes have approximately twice the risk of cardiovascular (CV) disease compared with those without diabetes. Patients are also at increased risk for diabetes‐specific complications including retinopathy and nephropathy. In the United Kingdom Prospective Diabetes Study (UKPDS),3 each 10 mm Hg decrease in mean systolic blood pressure (BP) was associated with reductions in risk of 12% for any complication related to diabetes, 15% for deaths related to diabetes, 11% for myocardial infarction, and 13% for microvascular complications.
In hypertensive patients, in particular those with comorbid diabetes, the benefits and risks associated with various therapies and treatment strategies (or combinations) must be carefully considered.2 For example, thiazide diuretics have been shown to promote the development of diabetes, while β‐blockers can cause worsening of metabolic control.4, 5 Blockers of the renin‐angiotensin system (RAS), such as angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), on the other hand, have been found to be beneficial and are preferred in patients with diabetes.2, 4, 6 Attempts, however, to utilize the potential benefit of concurrently administering multiple RAS‐blocking agents have been shown to be futile.6 Further, aliskiren, which has a positive benefit–risk ratio in patients with hypertension,7, 8, 9, 10, 11 did not show any benefit on cardiorenal outcomes, with a trend for increased rates of CV events in the Aliskiren Trial in Type 2 Diabetes Using Cardio‐Renal Disease Endpoints (ALTITUDE)12 when given in addition to RAS‐based treatment strategies (ie, ACE inhibitors or ARBs). Moreover, there was an increase in the rate of hyperkalemia, hypotension, and renal dysfunction in the aliskiren group, which is in line with its mechanism of action.12 The use of aliskiren in combination with either an ACE inhibitor or an ARB is therefore now contraindicated in patients with diabetes. In addition, the Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT)13, 14 found that patients with diabetes who received aliskiren initiated early after hospitalization for acute decompensated heart failure in combination with standard heart failure therapy, which included ACE inhibitors and ARBs, showed worse postdischarge outcomes than nondiabetic patients.
To assess real‐world evidence for the use of aliskiren in patients with hypertension, we retrospectively analyzed the Registry for Ambulant Therapy With RAS Inhibitors in Hypertension Patients in Germany (3A), which was established in Germany in 2008. The principal aim of the present analysis was to evaluate the benefits and risks associated with the use of aliskiren for treating hypertension in patients with type 2 diabetes.
Methods
Study Design
The 3A registry is a prospective, observational, noninterventional, multicenter registry that was established to investigate the characteristics of patients with hypertension and the effects of treatment with aliskiren. It was established in 2008 by the Institut für Herzinfarktforschung (Ludwigshafen, Germany). The design and patient criteria for the 3A registry are provided in detail elsewhere.15 All patients provided written informed consent prior to study inclusion. In addition, the study protocol was approved by the Landesärztekammer Rheinland‐Pfalz medical ethics committee (Mainz, Germany). This investigation was registered at clinicaltrials.gov (NCT01454583) and within the Verband forschender Arzneimittelhersteller (VfA) database.16
Patients and Treatments
Patient eligibility for the 3A registry was determined according to the following criteria: 18 years or older, presence of arterial hypertension (known or newly diagnosed), physician decision to initiate or modify treatment for hypertension, follow‐up availability, and written informed consent. The data presented here correspond to patients enrolled between August 2008 and April 2009.
The treating physician independently decided and per their best clinical judgment about the therapy for their patients. Prescription of drugs was in accordance with governing German regulations and reimbursement criteria; no drugs were provided for the participants. In order to avoid selection bias, physicians were asked to enroll patients in a consecutive manner. Patients were excluded based on participation in randomized controlled trials (RCTs) and inability to attend follow‐up visits.
Study participants were categorized into four groups based on physician‐selected treatment strategy: (1) aliskiren alone, (2) other RAS blocker (ACE inhibitor or ARB) alone, (3) aliskiren plus a RAS blocker, and (4) non–RAS‐blocking agents (either alone as monotherapy or in addition to an existing drug regimen). The primary goal of this investigation was to collect data regarding the compound aliskiren. Thus, physicians were asked to include consecutive patients into the registry in a ratio of four (aliskiren) to one (other RAS blocker with or without aliskiren) to one (non–RAS‐blocking agent).
Data Collection
A standardized Web‐based questionnaire (electronic case record form) was used for recording the collected data. Source data were verified for 10% of the patients, and 5% of the centers and patients were audited. Patient data were recorded during study inclusion (baseline) and 1 year later (prospectively), either during clinical examination or by reviewing the patient's chart. Outcomes were not centrally adjudicated, but were based on physician's diagnosis. Resistant hypertension was defined as an elevated BP level despite use of three antihypertensive agents of different classes including a diuretic.
Statistical Analyses
Continuous variables were based on the available data and summarized using descriptive statistics (ie, absolute numbers, means, standard deviations [SDs], or medians with 25th and 75th percentiles). Categorical data were presented based on the number and percentage of patients within each category. Treatment group comparisons were performed using Pearson chi‐square test (categorical variables) or Kruskal‐Wallis test (continuous data). For each parameter, percentages were calculated based on the available patient data (ie, missing values were not considered).
