Abstract
Studies identified from an updated systematic review (from June 2014 to May 2015) on the impact of dietary salt intake on clinical and population health are reviewed. Randomized controlled trials, cohort studies, and meta‐analyses of these study types on the effect of sodium intake on blood pressure, or any substantive adverse health outcomes were identified from MEDLINE searches and quality indicators were used to select studies that were relevant to clinical and public health. From 6920 studies identified in the literature search, 144 studies were selected for review, of which only three (n=233,680) met inclusion criteria. Between them, the three studies demonstrated a harmful association between excess dietary salt and all‐cause mortality, noncardiovascular and cardiovascular disease mortality, and headache. None of the included studies found harm from lowering dietary salt. The findings of this systematic review are consistent with the large body of research supportive of efforts to reduce population salt intake and congruent with our last annual review from June 2013 to May 2014.
Habitual excess salt consumption is a well‐established determinant of increased blood pressure (BP),1 which in 2010 caused approximately 19% of all global deaths.2 In addition to the adverse effects of salt on BP and vascular risk, a range of other serious health problems are also implicated including gastric cancer and osteoporosis.3 On the basis of the evidence linking salt, BP, and vascular risk, the World Health Organization (WHO) recommends that all member states implement a salt reduction program. A 30% lowering in mean population salt intake by 2025 has been included as one of the targets of the “25 by 25” World Health Assembly/WHO initiative for the control of noncommunicable diseases.4
Background
While there are many comprehensive reviews on the adverse effects associated with excess salt intake and the likely positive impact of salt reduction, there are several studies that still challenge the benefits of reducing dietary salt.5, 6, 7 Several international health and scientific organizations have expressed concerns that low‐quality research (including studies with inadequate statistical power, observational studies prone to confounding, and reverse causality) and financial conflicts of interest are sources of controversy associated with reducing dietary salt.8 In addition, there are approximately 100 publications per week identified by regularly updated MEDLINE searches.9 Hence, there is a critical need to continually update the evidence base and ensure that new studies are assessed for quality to track developments in the science of salt to effectively inform clinical and public health interventions. This annual review uses quality filters to exclude low‐quality research and updates a previous annual review.9 Future reviews will be replaced by a more regularly updated review published every few months.10
Aims
The aim of this review is to provide an update to the June 2013 to May 2014 annual review of recent evidence on the impact of dietary salt on public health using the Science of Salt search methodology.10
Methods
Search Strategy and Selection Criteria
The existing automated search strategy (Table 1) established for the weekly Science of Salt eNewsletter publication was used (www.hypertensiontalk.com/science-of-salt-weekly). MEDLINE (Ovid) searches are conducted weekly to identify studies on dietary sodium according to various predefined quality criteria. The criteria were adapted from the systematic reviews conducted to develop WHO dietary salt recommendations. For the current review, studies were included from searches conducted between June 3, 2014, and May 26, 2015.
Table 1.
Weekly MEDLINE (Ovid) Search Strategy Years: 1946–Present
| Step | Search |
|---|---|
| 1 | Exp Sodium Glutamate |
| 2 | (Monosodium glutamate or msg).tw. |
| 3 | (Salt or sodium).tw. |
| 4 | 1 or 2 or 3 |
| 5 | Exp Diet |
| 6 | (Diet* or food or intake).tw. |
| 7 | 5 or 6 |
| 8 | 4 and 7 |
| 9 | Exp Sodium, Dietary |
| 10 | Exp Diet, Sodium‐Restricted |
| 11 | 8 or 9 or 10 |
| 12 | Limit 11 to English language |
| 13 | Limit 12 to yr=“2013–Current” |
| 14 | Limit 13 to humans |
The Science of Salt Weekly MEDLINE (Ovid) Search Strategy is a modified version of the search strategy developed by a Cochrane librarian to support the development of the Canadian Hypertension Education Program recommendations regarding dietary sodium.
