Abstract
Hypertension is one of the major side effects of sorafenib, and reported incidences vary substantially among clinical trials. A systematic review was conducted using Medline, PubMed, Embase, and the Cochrane Library for all longitudinal studies to investigate the incidence and risk of hypertension events in cancer patients treated with sorafenib. A total of 14 randomized controlled trials and 39 prospective single‐arm trials involving 13,555 patients were selected for the meta‐analysis. The relative risk of all‐grade and high‐grade hypertension associated with sorafenib were 3.07 (95% confidence interval [CI], 2.05–4.60; P<.01) and 3.31 (95% CI, 2.21–4.95; P<.01), respectively. The overall incidence of sorafenib‐induced all‐grade and high‐grade hypertension were 19.1% (95% CI, 15.8%–22.4%) and 4.3% (95% CI, 3.0%–5.5%), respectively. A significantly higher incidence of hypertension was noted in patients with renal cell carcinoma (RCC) compared with those with non‐RCC malignancies (all‐grade: 24.9% [95% CI, 19.7%–30.1%] vs 15.7% [95% CI, 12.1%–19.3%]; P<.05; high‐grade:8.6% [95% CI, 6.0%–11.2%] vs 1.8% [95% CI, 0.9%–2.6%]; P<.05). The trials with median progression‐free survival (PFS) longer than 5.3 months (mean PFS) demonstrated a significantly higher incidence of high‐grade hypertension than trials with shorter PFS (6.3% [95% CI, 4.1%–8.5%] vs 2.6% [95% CI, 1.4%–3.8%]; P<.05). Findings of the meta‐analysis indicated a significantly high risk of sorafenib‐induced hypertension. Patients with RCC have a significantly higher incidence of hypertension and the occurrence of hypertension may be associated with improved prognosis.
Targeted agents have substantially improved the outcome of patients with cancer. Sorafenib, as the first multikinase inhibitor and one of the most widely used small‐molecule oral‐targeted drugs, has a broad spectrum of antitumor activity that induces both tumor apoptosis and disruption of the tumor vasculature.1 At present, sorafenib is recommended as the standard first‐line treatment for advanced hepatocellular carcinoma (HCC),2 the second‐line treatment for advanced renal cell carcinoma (RCC),3 and, more recently, the treatment of late‐stage (metastatic) differentiated thyroid cancer (http://www.fda.gov). New indications and treatment modalities of sorafenib are being explored by current clinical research for a wide range of tumors, for instance, prostate cancer and melanoma.4, 5, 6
Hypertension is one of the common side effects associated with sorafenib and has been noted in clinical trials with high incidence. In clinical trials, the onset of hypertension in sorafenib‐treated patients can occur at any time during therapy and the reported incidences of hypertension associated with sorafenib therapy vary substantially. Although a previous meta‐analysis7 reported a significant increase in the risk of hypertension with sorafenib, these estimates are based on only a small number of studies of varying quality, and, as much of the evidence relates to patients with RCC, they may not be applicable for patients with other types of malignancies. Moreover, the risk factors for the development of hypertension, an important issue in reducing the risk of occurrence, have not been elucidated. In the past 5 years, many more prospective clinical trials with larger sample size and various types of malignancies have been performed. We proposed that pooling the analyses of recent studies could provide a better understanding on the overall risk of hypertension and the underlying risk factors. Therefore, we performed a systematic review and meta‐analysis of the published prospective studies to further investigate the incidence and risk of hypertension associated with sorafenib.
Materials and Methods
Data Sources
We systematically searched the electronic databases Medline, PubMed, EMBASE, Society of Clinical Oncology annual meetings (http://www.asco.org),and the Cochrane Library for Central Register of Clinical Trials, using the MESH terms “sorafenib,” with the key words “cancer” and “clinical trial.” We limited our search to studies in human patients and English language in peer‐reviewed journals from 1966 to October 2013. The reference lists of identified articles and bibliographies of original articles were also reviewed. The articles that were not freely available to us were requested from the authors.