The treatment groups displayed different baselines with regard to BP. Thus, we selected a unique statistical approach to analyze this variance and to assess the follow‐up data. Specifically, individual patient means, and the linear function linking baseline means and other confounders to the follow‐up means, were estimated for the three treatment groups using SAS software (NLMIXED procedure; SAS Institute Inc, Cary, NC). Notably, this technique allowed us to maximize approximations to likelihood (quasi‐Newton algorithm) and integrate them over the random effects (adaptive Gauss‐Hermite quadrature). In order to define adjusted changes in BP, we chose three pretreatment values (ie, the borders between the four quartiles) derived from all patients with diabetes. Adjustments were made for age, sex, smoking, dyslipidemia, glomerular filtration rate (GFR) <60, CV disease, severity of hypertension, number of additional antihypertensive drugs, body mass index (BMI), family history of coronary artery disease, and hypertension duration.
All events were documented without dates and were appropriately analyzed either separately or in combination (eg, major CV events and major adverse CV and cerebrovascular events [MACCEs]). The data presented are unadjusted. In an exploratory analysis, MACCE rates were analyzed in a Cox proportional hazard regression model adjusting for age, sex, smoking, dyslipidemia, GFR ≥60, CV disease, hypertension stage, and number of additional antihypertensive drugs. In addition, in a stepwise regression, BMI, family history of CAD, and duration of hypertension were considered given their α was ≤.1.
All P values were determined by two‐sided tests, with P values ≤.05 considered significant. Statistical analyses were performed using SAS 9.2 software (SAS Institute Inc).
Results
A total of 14,986 patients were enrolled in the 3A registry (Figure). Of those, 13,424 had 12‐month follow‐up information available (89.6%). A total of 3772 (28%) individuals had type 2 diabetes (mean duration 7.7±6.8 years), as diagnosed through glycated hemoglobin (HbA1c) levels ≥6.5%. Characteristics of patients with diabetes compared with those without diabetes, who were excluded, are presented in Tables S1 and S2. Patients with diabetes were older, less often female, and had a higher bodyweight/BMI. Resistant hypertension was more often diagnosed (49.5% vs 28.3%; P<.0001) and the rate of overall CV disease at baseline was higher (44.6% vs 26.1%, P<.0001). Furthermore, renal failure (16.3% vs 5.9%; P<.0001) and low estimated GFRs (29.2% vs 18.9%; P<.0001) were found to be more prevalent in diabetic individuals. Finally, HbA1c (7.0±1.2 vs 5.6±0.4; P<.0001) and glucose fasting values (133.3±43.7 vs 91.5±18.2; P<.0001) were higher in patients with diabetes than without (Table S2).
Figure 1.
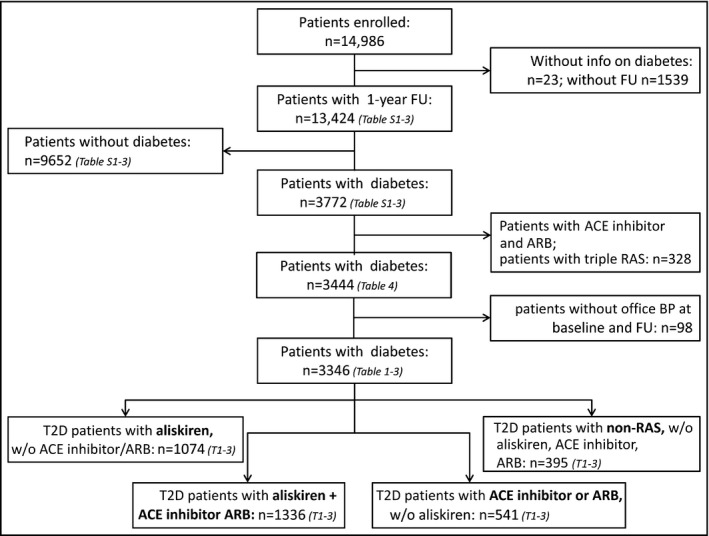
Patient groups considered for the analyses. FU indicates follow‐up; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; T2D, type 2 diabetes; RAS, renin‐angiotensin system.
Characteristics of Patients With Type 2 Diabetes by Treatment Group
Among the group of patients with type 2 diabetes, 1074 patients received aliskiren only (28.5%), 541 ACE inhibitors/ARBs only (14.3%), 1336 aliskiren on top of ACE inhibitors/ARBs (35.4%), and 395 patients received antihypertensive drugs other than RAS‐blocking agents (10.5%) (Table 1). While there were only minor differences in patient characteristics, noteworthy differences included higher BP values in both the aliskiren and the aliskiren + ACE inhibitor/ARB groups compared with the other two treatment groups and a higher rate of comorbid disease (heart failure, coronary artery disease, peripheral artery disease, and renal disease) in patients receiving aliskiren + ACE inhibitor/ARB.
Table 1.