Inclusion/Exclusion Criteria
Any randomized controlled trials (RCTs) or cohort studies in nonacutely ill adults that examined sodium intake (excluding studies with participants who had type II diabetes, chronic kidney disease, or chronic heart failure) and were published in English were included. Specifically, RCTs evaluating the association between sodium intake and BP were included if the following criteria were met: there was a minimum intervention period of 4 weeks, the intervention was composed of at least one group receiving decreased sodium intake compared with a control group, there was a difference of at least 2.3 g of salt (sodium 920 mg) per day between the intervention and the control groups, and sodium intake was measured by 24‐hour urinary excretion. RCTs were excluded if additional cointerventions were part of the study (including nondrug interventions, antihypertensives, or other drugs), thus preventing the independent assessment of the effects of reducing dietary salt. Cohort studies and RCTs evaluating the association between sodium intake and any health outcome were included if they were prospective in design, lasted at least 1 year, and measured sodium intake for at least 24 hours. Substantive adverse health outcomes of interest were all‐cause mortality, cardiovascular disease (CVD), stroke, coronary heart disease, kidney disease, and cancer. Other health outcomes, such as headache, renal stones, and obesity, as well as surrogate markers including endothelium‐dependent dilation, urinary marinobufagenin excretion, aortic pulse wave velocity, plasma, and urinary nitrate/nitrite were assessed. See Table 2 for the rationale of criteria for inclusion and exclusion of studies.
Table 2.
Rationale for Selection Criteria (Inclusion/Exclusion)
| Inclusion/Exclusion Criteria | Rationale |
|---|---|
| Randomized controlled trials (RCTs; blood pressure [BP]–lowering minimum standards) | |
| Achieved an intake difference of ≥920 mg sodium/2.3 g salt between intervention and control | Established by World Health Organization (WHO) Nutrition Guidance Expert Advisory Group Subgroup on Diet and Health as criteria for the WHO guideline on sodium intake to ensure that there is a substantive difference in intervention groups. The Global Burden of Disease Study uses 460 mg sodium/1.15 g salt as a surrogate for adequate statistical power to assess effectiveness of the intervention. |
| Duration of ≥4 weeks | An estimated minimum period required to observe maximum BP‐lowering effect of reduced dietary salt.47 |
| No additional co‐interventions (non‐drug, antihypertensives, other drug) | Required for the independent assessment of the effects of reducing dietary salt. |
| Sodium intake measured by 24‐hour urinary excretion | Minimum standard for sodium intake assessment in an individual, accurate assessment requires multiple days of 24‐hour urinary sodium measurements.18 |
| Cohort and RCT studies for other health outcomes | |
| Prospective design | Assessment of outcomes relating to dietary salt intake require methodologically robust study design that sets out to measure the association, ie, retrospective designs generally used to generate a hypothesis. |
| Duration of ≥1 year | A minimum period for many health events to occur and to achieve adequate statistical power to detect a change in an outcome of interest, eg, stroke and cardiovascular disease. Other outcomes such as renal failure and cancer may require substantially longer observation periods (ie, decades). |
| Sodium intake measured for 24 hours | Minimum standard for sodium intake assessment in an individual, accurate assessment requires multiple days of 24‐hour urinary sodium measurements. |
Data Extraction and Quality Assessment
Two reviewers (CJ and TSR) independently evaluated and excluded articles at the title/abstract review stage. Full‐text articles whose abstracts met the inclusion criteria were then reviewed. An Excel data extraction template was developed, tested, and then refined. This was used to populate data from articles that met the final inclusion criteria. For the RCT, methodological quality was assessed using the Cochrane risk of bias (ROB) assessment tool. The Cochrane ROB was modified with components of the National Heart, Lung, and Blood Institute Quality Assessment Tool for the assessment of the cohort study,11 and the AMSTAR tool12 was used to assess the quality of the systematic review. Discrepancies in article inclusion, data extraction, and bias assessment were solved by team consensus.
Results
A total of 6920 studies were identified, of which 144 were selected for full review (Figure). Of these, 141 were excluded for the following reasons: 128 studies were cross‐sectional or other excluded designs. One meta‐analysis13 and two RCTs were excluded because they included chronically ill populations: one involving patients with chronic disease (who also had hypertension) and one involving patients with chronic kidney disease (who were nondiabetic with hypertension).14, 15 Eight cohort studies did not investigate an outcome of interest for this review: one measured demographic determinants of subgroups with high sodium intake,16 one measured adherence to recommendations,17 one cohort study included unhealthy participants and did not investigate an outcome of interest,18 and six studies included unhealthy populations including those with kidney disease, high cardiovascular disease risk, type 1 and 2 diabetes, and multiple sclerosis.19, 20, 21, 22, 23, 24 In addition, two RCTs were excluded: one was an implementation trial that did not investigate BP or a health outcome25 and one study intervention was less than 4 weeks' duration.26 See Figure for details on excluded studies.
Figure 1.