Study Selection
Trials that met the following criteria were chosen for analysis: (1) randomized controlled trials (RCTs) that directly compared cancer patients treated with and without sorafenib; prospective uncontrolled single‐arm trials in which sorafenib as a single systematic administration was given at a starting dose of 400 mg twice a day; (2) safety data available for the events or incidences of hypertension; and (3) at least 20 patients were enrolled in every clinical trial. Data extraction was conducted independently by two investigators (LY and LS) and according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement (www.prisma-statement.org). Any discrepancies were resolved by consensus. We mainly extracted the following information: first author's name; year of publication; trial design; number of enrolled patients; treatment arms; number of cases in the treatment and placebo groups (when available); underlying malignant disease; median age; median treatment duration; median progression‐free survival (PFS); adverse outcomes of hypertension; and doses and schedules used.
Clinical Endpoints
Clinical endpoints were selected from the safety profile of each trial. Hypertension was recorded according to versions 2 or 3 of the Common Terminology Criteria for Adverse Events (CTCAE; http://ctep.cancer.gov): grade 1, asymptomatic, transient (<24 hours) increase in blood pressure (BP) by >20 mm Hg (diastolic) or to >150/100 mm Hg if previously within normal limits, intervention not indicated; grade 2, recurrent or persistent (>24 hours) or symptomatic increase by >20 mm Hg (diastolic) or to >150/100 mm Hg if previously within normal limits, monotherapy might be indicated; grade 3, >1 drug needed for treatment or more intensive treatment than used previously; and grade 4, life‐threatening consequences (eg, hypertensive crisis). We included the incidence of hypertension of grade I or above in our analysis. We included the incidence of all‐grade and high‐grade hypertension (grade 3 or above) in our analysis.
Statistical Analysis
Stata statistical software package (release 11.2; Stata Corporation, College Station, TX) was used for statistical analysis. The proportion of both all‐grade and high‐grade (grade 3 and 4) hypertension were derived from each study. For studies with a control group, the relative risk (RR) of hypertension was also calculated. For the meta‐analysis, we used a fixed‐effects (weighted with inverse variance) or random‐effects model.8 The Cochran's Q statistic and I² statistics were first calculated to assess the heterogeneity among the proportions of the included trials. If the P value was <.1, the assumption of homogeneity was deemed invalid, and the random‐effects model was reported after exploring the causes of heterogeneity.9 Otherwise, the fixed‐effects model was reported. Subgroup analyses were performed to identify risk factors with sorafenib‐based therapy. Publication bias was quantified by Begg's test and Egger's test. A 2‐tailed P value <.05 was considered statistically significant. We also used funnel plots to evaluate the publication bias.
Results
Patient Characteristics
Our search yielded 264 clinical studies, of which 211 were initially excluded. After evaluating each remaining study, a total of 53 studies with 13,555 patients were available for analysis (Figure 1). The main characteristics of the included trials are presented in Table 1. The baseline Eastern Cooperative Oncology Group performance status for most patients was between 0 and 1. Malignant diseases included were RCC (12 studies), HCC (7 studies), prostate cancer (5 studies), thyroid cancer (3 studies), sarcomas (3 studies), melanoma (4 studies), non–small‐cell lung cancer (6 studies), breast cancer (2 studies), gallbladder carcinoma and cholangiocarcinoma (1 study), squamous cell carcinoma of the head and neck (1 study), neuroendocrine tumor (1 study), gastric stromal tumor (1 study), squamous cell carcinoma of the head and neck/nasopharyngeal carcinoma (1 study), uterine carcinoma/carcinosarcoma (1 study), osteosarcoma (1 study), mesothelioma (1 study), lymphoma (1 study), and acute myelocytic leukemia (1 study). The starting dose and schedule of sorafenib was based on that currently approved by the US Food and Drug Administration (400 mg, orally, twice daily) in each trial.
Figure 1.
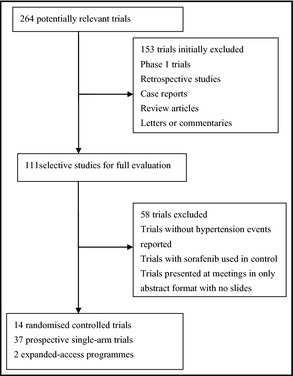
Selection process for trials.