Comparison of Baseline Data Between Treatment Groups in Patients With Type 2 Diabetes (n=3346)
| Aliskiren Only (n=1074) | ACE Inhibitor/ARB Only (n=541) | P Value vs Aliskiren | Aliskiren + ACE Inhibitor/ARB (n=1336) | P Value vs Aliskiren | Non‐RAS (n=395) | P Value vs Aliskiren | |
|---|---|---|---|---|---|---|---|
| Age, y | 66.0±10.8 | 67.4±10.0 | <.05 | 67.0±9.9 | <.05 | 66.0±11.0 | .85 |
| Female, % | 46.6 | 42.5 | .12 | 42.6 | <.05 | 46.1 | |
| Body weight, kg | 88±17 | 89±18 | .35 | 91±18 | <.0001 | 87±18 | .46 |
| BMI, kg/m2 | 30.5±5.2 | 30.7±5.6 | .95 | 31.5±5.8 | <.0001 | 30.3±5.2 | .40 |
| Diabetes duration, y | 7.2±6.6 | 7.0±6.5 | .87 | 8.3±6.9 | <.0001 | 6.3±5.7 | .06 |
| HTN | |||||||
| Age at HTN onset, y | 57.9±11.4 | 58.4±11.0 | .40 | 56.2±11.0 | <.001 | 58.7±11.3 | .26 |
| History of HTN, y | 9.1±7.3 | 9.6±7.9 | .40 | 10.8±8.2 | <.0001 | 8.2±7.3 | <.01 |
| Office BP systolic, mm Hg | 157±19.0 | 152±19.6 | <.0001 | 159±20.0 | .10 | 151±18.4 | <.0001 |
| Office BP diastolic, mm Hg | 89.8±11.0 | 86.9±11.4 | <.0001 | 88.5±11.6 | <.01 | 87.2±11.1 | <.001 |
| Mean ABPM systolic BP, mm Hg | 147±14.7 | 144±15.3 | <.01 | 149±15.9 | .27 | 143±16.8 | <.01 |
| Mean ABPM diastolic BP, mm Hg | 85.3±11.1 | 82.5±10.1 | <.01 | 83.2±11.0 | <.01 | 83.9±10.9 | .12 |
| Resistant HTN, % | 35.1 | 43.7 | <.001 | 65.1 | <.0001 | 24.9 | <.001 |
| Comorbidity | |||||||
| Heart failure, % | 19.7 | 21.8 | .31 | 24.6 | <.01 | 16.5 | .16 |
| CAD, % | 25.0 | 28.3 | .16 | 32.2 | <.001 | 27.6 | .31 |
| Prior stroke, % | 6.1 | 5.6 | .70 | 7.7 | .14 | 6.5 | .81 |
| PAD, % | 8.5 | 9.8 | .39 | 14.2 | <.0001 | 6.8 | .28 |
| Renal function | |||||||
| Renal failure, %a | 12.7 | 13.0 | .86 | 20.4 | <.0001 | 10.8 | .34 |
| Microalbuminuria/macroalbuminuria, % | 20.7 | 20.5 | .92 | 29.9 | <.0001 | 16.5 | .09 |
| eGFR <60/mL/min/1.732, % | 28.1 | 26.4 | .46 | 31.1 | .11 | 22.8 | <.05 |
| Concomitant treatment | |||||||
| ACE inhibitor | 0 (0.0) | 266 (49.2) | NA | 711 (53.2) | NA | 0 (0.0) | NA |
| ARB | 0 (0.0) | 275 (50.8) | NA | 625 (46.8) | NA | 0 (0.0) | NA |
| β‐Blocker | 519 (48.3) | 287 (53.1) | .07 | 838 (62.7) | <.0001 | 262 (66.3) | <.0001 |
| Diuretics | 467 (43.5) | 284 (52.6) | <.001 | 869 (65.1) | <.0001 | 182 (46.1) | .38 |
| Calcium channel blocker | 327 (30.5) | 209 (38.7) | <.001 | 745 (55.8) | <.0001 | 164 (41.5) | <.0001 |
Abbreviations: ABPM, ambulatory blood pressure measurement; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CV, cardiovascular disease; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; HTN, hypertension; NA, not available; PAD, peripheral artery disease; RAS, renin‐angiotensin system. aRenal failure was obtained from the physician but not predefined by the protocol. Values are indicated in percentage, median (interquartile range), or mean±standard deviation.
BP Reduction by Treatment Group in Patients With Type 2 Diabetes Mellitus
Office BP in patients with diabetes was 156±19.5/90.5±11.0 mm Hg with only minor differences compared with those without diabetes (Table S1). To adjust for baseline differences in BP among the treatment groups, three pretreatment office BP values were chosen (145/80 mm Hg, 155/90 mm Hg, and 170/95 mm Hg) representing the three borders between four quartiles (Table 2). There was no significant difference in BP lowering in the ACE inhibitor/ARB, aliskiren + ACE inhibitor/ARB, or the non‐RAS group compared with the patient group receiving aliskiren only.
Table 2.