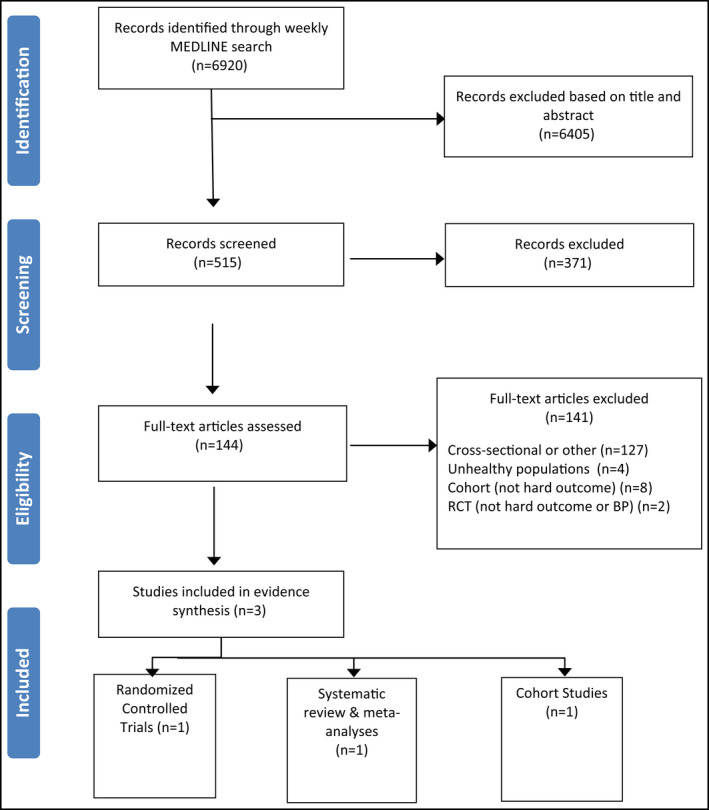
Included/excluded studies.
In all, three studies were included in the final review, as summarized in Table 3 (the ROB table is shown in Appendix S1). They include one RCT on the occurrence and severity of headache27; one cohort study on substantive patient outcomes including all‐cause mortality, CVD, and/or mortality28; and one systematic review and meta‐analysis on CVD mortality.29 The included RCT and cohort studies were conducted in the United States and the meta‐analysis included studies from Japan, the United States, Belgium, Scotland, the Netherlands, and Finland.
Table 3.
Included Studies
| Author | Country | Outcome | Population | Intervention/Follow‐Up | Measurement Method | Results | Comments |
|---|---|---|---|---|---|---|---|
| Surrogate markers (one RCT) | |||||||
| Amer et al27 | United States | Headache | 390 participants with prehypertension or stage one hypertension aged ≥22 y | Three levels of dietary sodium intake and two diet patterns (the DASH diet and a control diet). Three 30‐day periods, each at “high” sodium diet with 8.6 g salt (sodium 3400 mg)/d, “intermediate” sodium diet with 5.75 g salt (sodium 2300 mg)/d, and “low” sodium diet with 2.8 g salt (sodium 1120 mg)/d | 24‐h urinary sodium excretion; self‐administered questionnaire to assess severity of headache | Lower risk of headache on the “low” sodium diet with 2.8 g salt (sodium 1120 mg)/d, compared with “high” sodium diet with 8.6 g salt (sodium 3400 mg)/d, both on the control (OR, 0.69; 95% CI, 0.49–0.99; P=.05) and the DASH (OR, 0.69; 95% CI, 0.49–0.98; P=.04) diets | No significant association of diet pattern (DASH vs control) with headache on any sodium level. Headaches not a hard outcome |
| Substantive patient outcomes (two cohort, one systematic review and meta‐analysis, and one meta‐analysis [total of four studies]) | |||||||
| Singer et al28 | United States | All‐cause mortality; cardiovascular mortality | 3505 hypertensive participants | Follow‐up of 18.6 y | 24‐h urinary sodium excretion | There was a significant association between sodium and all‐cause mortality (Q1 vs Q4: HR, 0.81; 95%, CI, 0.66–1.00; P=.05). A significant association between dietary sodium and noncardiovascular mortality (Q1 vs Q4 ratio: HR, 0.57; 95% CI, 0.42–0.80; P=.001) | Exposure was only assessed at baseline. |
| Poggio et al29 | Japan, United States, Belgium, Scotland, the Netherlands, and Finland | Cardiovascular mortality | 229,785 participants | Average follow‐up period of 13.37 y (range 5.5–19 y) | Different methods used for assessment of sodium intake (24‐h urine, 24‐h dietary recall, food Frequency questionnaire and 3‐d dietary record) | There was a significant association between higher sodium intake and cardiovascular mortality (RR, 1.12; 95% CI, 1.06–1.19). Every increase of sodium 0.57 salt (sodium 230 mg)/d in sodium intake, CVD mortality increased by 1% (P=.016) | |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; HR, hazard ratio; OR, odds ratio; Q, quartile; RCT, randomized controlled trial; RR, relative risk.