Table 1.
Characteristics of Clinical Trials and Patients Included in the Meta‐Analysis
| Trial | Trial Design | Patients Enrolled, No. | Sample Size, No. | Treatment Arms | Median Age, y | Underlying Malignancy | Median TD, mo | Median PFS, mo |
|---|---|---|---|---|---|---|---|---|
| Ratain30 | Single‐arm phase II | 202 | 202 | Sorafenib 400 mg BID | 58 | RCC[Link] | 3 | 6 |
| Escudier31 | Randomized phase III | 903 | 451 | Sorafenib 400 mg BID/placebo | 58 | RCC | 5.7 | 5.5 |
| Stadler32 | EAP | 2502 | 2502 | Sorafenib 400 mg BID | 63 | RCC | 5.5 | 9 |
| Escudier33 | Randomized phase II | 189 | 97 | Sorafenib 400 mg bid/placebo | 62 | RCC | 6 | 5.7 |
| Procopio34 | Single‐arm phase II | 128 | 62 | Sorafenib 400 mg BID | 62 | RCC | 6.8 | 7 |
| Rini35 | Randomized phase III | 723 | 362 | Sorafenib 400 mg BID/axitinib | 61 | RCC | 5 | 4.7 |
| Di Lorenzo36 | Single‐arm phase II | 52 | 52 | Sorafenib 400 mg BID | 60 | RCC | 4.3 | 4 |
| Akaza37 | Single‐arm phase II | 131 | 131 | Sorafenib 400 mg BID | 63 | RCC | 7 | 7.5 |
| Beck38 | EAP | 1159 | 1145 | Sorafenib 400 mg BID | 62 | RCC | NR | 6.6 |
| Zhang39 | Single‐arm phase II | 98 | 98 | Sorafenib 400 mg BID | NR | RCC | 19 | 15 |
| Garcia40 | Single‐arm phase II | 47 | 47 | Sorafenib 400 mg BID | 64 | RCC | 3 | 4.4 |
| Motzer41 | Randomized phase III | 517 | 257 | Sorafenib 400 mg BID/tivozanib | 50 | RCC | NR | 9.1 |
| Kudo42 | Randomized phase III | 458 | 229 | Sorafenib 400 mg BID/placebo | 69 | HCC[Link] | 4 | 5.4[Link] |
| Llovet43 | Randomized phase III | 602 | 297 | Sorafenib 400 mg BID/placebo | 65 | HCC | NR | 4.1 |
| Cheng44 | Randomized phase III | 271 | 149 | Sorafenib 400 mg BID/placebo | 51 | HCC | NR | 2.8[Link] |
| Sansonno45 | Randomized phase II | 80 | 40 | Sorafenib 400 mg BID/placebo | 73 | HCC | NR | 9.2[Link] |
| Yau46 | Single‐arm phase II | 51 | 51 | Sorafenib 400 mg BID | 56 | HCC | 3 | 3 |
| Di Costanzo47 | Single‐arm phase II | 116 | 116 | Sorafenib 400 mg BID | 67 | HCC | 3 | 12[Link] |
| Duan48 | Single‐arm phase II | 52 | 52 | Sorafenib 400 mg BID | 51 | HCC | NR | 10 |
| Steinbild49 | Single‐arm phase II | 57 | 55 | Sorafenib 400 mg BID | 70 | Prostate cancer | 3 | 2 |
| Safarinejad6 | Single‐arm phase II | 64 | 64 | Sorafenib 400 mg BID | 69 | Prostate cancer | 1.5 | 2.9 |
| Aragon‐Ching50 | Single‐arm phase II | 24 | 24 | Sorafenib 400 mg BID | 66 | Prostate cancer | NR | 3.7 |
| Dahut51 | Single‐arm phase II | 22 | 22 | Sorafenib 400 mg BID | 64 | Prostate cancer | 1 | 1.8 |