BP Reduction Among Diabetic Patients Within Each Treatment Group (n=3346) at 1 Year
| Office BP | Aliskiren Only (n=1074) | ACE Inhibitor/ARB Only (n=541) | P Value vs Aliskiren | Aliskiren + ACE Inhibitor/ARB (n=1336) | P Value vs Aliskiren | Non‐RAS (n=395) | P Value vs Aliskiren |
|---|---|---|---|---|---|---|---|
| Low baseline BPa | |||||||
| Systolic (145 mm Hg) | 8.3±0.7 | 8.8±0.8 | .65 | 7.7±0.7 | .39 | 9.6±0.9 | .21 |
| Diastolic (80 mm Hg) | 0.6±0.4 | 0.5±0.5 | .84 | 0.4±0.4 | .59 | 0.5±0.5 | .89 |
| Intermediate baseline BPa | |||||||
| Systolic (155 mm Hg) | 16.5±0.5 | 17.1±0.7 | .40 | 15.8±0.5 | .33 | 17.7±0.8 | .16 |
| Diastolic (90 mm Hg) | 8.7±0.3 | 8.9±0.4 | .65 | 8.6±0.3 | .78 | 8.4±0.5 | .55 |
| High baseline BPa | |||||||
| Systolic (170 mm Hg) | 28.6±0.7 | 29.7±1.0 | .30 | 28.0±0.7 | .43 | 30.0±1.1 | .27 |
| Diastolic (95 mm Hg) | 12.8±0.3 | 13.1±0.5 | .48 | 12.7±0.3 | .94 | 12.3±0.5 | .48 |
| 24‐h ABPM | Aliskiren Only (n=1074) | ACE Inhibitor/ARB Only (n=541) | P Value vs Aliskiren | Aliskiren + ACE Inhibitor/ARB (1336) | P Value vs Aliskiren | Non‐RAS (n=395) | P Value vs Aliskiren |
|---|---|---|---|---|---|---|---|
| Low baseline BP | |||||||
| Systolic (138 mm Hg) | 2.6±1.0 | 4.1±1.4 | .34 | 3.8±1.0 | .35 | 2.5±1.8 | .98 |
| Diastolic (79 mm Hg) | 0.6±0.6 | 1.1±0.9 | .60 | 2.8±0.5 | .004 | 0.5±1.2 | .91 |
| Intermediate baseline BP | |||||||
| Systolic (149 mm Hg) | 10.6±0.8 | 12.0±1.3 | .34 | 10.5±0.8 | .99 | 8.0±1.6 | .14 |
| Diastolic (85 mm Hg) | 4.5±0.5 | 5.2±0.8 | .45 | 5.9 ± 0.5 | .04 | 3.2±1.0 | .21 |
| High baseline BP | |||||||
| Systolic (158 mm Hg) | 17.1±1.0 | 18.5±1.7 | .48 | 16.1±1.0 | .41 | 12.5±1.8 | .02 |
| Diastolic (92 mm Hg) | 9.8±0.6 | 10.6±1.0 | .48 | 10.0±0.6 | .73 | 6.9±1.0 | .01 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; RAS, renin‐angiotensin system. aTo illustrate adjusted changes in blood pressure (BP), three pretreatment BP values were chosen to represent the three borders between four quartiles derived from all patients with diabetes. Adjustments were made for age, sex, smoking, dyslipidemia, glomerular filtration rate <60, cardiovascular disease, severity of hypertension, number of additional antihypertensive drugs, body mass index, family history of coronary artery disease, and hypertension duration. Bold values indicate significance.
Based on 24‐hour ambulatory BP monitoring (ABPM), there was a stronger diastolic BP reduction with aliskiren + ACE inhibitor/ARB than with aliskiren alone for both the low (2.8±0.5 vs 0.6±0.6; P=.004) and intermediate (5.9±0.5 vs 4.5±0.5; P=.04) baseline BP groups. Further, there was a lesser BP reduction observed for patients receiving non‐RAS in the high baseline BP category for both the systolic (12.5±1.8 vs 17.1±1.0; P=.02) and diastolic (6.9±1.0 vs 9.8±0.6; P=.01) ABPM readings.
Changes in Laboratory Values Following 1 Year of Treatment
With regard to laboratory values, the main differences between diabetic and nondiabetic patients were increased HbA1c and fasting blood glucose levels as well as decreased GFR (Table S2). These values showed only slight differences following 1 year of treatment with the most apparent being the rise in fasting glucose in patients with diabetes (5.9±43.8 mg/dL) and a mean nominal reduction in those without diabetes (−2.3±22.4 mg/dL; P<.0001).
Grouped by treatment in patients with diabetes (Table 3), baseline laboratory values were largely comparable, with only the difference in HbA1c between the aliskiren and the ACE inhibitor/ARB group (P<.05) and the fasting blood glucose between non‐RAS and aliskiren (P<.01) reaching statistical significance. Within the 1 year of follow‐up, changes in HbA1c, fasting glucose, creatinine, and potassium were either statistically not significant or clinically not relevant. The only noteworthy difference was a steeper decline in estimated GFR in the aliskiren + ACE inhibitor/ARB group (2.1±16.5 mL/min/1.73 m² vs 0.3±22.1; P<.01), which was also present in the non‐RAS group (1.9±16.1 mL/min/1.73 m²), although it did not reach statistical significance (P=.09).
Table 3.