Substantive Adverse Health Outcomes
One cohort study and one systematic review and meta‐analysis of cohort studies examined the relationship between salt intake and adverse health outcomes. In a worksite cohort study conducted in New York, Singer and colleagues28 analyzed data from 3505 people with hypertension, including 2243 men and 1262 women aged 52±10 years recruited between 1978 and 1999. The primary aim was to determine the association between baseline sodium intake and all‐cause and cardiovascular mortality over an average 18.6‐year follow‐up period. Sodium intake was assessed by a single 24‐hour urine collection during the study's pretreatment period during which participants were instructed to “avoid excessively salty food.” Mean 24‐hour urinary sodium was 7.47±3.96 g salt (sodium 2988±1584 mg)/d. Participants were classified into four quartiles based on the 24‐hour urinary sodium excretion: quartile 1 (Q1): 3.16±1.15 g salt (sodium 1264±460 mg)/d; quartile 2 (Q2): 5.86±0.97 g salt (sodium 2322±388 mg)/d; quartile 3 (Q3): 8.22±1.15 g salt (sodium 3288±460 mg)/d; and quartile 4 (Q4): 12.7±3.22 g salt (sodium 5080±1288 mg)/d. There was no reported marker for assessment of the completeness of urine collections, but sensitivity analyses were undertaken by excluding the top and bottom 1% of urinary sodium values and those with creatinine not between ±35% of estimated glomerular filtration rate, which did not substantially change the results. Unadjusted and multivariable‐adjusted Cox proportional hazards were performed to examine the association of sodium quartiles with mortality. There were 1013 deaths, of which 399 were from cardiovascular causes (128 cardiovascular deaths in Q1 vs 78 in Q4). Deaths caused by cancer (n=198) occurred in nearly half of known causes of noncardiovascular deaths (n=394). After adjustment, sodium intake was not significantly associated with cardiovascular mortality (Q1 vs Q4: hazard ratio [HR], 1.00; 95% confidence interval [CI], 0.71–1.42; P=.99), but there were fewer deaths overall in the lower vs higher salt consumption quartile (QI vs Q4: HR, 0.81; 95% CI, 0.66–1.00; P=.05). The adjusted model also showed a significant association between dietary sodium and noncardiovascular mortality (QI vs Q4: HR, 0.57; 95% CI, 0.42–0.80; P=.001). Therefore, higher baseline sodium intake (24‐hour urine sodium) was directly associated with harm from non‐CVD and total mortality, but was not significantly associated with any measured cardiovascular outcomes, which may have been subject to measurement bias or classification errors.
Poggio and colleagues29 conducted a systematic review and meta‐analysis of prospective studies representing the general population to assess whether elevated dietary sodium intake is associated with cardiovascular mortality. Authors reviewed data from 11 studies that assessed the relationship between daily sodium intake and mortality including 229,785 participants. There were 9346 CVD deaths after a mean follow‐up period of 13.37 years (range 5.5–19 years). The results indicated that higher sodium intake was associated with higher cardiovascular mortality (relative risk [RR], 1.12; 95% CI, 1.06, 1.19). An increase in cardiovascular mortality by 1% was seen per incremental increase of 0.57 g/d salt (230 mg/d sodium) (P=.016). Furthermore, there was a significant positive association between age, hypertensive status, length of follow‐up, and cardiovascular mortality. In the sensitivity analyses, it was found that higher sodium intake was significantly associated with higher cardiovascular mortality even when studies with a strong positive association and relative study weights were excluded (RR, 1.08; 95% CI, 1.01–1.15). The study results are consistent with higher sodium intake being associated with cardiovascular mortality in the general population.