| Chi52 | Single‐arm phase II | 28 | 28 | Sorafenib 400 mg BID | 67 | Prostate cancer | 2 | 2.3 |
| Scagliotti53 | Randomized phase II | 922 | 43 | CP+sorafenib 400 mg BID/CP+placebo | 62 | NSCLC[Link] | 3.9 | 4.6 |
| Paz‐Ares54 | Randomized phase III | 769 | 385 | GC+sorafenib 400 mg BID/GC+placebo | 60 | NSCLC[Link] | 4 | 6 |
| Dingemans55 | Single‐arm phase II | 59 | 59 | Sorafenib 400 mg BID | 58.5 | NSCLC[Link] | 2.1 | 2.3 |
| Wakelee56 | Single‐arm phase II | 333 | 333 | Sorafenib 400 mg BID | 64 | NSCLC[Link] | 2 | 2.3 |
| Blumenschein57 | Single‐arm phase II | 54 | 52 | Sorafenib 400 mg BID | NR | NSCLC[Link] | 2.3 | 2.7 |
| Spigel58 | Randomized phase II | 165 | 111 | Erlotinib+sorafenib 400 mg BID/erlotinib+placebo | 65 | NSCLC | NR | 3.9 |
| Eisen59 | Single‐arm phase II | 502 | 37 | Sorafenib 400 mg BID | 53 | Melanoma | 3 | 2.6 |
| Ott5 | Single‐arm phase II | 36 | 36 | Sorafenib 400 mg BID | 64 | Melanoma | 2 | NR |
| McDermott29 | Randomized phase II | 101 | 51 | DTIC+sorafenib 400 mg BID/DTIC+placebo | 58 | Melanoma | 4.5 | 4.9 |
| Flaherty60 | Randomized phase III | 790 | 393 | CP+sorafenib 400 mg BID/CP+placebo | 16 | Melanoma | 5 | 4.9 |
| Gupta‐Abramson4 | Single‐arm phase II | 30 | 30 | Sorafenib 400 mg BID | 63 | Thyroid cancer | 6.3 | 18.4 |
| Kloos61 | Single‐arm phase II | 56 | 56 | Sorafenib 400 mg BID | 61 | Thyroid cancer | 10.4 | 15 |
| Savvides62 | Single‐arm phase II | 20 | 20 | Sorafenib 400 mg BID | 59 | Thyroid cancer | 2 | 1.9 |
| Moreno‐Aspitia63 | Single‐arm phase II | 23 | 23 | Sorafenib 400 mg BID | 54 | Breast cancer | 2 | 2 |
| Baselga64 | Randomized phase II | 224 | 112 | Capecitabine+sorafenib 400 mg BID/capecitabine+placebo | 55 | Breast cancer | 7.7 | 6.4 |
| Elser65 | Single‐arm phase II | 27 | 26 | Sorafenib 400 mg BID | 53 | SCCHN/NPC | 2 | 1.8[Link] |
| Williamson66 | Single‐arm phase II | 41 | 41 | Sorafenib 400 mg BID | 63.5 | SCCHN[Link] | NR | 4 |
| Maki67 | Single‐arm phase II | 145 | 144 | Sorafenib 400 mg BID | 55 | Sarcomas | 3 | 3.2 |
| Grignani68 | Single‐arm phase II | 35 | 35 | Sorafenib 400 mg BID | 21 | Osteosarcoma | 4.4 | 4 |
| von Mehren69 | Single‐arm phase II | 51 | 37 | Sorafenib 400 mg BID | 63 | Sarcomas | NR | 3 |
| Santoro70 | Single‐arm phase II | 100 | 100 | Sorafenib 400 mg BID | 54 | Sarcomas | 1 | 4.2 |
| Hobday71 | Single‐arm phase II | 93 | 93 | Sorafenib 400 mg bid | 59 | NET | NR | NR |
| Nimeiri72 | Single‐arm phase II | 56 | 56 | Sorafenib 400 mg BID | 64 | UC/CS | 3 | 3.2 |
| El‐Khoueiry73 | Single‐arm phase II | 36 | 31 | Sorafenib 400 mg BID | 57 | GC/CC | 2 | 3 |