Laboratory Values in Patients With Diabetes at BL and at 1 Year FU (n=3346)
| Aliskiren Only (n=1074) | ACE Inhibitor/ARB Only (n=541) | P Value vs Aliskiren | Aliskiren + ACE Inhibitor/ARB (n=1336) | P Value vs Aliskiren | Non‐RAS (n=395) | P Value vs Aliskiren | |
|---|---|---|---|---|---|---|---|
| HbA1c, % | |||||||
| BL | 7.0±1.2 | 6.9±1.2 | <.05 | 7.1±1.2 | .11 | 7.0±1.3 | .28 |
| ∆BL‐FU | −0.2±1.6 | −0.1±1.6 | .06 | −0.1±1.9 | <.05 | 0.1±1.4 | .18 |
| Glucose fasting, mg/dL | |||||||
| BL | 135.9±49.7 | 132.4±42.2 | .16 | 134.2±40.0 | .80 | 128.7±42.4 | <.01 |
| ∆BL‐FU | 7.8±51.0 | 4.8±39.7 | .23 | 4.4±41.0 | .10 | 6.4±36.6 | .97 |
| Creatinine, mg/dL | |||||||
| BL | 1.27±1.53 | 1.13±1.13 | .96 | 1.14±0.99 | <.05 | 1.09±1.14 | .13 |
| ∆BL‐FU | 0.1±1.7 | 0.0±1.4 | .36 | −0.1±1.3 | <.01 | −0.0±1.0 | .13 |
| Potassium, mmol/L | |||||||
| BL | 4.5±0.6 | 4.5±0.5 | .72 | 4.5±0.6 | .09 | 4.5±0.5 | .48 |
| ∆BL‐FU | −0.0±0.5 | 0.1±0.5 | .53 | 0.0±0.6 | .38 | −0.1±0.6 | .72 |
| eGFR, mL/min/1.73 m² | |||||||
| BL | 71.2±23.5 | 72.1±21.1 | .84 | 70.2±21.5 | .07 | 73.8±20.3 | .16 |
| ∆BL‐FU | 0.3±22.1 | 0.9±19.8 | .17 | 2.1±16.5 | <.01 | 1.9±16.1 | .09 |
Abbreviations: ∆, change; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BL, baseline; eGFR, estimated glomerular filtration rate; FU, follow‐up; HbA1c, glycated hemoglobin; RAS, renin‐angiotensin system. Values are presented as median (interquartile range), mean±standard deviation, or percentage. Bold values indicate significance.
Event Rates During the 1‐Year Follow‐Up
Principal differences between patients with or without diabetes at 1 year were higher rate of death (odds ratio [OR], 2.63; 95% confidence interval [CI], 1.79–3.88]) and higher rate of MACCE (death and/or myocardial infarction and/or stroke (OR, 2.14; 95% CI, 1.58–2.90) (Table S3).
Event rates by treatment groups in patients with diabetes (Table 4) were low and the combined endpoint MACCE showing the highest rates, with 2.5% in the non‐RAS group and 2.2% in the aliskiren only and aliskiren + ACE inhibitor/ARB groups, respectively, and 1.3% in those receiving ACE inhibitor/ARB only. Differences vs aliskiren alone were, however, nonsignificant for either group with an OR of 1.74 (95% CI, 0.75–4.07) for the ACE inhibitor/ARB group and ORs slightly less than 1 for the two other groups.
Table 4.
Event Rates in Patients With Diabetes at 1‐Year Follow‐Up (n=3444)
| Aliskiren Only (n=1102) | ACE Inhibitor/ARB Only (n=555) | Aliskiren + ACE Inhibitor/ARB (n=1381) | Non‐RAS (n=406) | ||||
|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | OR (95% CI)b | No. (%) | OR (95% CI) | No. (%) | OR (95% CI)b | |
| Cardiovascular events, % | |||||||
| MACCE | 24 (2.2) | 7 (1.3) | 1.74 (0.75–4.07)a | 31 (2.2) | 0.97 (0.57–1.66)a | 10 (2.5) | 0.88 (0.42–1.86)a |
| Death | 16 (1.5) | 5 (0.9) | 1.62 (0.59–4.45) | 19 (1.4) | 1.06 (0.54–2.06) | 8 (2.0) | 0.73 (0.31–1.73) |
| Myocardial infarction | 6 (0.5) | 1 (0.2) | 3.04 (0.36–25.3) | 6 (0.4) | 1.26 (0.40–3.90) | 0 (0.0) | – |
| Stroke | 6 (0.5) | 1 (0.2) | 3.04 (0.36–25.3) | 10 (0.7) | 0.75 (0.27–2.07) | 2 (0.5) | 1.11 (0.22–5.51) |
| PCI | 6 (0.5) | 4 (0.7) | 0.76 (0.21–2.69) | 13 (0.9) | 0.58 (0.22–1.52) | 1 (0.2) | 2.22 (0.27–18.46) |
| CABG | 3 (0.3) | 0 (0.0) | – | 2 (0.1) | 1.88 (0.31–11.28) | 0 (0.0) | – |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CI, confidence interval; MACCE, major adverse cardiovascular or cerebrovascular event (death and/or myocardial infarction and/or stroke); OR, odds ratio; PCI, percutaneous coronary intervention; RAS, renin‐angiotensin system. aNonsignificant even after a Cox proportional hazard regression model adjusting for age, sex, smoking, dyslipidemia, glomerular filtration rate ≥60, cardiovascular disease, hypertension stage, and number of additional antihypertensive drugs. In addition, in a stepwise regression, body mass index, family history of coronary artery disease, and duration of hypertension were considered given their α was ≤0.1. bUnadjusted.