Other Health Outcome—Headache
One study assessed the relationship between salt intake and headache.30 In that study, Amer and colleagues27 conducted a post hoc analysis of the Dietary Approaches to Stop Hypertension (DASH)–Sodium trial, a randomized, multicenter clinical study based in the United States. The trial included 390 healthy adults with systolic BPs between 120 and 159 mm Hg and diastolic BPs between 80 and 95 mm Hg, aged 22 years and older, with recruitment between September 1997 and November 1999. The investigators retrospectively analyzed the data to assess the association between the occurrences of headache and three levels of dietary sodium intake (ie, high [8.6 g salt, 3440 mg sodium/d], intermediate [5.75 g salt, 2300 mg sodium/d], and low [2.8 g salt, 1120 mg sodium/d]) and two diet patterns (ie, the DASH diet, which is rich in fruits, vegetables, and low‐fat dairy products with reduced saturated and total fat; and a control diet typical of Western consumption patterns). Participants were randomized to either the DASH diet group (n=208) or control group (n=204) using a parallel‐group design. During three 30‐day periods, participants consumed diets with varying amounts of sodium (ie, high, intermediate, and low) in random order. The occurrence and severity of headaches among participants were ascertained from self‐administered questionnaires completed at the end of each 30‐day period. There was a lower risk of headache with the low, compared with the high sodium intake exposures, and this was consistent with both the control (OR, 0.69; 95% CI, 0.49–0.99; P=.05) and the DASH (OR, 0.69; 95% CI, 0.49–0.98; P=.04) diets. These findings are consistent with the hypothesis that reduced sodium intake significantly lowers risk of headache.
Discussion
This annual systematic review has added to the evidence from our previous review of quality evidence on dietary salt (2013–2014), which identified 11 studies that confirmed an association between increasing dietary salt and increased BP and several adverse health outcomes. The three studies in our current 2014 to 2015 analysis that met our predefined quality criteria all demonstrated an increased risk of harm associated with higher dietary salt intake. One retrospective analysis showed that reduced dietary salt was associated with a lower risk of headaches, although further research is required to confirm this hypothesis. Two studies demonstrated associations between salt intake and mortality. The association of salt intake to total mortality and noncardiovascular (mostly cancer) deaths in the Singer study is particularly important, given the long length of follow‐up. It strengthens the evidence from previous associations of salt intake to gastric and renal cell cancer and relates to biomedical mechanisms of higher salt intake promoting cancer.31, 32, 33, 34
The systematic review excluded many studies based on failure to achieve our quality criteria. Low‐quality research methods (including short duration studies, clinically insignificant reductions in salt intake, flawed measurement methods such as spot urine samples to measure long‐term individual salt intake, and cohort studies in people with disease) have resulted in controversy over the impact of changing dietary salt on health.35, 36
The excluded studies reported both adverse and beneficial health effects associated with higher dietary salt intake. Four studies with low‐quality designs and including unhealthy participants showed an association between increased salt intake and adverse health outcomes and three suggested harm in people who consumed lower salt. Notably, to this effect, Graudal and colleagues13 published a meta‐analysis of cohort studies that included unhealthy populations who may consume less but who are also at higher risk for death. This means the study was predisposed to reverse causation (they are eating less food (and hence sodium) and more likely to die because they are sick rather than being sick and dying because they are eating less sodium). Consistent with this, the meta‐analysis found a U‐shaped relationship between dietary salt and all‐cause mortality and cardiovascular mortality. Many of the studies included in the meta‐analysis used spot urine samples and 24‐hour dietary recalls for measuring sodium intake. A previously reported examination of a subanalysis of the Graudal meta‐analysis identified a number of data extraction errors and inconsistent classification of data.10
Two papers based on data from the Prospective Urban Rural Epidemiology (PURE) study were also excluded from this review as they did not meet the inclusion criteria for measuring sodium intake. The PURE study used a single spot urine sample to estimate individuals' long‐term salt intake. There are multiple factors regulating short‐term sodium excretion that affect the amount of sodium in single spot urine samples. These factors may also predict individual cardiovascular risk and include creatinine, dietary potassium, calcium and magnesium, renin, angiotensin, aldosterone, sympathetic activation, and hydration37, 38, 39, 40 The U‐shaped relationship between salt intake and death or cardiovascular events and the curvilinear relationship between salt intake and BP that were purportedly shown by the PURE study may have been influenced by one or more of these factors. Likewise, the equation used to estimate 24‐hour salt intake from the spot urine samples in the PURE study also utilized age, sex, and creatinine values, which are well‐established confounding health risks. This means it is not appropriate to correlate health outcomes with salt intake based on salt intake estimated from spot urines using these equations. Furthermore, the validation study in the PURE study had four errata in relating the spot urine sodium estimates of salt intake to 24‐hour urine samples and has also been critiqued for multiple methodological flaws. These include a very high rate of incomplete 24‐hour urine samples, lack of a relationship of PURE BP results to high‐quality BP surveys, and the potential for reverse causality.41, 42, 43 More recently, data from the PURE study conducted in China found a much lower association of spot urine to 24‐hour urine sodium than the main PURE study confirming prior observations that a single spot urine sample may be a reliable and reproducible estimate of individual 24‐hour urinary salt intake.44