| Dubey74 | Single‐arm phase II | 51 | 50 | Sorafenib 400 mg BID | 69 | Mesothelioma | 3 | 3.6 |
| Park75 | Single‐arm phase II | 31 | 31 | Sorafenib 400 mg BID | 59 | GST[Link] | 5.7 | 4.9 |
| Guidetti76 | Single‐arm phase II | 30 | 30 | Sorafenib 400 mg BID | 61 | Lymphoma | 4 | 4 |
| Serve77 | Randomized phase II | 197 | 102 | SC+sorafenib 400 mg BID/SC+placebo | NR | AML | NR | 7 |
| Goncalves78 | Randomized phase III | 102 | 50 | Gemcitabine+sorafenib 400 mg BID/gemcitabine+placebo | 61 | Pancreatic cancer | 2 | 3.8 |
Abbreviations: A, Asian population; AML, acute myelocytic leukemia; BID, twice daily; C, Caucasian; CP, carboplatin and paclitaxel chemotherapy; EAP, expanded access program; GC, gemcitabine and cisplatin chemotherapy; GC/CC, gallbladder carcinoma and cholangiocarcinoma; GST, gastric stromal tumor; HCC, hepatocellular carcinoma; M, multiple race; NET, neuroendocrine tumor; NR, not reported; NSCLC, non–small‐cell lung carcinoma; PFS, progression‐free survival; RCC, renal cell cancer; SCCHN, squamous cell carcinoma of the head and neck; SCCHN/NPC, squamous cell carcinoma of the head and neck/nasopharyngeal carcinoma; TD, treatment duration; UC/CS, uterine carcinoma/carcinosarcoma; SC, standard 7 + 3 induction chemotherapy plus two cycles of consolidation therapy with intermediate dose (6×1 g/sqm) AraC. Only Time to Progression was reported.
RR of Hypertension Events
We calculated the RR of all‐grade and high‐grade hypertension associated with sorafenib treatment compared with the control treatment from 14 randomized controlled trials (2930 patients in the sorafenib group and 2790 patients in the control group). Using a random‐effects model, the RR of all‐grade hypertension associated with sorafenib vs control was 3.07 (95% confidence interval [CI], 2.05–4.60; P<.01; Figure 2). The RR of high‐grade hypertension associated with sorafenib vs control was 3.31 (95% CI, 2.21–4.95; P<.01) as calculated using the fixed‐effects model (Figure 3). Stratified analysis by the presence or not of concomitant chemotherapy demonstrated similar risks (Table 2).
Figure 2.
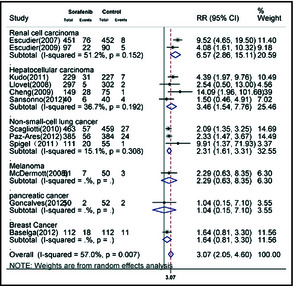
Meta‐analysis of the relative risk (RR) of developing all‐grade hypertension in cancer patients receiving sorafenib. CI indicates confidence interval.
Figure 3.
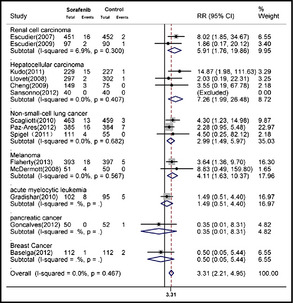
Meta‐analysis of the relative risk (RR) of developing high‐grade hypertension in cancer patients receiving sorafenib. CI indicates confidence interval.
Table 2.