Furthermore, we investigated the incidence of side effects when aliskiren was given alone or in combination with ACE inhibitors or ARBs. None of the parameters, as displayed in Table 5 had a statistically significant different rate in either group with hypotension (seven cases in the aliskiren group (0.6%); 18 cases in the aliskiren + ACE inhibitor/ARB group (1.4%) showing the highest nominal difference (P=.07).
Table 5.
Adverse Event Comparison of Aliskiren Either Alone or in Dual RAS Combination With an ACE Inhibitor or ARB
| Adverse Event, No. (%) | Aliskiren Only (n=1102) | Aliskiren + ACE Inhibitor/ARB (n=1381) | P Value |
|---|---|---|---|
| Diarrhea | 3 (0.3) | 4 (0.3) | .94 |
| Angioedema | 5 (0.5) | 2 (0.1) | .15 |
| Hyperkalemia | 2 (0.2) | 8 (0.6) | .12 |
| Renal dysfunction | 14 (1.3) | 21 (1.5) | .60 |
| Peripheral edema | 3 (0.3) | 2 (0.1) | .48 |
| Hypotension | 7 (0.6) | 19 (1.4) | .07 |
| Cough | 0 (0.0) | 1 (0.1) | .37 |
| Cardiac arrhythmia | 11 (1.0) | 19 (1.4) | .39 |
| Hypertensive crisis | 9 (0.8) | 16 (1.2) | .40 |
| Dizziness | 2 (0.2) | 4 (0.3) | .59 |
| Headache | 0 (0.0) | 1 (0.1) | .37 |
| Elevated liver enzymes | 5 (0.5) | 5 (0.4) | .72 |
Abbreviations: ACE, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; RAS, renin‐angiotensin system. Rates were derived from reporting of adverse events to the manufacturer of aliskiren rather than being part of the electronic case record form. No rates are available for patient groups not receiving aliskiren.
Discussion
In a real‐world cohort of patients with type 2 diabetes, in which there was a stratification of patients to aliskiren, other RAS‐blocking agents, and non–RAS‐blocking agents in a 4:1:1 ratio, 55.4% of aliskiren patients received the drug in combination with ACE inhibitors/ARBs. This proportion may seem to be high, given the contraindication of aliskiren for the combined use with other RAS‐blocking agents, but reflects clinical practice at the time the registry was conducted, when the results of ASTRONAUT and ALTITUDE were not yet available.12, 13, 14 The data illustrate (1) the increased risk profile of patients with diabetes and their increased CV event rate at 1 year vs those without diabetes; (2) no substantial difference in the BP‐lowering effect of each treatment strategy and either aliskiren or its combination with ACE inhibitors/ARBs in particular; and (3) a steeper decline of the estimated GFR in the aliskiren and ACE inhibitor/ARB group vs aliskiren alone, but no further observed difference in adverse effects of either option on laboratory values, CV endpoints, and adverse events.
Aliskiren Effectiveness With or Without Concurrent ACE Inhibitor/ARB Use
In the present study, we specifically analyzed the benefits and risks associated with aliskiren treatment both as monotherapy and in combination with ACE inhibitors and ARBs (now contraindicated) in patients with type 2 diabetes. Following 1 year of follow‐up, we observed a comparable overall reduction in office BP between treatment groups when baseline BP was considered as a covariate. Using ABPM, however, there was a more pronounced diastolic BP–lowering effect in the aliskiren plus ACE inhibitor/ARB group in both the low and intermediate baseline BP group compared with aliskiren alone, and a lesser effect of non‐RAS treatment compared with aliskiren alone in the high baseline BP group. This is important because ABPM is more closely related to organ damage17, 18 and CV morbidity19, 20 than office BP.
This finding has to be interpreted with caution, however, since it was observed on an intense BP‐lowering treatment scheme including the concomitant prescription of β‐blockers, diuretics, and calcium channel blockers in each group (Table 1). Furthermore, this was a not randomized comparison of treatment groups, with physician rather than random assignment used to form treatment groups, suggesting that physicians may have other patient‐related variables in mind when selecting a particular treatment option. Finally, it was observed on the background of significant differences in baseline BP values for both the office BP and APBM. We aimed to adjust for these differences by applying a “nonlinear additive multivariate mixed model” that groups baseline BP values into quartiles and uses the cutoffs between the quartiles to illustrate the corresponding BP‐lowering effect at a low, intermediate, and high baseline BP. Taken together, however, our results in diabetic patients are consistent with previous studies demonstrating the antihypertensive profile of aliskiren on BP,7, 8, 9, 10, 11, 21 indicating even superiority in some studies in terms of BP reduction vs the comparators.22, 23, 24
Safety and Tolerability of Aliskiren With or Without Concurrent ACE Inhibitor/ARB Use
In the present study, we observed a steeper decline of estimated GFR in patients receiving aliskiren on top of an ACE inhibitor/ARB (2.1±16.5) compared with aliskiren alone (0.3±22.1; P<.01). The degree of the estimated GFR decline with aliskiren on top of an ACE inhibitor/ARB was, however, comparable to the decline observed with a regimen containing a non–RAS‐blocking agent (1.9±16.1). There was no difference in predefined CV events (death, myocardial infarction, stroke, percutaneous coronary intervention, or coronary artery bypass grafting), or in the rate of further adverse events.