The review here used modest quality‐control criteria for including studies. Use of more rigorous criteria could increase reliability, but very few studies would be eligible. Accurately assessing individual salt intake requires several days of complete 24‐hour urine collections while our criteria only required a single 24‐hour period to assess salt intake. Accurate assessment of salt intake relies on complete collections of urine over a 24‐hour period but in many studies 24‐hour urine collections are not complete.18, 45 The use of adequate criteria for assessing salt intake is important as otherwise inaccurate classification of an individual's salt consumption level will predispose to the null hypothesis or potentially U‐shaped relationships if those who are noncompliant with 24‐hour urine collection are falsely classified as consuming low amounts of salt.45, 46 In the Singer and colleagues28 study, high salt intake was not associated with CVD but was associated with total mortality; however, misclassifications of causes of death are common and may have covered the relationship between salt intake and cardiovascular deaths. While these challenges will continue to be present, researchers should endeavor to use rigorous research methods to ensure reliable reproducible results, and granting agencies and journals should be more diligent in reviewing studies that lack rigor in their research methodology.
As in the previous iteration of this review, the fact that only literature published within a single year is covered is a potential limitation. In addition, cohort studies conducted in people with chronic disease (which are prone to reverse causation) were excluded. However, the inclusion of recent comprehensive systematic reviews on the impact of high dietary salt on cardiovascular patient outcomes, BP, and some surrogate markers in this and last year's systematic reviews represents much of the relevant research.
Reiterating the international concerns about low‐quality research on dietary salt, we note the very high number of publications excluded based on failure to achieve what are very modest quality criteria compared with the few studies that met the criteria.8 This supports the development of minimum research standards on dietary salt under the auspices of an international consortium of health and scientific organizations.
The findings of this updated systematic review of studies that are relevant to clinical and population efforts are in support of current recommendations to reduce dietary salt and, specifically, the recommendations of the WHO, World Hypertension League, International Society of Hypertension, and multiple other international and national governmental and nongovernmental organizations.
Conclusions
This systematic review has further added to the evidence that higher salt intake is associated with adverse health outcomes and supports the current global targets for a reduction in dietary salt intake.
Disclosures
NC has a contract with Novartis Foundation to assist in hypertension control interventions in low resource settings and is a member of World Action on Salt and Health (a dietary salt reduction organization). JA, CJ, TSR, KT, RM, and AL have no conflicts of interest to disclose. JW is Director of the WHO Collaborating Centre on Population Salt Reduction and is supported by a National Health and Medical Research Council/National Heart Foundation Career Development Fellowship on international strategies to reduce salt. JW has funding from WHO, VicHealth, and the Australian National Health and Medical Research Council of Australia for research on salt reduction. MW is a research consultant with Arbor Research Collaborative for Health.
Supporting information
Appendix S1. Risk of bias tables for included studies.
Acknowledgments
The process to provide regular updates on the science of sodium is supported by the World Hypertension League, WHO Collaborating Centre on Population Salt Reduction (George Institute for Global Health), Pan American Health Organization/WHO Technical Advisory Group on Cardiovascular Disease Prevention through Dietary Sodium, World Action on Salt and Health, and the HSF CIHR Chair in Hypertension Prevention and Control.
J Clin Hypertens (Greenwich). 2016;18:832–839. DOI: 10.1111/jch.12877. © 2016 Wiley Periodicals, Inc.
References
- 1. Asaria P, Chisholm D, Mathers C, et al. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–2053. [DOI] [PubMed] [Google Scholar]
- 2. Institute for Health Metrics and Evaluation (IHME) . GBD Compare. http://vizhub.healthdata.org/gbd-compare. Accessed Dec 22, 2015.
- 3. World Health Organisation . Reducing salt intake in populations: report of a WHO forum and technical meeting 5–7 October 2006. 2007 Paris 2007.
- 4. World Health Organisation . Draft comprehensive global monitoring framework and targets for the prevention and control of noncommunicable diseases: Formal Meeting of Member States to conclude the work on the comprehensive global monitoring framework, including indicators, and a set of voluntary global targets for the prevention and control of noncommunicable diseases. A/NCD/INF./1. November 5–7; 2012.