The lack of differences in the observed safety and tolerability between the treatment groups is important when compared with the results of the ALTITUDE study.12 In this RCT, aliskiren or placebo were added to standard RAS‐blocking therapy (ie, ACE inhibitors or ARBs) in patients with hypertension and type 2 diabetes, in addition to chronic kidney disease and/or CV disease. They identified a slightly higher rate of CV and renal events in the aliskiren group than in the placebo group, with the primary composite outcome reached by 18.3% of the aliskiren patients and 17.1% of those receiving the placebo (hazard ratio [HR], 1.08; 95% CI, 0.98–1.20; P=.12). Furthermore, cardiac death with resuscitation was found to be significantly more likely in the aliskiren group (0.4% vs 0.2%; P=.04). The findings of this trial led to its early discontinuation and to the Food and Drug Administration advising against the use of aliskiren in patients with hypertension in addition to type 2 diabetes who were at increased risk for a CV or renal event. In the diabetes subgroup of ASTRONAUT, which included patients hospitalized for heart failure (82% with hypertension; 63% ACE inhibitor and 23% ARB), aliskiren was associated with a slightly higher but nonsignificant rate of the combined endpoint of CV death or hospitalization for heart failure at 1 year (42.3% vs 39.7%; HR, 1.16; 95% CI, 0.91–1.47).14
On the other hand, the recent Valsartan Aliskiren Hypertension Diabetes (VIvID) study21 conducted in hypertensive participants with type 2 diabetes and stage 1 or 2 chronic kidney disease did not result in significant safety concerns upon addition of aliskiren to valsartan. In addition, the Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) trial25 did not report serious adverse events in patients with hypertension, type 2 diabetes, and advanced nephropathy upon aliskiren treatment (in combination with losartan).
In the present observational study, while MACCE rates were numerically lower in the group receiving a RAS‐blocking agent alone in comparison to the groups receiving aliskiren, either alone or in combination with an ACE inhibitor or ARB, the ORs were not statistically significant and the 95% CIs were wide. This lack of difference in event rates does not necessarily contradict the findings of ALTITUDE.12 Firstly, their time‐to‐event data indicate that the divergence in rates did not occur until after 1 year, the time at which our endpoint data were collected. Furthermore, the present study could merely be underpowered to identify significant differences. It should also be noted that the inclusion criteria varied between the studies, with patients in ALTITUDE being at increased risk for a CV or renal event. The lower event rate found in our study may therefore not be able to fully capture the differential risk profile of aliskiren alone or in combination with other RAS‐blocking agents.
Limitations
This investigation has some limitations, including a potential selection bias related to the participating study sites. Indeed, specific centers may have taken part in the study because of a particular interest in hypertension therapy. Furthermore, patients were not randomly assigned to treatment groups, which results in an imbalance of patient characteristics between groups. This likely introduced a degree of error into the treatment comparisons. Furthermore, it is known that registry data tend to be less complete compared with data collected in RCTs. In addition, myocardial infarctions and strokes were not adjudicated, which could have resulted in misclassification of major CV events. Finally, it is possible that the follow‐up duration was not sufficient for accurate appraisal of the association between event rates and the various treatment strategies.
Conclusions
Our analysis of a large, unselected cohort of patients with hypertension and type 2 diabetes revealed that aliskiren was beneficial in lowering BP, with no observed increase in risk of major adverse effects after 1 year of treatment. The only noteworthy difference in laboratory values was a steeper decline in estimated GFR in the aliskiren + ACE inhibitor/ARB group compared with the aliskiren alone group. These clinical practice data add to those obtained from RCTs.
Disclosures
The study was funded by Novartis Pharma GmbH, Germany. UZ, RD, PB, DP, JS, and RES received speaker's fees and/or consulting honoraria from Novartis and other manufacturers of antihypertensive drugs. IH is a full‐time employee of Novartis Pharma GmbH Germany. The other authors report no conflicts of interest.
Supporting information
Table S1. Comparison of baseline data between diabetic and nondiabetic patients
Table S2. Change in laboratory values at baseline and 1‐year follow‐up in patients with or without diabetes (n=13,424)
Table S3. Event rates at the 1‐year follow‐up in patients with or without diabetes (n=13,424)
J Clin Hypertens (Greenwich). 2016;18:1045–1053. DOI: 10.1111/jch.12828. © 2016 Wiley Periodicals, Inc.
The results have been presented at the European Society of Hypertension, Milan, June 2011.
References
- 1. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 3. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 4. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta‐analysis. Lancet. 2007;369:201–207. [DOI] [PubMed] [Google Scholar]
- 5. Zidek W, Schrader J, Luders S, et al. Ramipril‐based versus diuretic‐based antihypertensive primary treatment in patients with pre‐diabetes (ADaPT) study. Cardiovasc Diabetol. 2012;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure‐lowering agents in adults with diabetes and kidney disease: a network meta‐analysis. Lancet. 2015;385:2047–2056. [DOI] [PubMed] [Google Scholar]
- 7. Gradman AH, Schmieder RE, Lins RL, et al. Aliskiren, a novel orally effective renin inhibitor, provides dose‐dependent antihypertensive efficacy and placebo‐like tolerability in hypertensive patients. Circulation. 2005;111:1012–1018. [DOI] [PubMed] [Google Scholar]
- 8. Nussberger J, Gradman AH, Schmieder RE, et al. Plasma renin and the antihypertensive effect of the orally active renin inhibitor aliskiren in clinical hypertension. Int J Clin Pract. 2007;61:1461–1468. [DOI] [PubMed] [Google Scholar]
- 9. O'Brien E, Barton J, Nussberger J, et al. Aliskiren reduces blood pressure and suppresses plasma renin activity in combination with a thiazide diuretic, an angiotensin‐converting enzyme inhibitor, or an angiotensin receptor blocker. Hypertension. 2007;49:276–284. [DOI] [PubMed] [Google Scholar]
- 10. Oh BH, Mitchell J, Herron JR, et al. Aliskiren, an oral renin inhibitor, provides dose‐dependent efficacy and sustained 24‐hour blood pressure control in patients with hypertension. J Am Coll Cardiol. 2007;49:1157–1163. [DOI] [PubMed] [Google Scholar]
- 11. Oparil S, Yarows SA, Patel S, et al. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double‐blind trial. Lancet. 2007;370:221–229. [DOI] [PubMed] [Google Scholar]
- 12. Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213. [DOI] [PubMed] [Google Scholar]
- 13. Gheorghiade M, Bohm M, Greene SJ, et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. [DOI] [PubMed] [Google Scholar]
- 14. Maggioni AP, Greene SJ, Fonarow GC, et al. Effect of aliskiren on post‐discharge outcomes among diabetic and non‐diabetic patients hospitalized for heart failure: insights from the ASTRONAUT trial. Eur Heart J. 2013;34:3117–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeymer U, Dechend R, Deeg E, et al. Aliskiren for the treatment of essential hypertension under real‐life practice conditions: design and baseline data of the prospective 3A registry. Int J Clin Pract. 2012;66:251–261. [DOI] [PubMed] [Google Scholar]
- 16. Verband der forschenden Arzneimittelhersteller (VfA) . Registry of non‐interventional studies (NIS): 3A Register. Internet: http://www.vfa.de/de/arzneimittel-forschung/datenbanken-zu-arzneimitteln/nisdb/nis-details/_616. Accessed October 9, 2011.
- 17. Fagard RH, Staessen JA, Thijs L. Relationships between changes in left ventricular mass and in clinic and ambulatory blood pressure in response to antihypertensive therapy. J Hypertens. 1997;15:1493–1502. [DOI] [PubMed] [Google Scholar]
- 18. Schmieder RE, Ruilope LM, Ott C, et al. Interpreting treatment‐induced blood pressure reductions measured by ambulatory blood pressure monitoring. J Hum Hypertens. 2013;27:715–720. [DOI] [PubMed] [Google Scholar]
- 19. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. [DOI] [PubMed] [Google Scholar]
- 20. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow‐up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. [DOI] [PubMed] [Google Scholar]
- 21. Bakris GL, Oparil S, Purkayastha D, et al. Randomized study of antihypertensive efficacy and safety of combination aliskiren/valsartan vs valsartan monotherapy in hypertensive participants with type 2 diabetes mellitus. J Clin Hypertens (Greenwich). 2013;15:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmieder RE, Philipp T, Guerediaga J, et al. Long‐term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: a 12‐month randomized, double‐blind comparator trial with hydrochlorothiazide. Circulation. 2009;119:417–425. [DOI] [PubMed] [Google Scholar]
- 23. Duprez DA, Munger MA, Botha J, et al. Aliskiren for geriatric lowering of systolic hypertension: a randomized controlled trial. J Hum Hypertens. 2010;24:600–608. [DOI] [PubMed] [Google Scholar]
- 24. Krone W, Hanefeld M, Meyer HF, et al. Comparative efficacy and safety of aliskiren and irbesartan in patients with hypertension and metabolic syndrome. J Hum Hypertens. 2011;25:186–195. [DOI] [PubMed] [Google Scholar]
- 25. Parving HH, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of baseline data between diabetic and nondiabetic patients
Table S2. Change in laboratory values at baseline and 1‐year follow‐up in patients with or without diabetes (n=13,424)
Table S3. Event rates at the 1‐year follow‐up in patients with or without diabetes (n=13,424)