- 5. Stolarz‐Skrzypek K, Kuznetsova T, Thijs L, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–1785. [DOI] [PubMed] [Google Scholar]
- 6. Taylor R, Ashton K, Moxham T, et al. Reduced dietary salt for the prevention of cardiovascular disease: a meta‐analysis of randomized controlled trials (Cochrane review). Am J Hypertens. 2011;24:843–853. [DOI] [PubMed] [Google Scholar]
- 7. Huang L, Crino M, Wu J, et al. Mean population salt intake estimated from 24‐h urine samples and spot urine samples: a systematic review and meta‐analysis. Int J Epidemiol 2016;45:239–250. [DOI] [PubMed] [Google Scholar]
- 8. Campbell N, Appel L, Cappuccio F, et al. A call for quality research on salt intake and health: from the World Hypertension League and supporting organizations. J Clin Hypertens (Greenwich). 2014;16:469–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson C, Raj T, Trudeau L, et al. The science of salt: a systematic review of clinical salt studies 2013 to 2014. J Clin Hypertens (Greenwich). 2015;17:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arcand J, Webster J, Johnson C, et al. Announcing “Up to Date in the Science of Sodium.” J Clin Hypertens (Greenwich). 2016; 18: 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies. NIH National Heart, Lung and Blood Institute; 2014. [Google Scholar]
- 12. Shea B, Grimshaw J, Wells G, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graudal N, Jürgens G, Baslund B, Alderman M. Compared with usual sodium intake, low‐and excessive‐sodium diets are associated with increased mortality: a meta‐analysis. Am J Hypertens. 2014;27:1129–1137. [DOI] [PubMed] [Google Scholar]
- 14. Ahn S, Kim S, Kim D, et al. Urinary sodium excretion has positive correlation with activation of urinary renin angiotensin system and reactive oxygen species in hypertensive chronic kidney disease. J Korean Med Sci. 2014;29(Suppl 2):S123–S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hwang J, Chin H, Kim S, et al. Effects of intensive low‐salt diet education on albuminuria among nondiabetic patients with hypertension treated with olmesartan: a single‐blinded randomized, controlled trial. Clin J Am Soc Nephrol. 2014;9:2059–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nerbass F, Pecoits‐Filho R, McIntyre N, et al. Demographic associations of high estimated sodium intake and frequency of consumption of high‐sodium foods in people with chronic kidney disease stage 3 in England. J Ren Nutr. 2014;24:236–242. [DOI] [PubMed] [Google Scholar]
- 17. Jonsdottir S, Brader L, Gunnarsdottir I, et al. Adherence to the Nordic Nutrition Recommendations in a Nordic population with metabolic syndrome: high salt consumption and low dietary fibre intake (The SYSDIET study). Food Nutr Res. 2013;57: 10.3402/fnr.v57i0.21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakaki M, Tsuchihashi T, Arakawa K, et al. Long‐term variability of urinary salt excretion and blood pressure in hypertensive patients. Hypertens Res. 2014;37:939–943. [DOI] [PubMed] [Google Scholar]
- 19. Horikawa C, Yoshimura Y, Kamada C, et al. Dietary sodium intake and incidence of diabetes complications in Japanese patients with type 2 diabetes: analysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab. 2014;99:3635–3643. [DOI] [PubMed] [Google Scholar]
- 20. Engelen L, Soedamah‐Muthu S, Geleijnse J, et al. Higher dietary salt intake is associated with microalbuminuria, but not with retinopathy in individuals with type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetologia. 2014;57:2315–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smyth A, Dunkler D, Gao P, et al. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014;86:1205–1212. [DOI] [PubMed] [Google Scholar]
- 22. Farez M, Fiol M, Gaitán M, et al. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 2015;86:26–31. [DOI] [PubMed] [Google Scholar]
- 23. Fan L, Tighiouart H, Levey A, et al. Urinary sodium excretion and kidney failure in nondiabetic chronic kidney disease. Kidney Int. 2014;86:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merino J, Guasch‐Ferré M, Martínez‐González MA, et al. Is complying with the recommendations of sodium intake beneficial for health in individuals at high cardiovascular risk? Findings from the PREDIMED study. Am J Clin Nutr. 2015;101:440–448. [DOI] [PubMed] [Google Scholar]
- 25. Markota N, Rumboldt M, Rumboldt Z. Emphasized warning reduces salt intake: a randomized controlled trial. J Am Soc Hypertens. 2015;9:214–220. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Liu F, Wang D, et al. Effect of salt intake and potassium supplementation on serum renalase levels in Chinese adults: a randomized trial. Medicine (Baltimore). 2014;93:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amer M, Woodward M, Appel LJ. Effects of dietary sodium and the DASH diet on the occurrence of headaches: results from randomised multicentre DASH‐Sodium clinical trial. BMJ Open. 2014;4:e006671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singer P, Cohen H, Alderman M. Assessing the associations of sodium intake with long‐term all‐cause and cardiovascular mortality in a hypertensive cohort. Am J Hypertens. 2015;28:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poggio R, Gutierrez L, Matta MG, et al. Daily sodium consumption and CVD mortality in the general population: systematic review and meta‐analysis of prospective studies. Public Health Nutr. 2014;22:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cooper W, Glover D, Hormbrey J, Kimber G. Headache and blood pressure: evidence of a close relationship. J Hum Hypertens. 1989;3:41–44. [PubMed] [Google Scholar]
- 31. Djamgoz M. Blood pressure and risk of cancer progression–a possible connection with salt and voltage‐gated sodium channel. Med Hypotheses. 2015;85:591–593. [DOI] [PubMed] [Google Scholar]
- 32. Deckers I, van den Brandt P, van Engeland M, et al. Long‐term dietary sodium, potassium and fluid intake; exploring potential novel risk factors for renal cell cancer in the Netherlands Cohort Study on diet and cancer. Br J Cancer. 2014;110:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park J, Kim Y, Koo H, et al. Estimated amount of 24‐hour urine sodium excretion is positively correlated with stomach and breast cancer prevalence in Korea. J Korean Med Sci. 2014;29(Suppl 2):S131–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang X, Terry P, Yan H. Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World J Gastroenterol. 2009;15:2204–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graudal N, Hubeck‐Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2011;(11):CD004022. [DOI] [PubMed] [Google Scholar]
- 36. IOM . Institute of Medicine Committee on Public Health Priorities to Reduce and Control Hypertension, Population‐Based Policy and Systems Change Approach to Prevent and Control Hypertension. Washington, DC: 2010. [Google Scholar]
- 37. Ji C, Sykes L, Paul C, et al. Systematic review of studies comparing 24‐hour and spot urine collections for estimating population salt intake. Revista Panamericana de Salud Pública. 2012;32:307–315. [DOI] [PubMed] [Google Scholar]
- 38. McLean R, Williams S, Mann J. Monitoring population sodium intake using spot urine samples: validation in a New Zealand population. J Hum Hypertens. 2014;28:657–662. [DOI] [PubMed] [Google Scholar]
- 39. Majoor C, Vogt L. Can sodium excretion from single fasting morning urine really be used for estimation of dietary sodium intake? J Hypertens. 2014;32:2500–2501. [DOI] [PubMed] [Google Scholar]
- 40. Pan American Health Organization . Salt‐Smart Americas: A Guide for Country‐Level Action. Washington, DC: PAHO; 2013. [Google Scholar]
- 41. Campbell N. Questionable scientific basis for relaxed dietary sodium recommendations. Can J Cardiol. 2016. doi: 10.1016/j.cjca.2015.10.027 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42. Campbell N, Lackland D, Niebylski M, Nilsson P. Is reducing dietary sodium controversial? Is it the conduct of studies with flawed research methods that is controversial? A perspective from the World Hypertension League Executive Committee. J Clin Hypertens (Greenwich). 2015;17:85–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He F, Ivkovic V, Jelakovic B, et al. Estimation of sodium excretion should be made as simple as possible, but not simpler: misleading papers and editorial on spot urines. J Hypertens. 2015;33:884–886. [DOI] [PubMed] [Google Scholar]
- 44. Peng Y, Li W, Wang Y, et al. Validation and assessment of three methods to estimate 24‐h urinary sodium excretion from spot urine samples in Chinese adults. PLoS One. 2016;11:e0149655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cogswell M, Maalouf J, Elliott P, et al. Use of urine biomarkers to assess sodium intake: challenges and opportunities. Annu Rev Nutr. 2015;35:349–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cook N, Appel L, Whelton P. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014; 129 : 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Law M, Frost C, Wald N. By how much does dietary salt reduction lower blood pressure? III–Analysis of data from trials of salt reduction. BMJ. 1991;302:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Risk of bias tables for included studies.