Relative Risk of Sorafenib‐Associated Hypertension vs Control From Randomized Controlled Trials of Cancer Patients Stratified by Underlying Malignancy and Concomitant Chemotherapy
| Subgroup | Study, No. | Sample Size | Relative Risk of Hypertension | ||||
|---|---|---|---|---|---|---|---|
| Sorafenib | Control | All‐Grade | 95% CI | High‐Grade | 95% CI | ||
| Stratified by underlying malignancy | |||||||
| RCC | 2 | 548 | 542 | 6.57 | 2.86–15.11 | 5.9 | 1.76–19.86 |
| HCC | 4 | 715 | 644 | 3.46 | 1.54–7.76 | 7.26 | 1.99–26.48 |
| NSCLC | 2/1 | 959 | 898 | 2.31 | 1.62–3.31 | 2.99 | 1.49–5.97 |
| Melanoma | 3 | 494 | 447 | 2.29 | 0.63–8.35 | 4.12 | 1.63–10.37 |
| Pancreatic cancer | 1 | 50 | 52 | 1.04 | 0.15–7.10 | 0.35 | 0.01–8.31 |
| Breast cancer | 1 | 112 | 112 | 1.64 | 0.81–3.31 | 0.5 | 0.05–5.44 |
| AML | 1 | 102 | 95 | – | – | 1.49 | 0.51–4.40 |
| Stratified by concomitant chemotherapy | |||||||
| Without | 6 | 1263 | 1186 | 4.59 | 2.50–8.40 | 6.54 | 2.70–15.84 |
| With | 8 | 1667 | 1604 | 2.14 | 1.62–2.82 | 2.57 | 1.62–4.07 |
Abbreviations: AML, acute myelocytic leukemia; CI, confidence interval; HCC, hepatocellular carcinoma; NSCLC, non–small‐cell lung carcinoma; RCC, renal cell carcinoma.
Incidence of Hypertension Events
In the incidence analysis, 7109 patients were included for all‐grade hypertension events, and 7853 were included for high‐grade events. This numerical difference was attributable to the fact that some trials reported only all‐grade events and others high‐grade events alone. Using a random‐effects model, we determined that the overall incidence of all‐grade hypertension in patients receiving sorafenib was 19.1% (95% CI, 15.8%–22.4%). The overall incidence of high‐grade hypertension was 4.3% (95% CI, 3.0%–5.5%; Figure 4).
Figure 4.
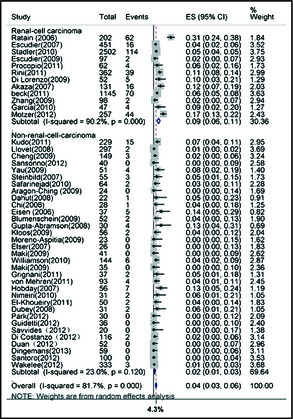
Meta‐analysis of incidence of high‐grade hypertension in cancer patients receiving sorafenib. ES indicates effect size; CI, confidence interval.
In the subgroup analysis, we first determined whether patients with RCC were associated with a higher risk for hypertension relative to other cancer patients. As shown in Table 3, a significant difference of the incidence of all‐grade hypertension was noted between patients with RCC and those with non‐RCC malignancies (24.9% [95% CI, 19.7%–30.1%] vs 15.7% [95% CI, 12.1%–19.3%], P<.05). The incidence of sorafenib‐associated high‐grade hypertension was also significantly higher for patients with RCC (8.6% [95% CI, 6.0%–11.2%]) than those with non‐RCC (1.8% [95% CI, 0.9%–2.6%]).
Table 3.
Incidence of Sorafenib‐Associated Hypertension in Cancer Patients Stratified by Underlying Malignancy, Median Treatment Duration, and PFS
| Subgroup | Study, No. | Sample Size | Incidence of Hypertension | |||
|---|---|---|---|---|---|---|
| All‐Grade/High‐Grade | All‐Grade/High‐Grade | All‐Grade, % | 95% CI | High‐Grade, % | 95% CI | |
| Stratified by underlying malignancy | ||||||
| Renal | 12/11 | 5406/5149 | 24.90 | 19.7–30.1 | 8.60 | 6.0–11.2 |
| Other | 28/34 | 2452/2704 | 15.7 | 12.1–19.3 | 1.80 | 0.9–2.6 |
| Stratified by treatment duration | ||||||
| >4.1 months | 13/13 | 4204/4205 | 21.50 | 15.7–27.3 | 4.60 | 2.7–6.5 |
| <4.1 months | 21/23 | 1779/1910 | 18.30 | 13.1–23.6 | 4.90 | 2.6–7.3 |
| Stratified by PFS | ||||||
| >5.3 months | 16/15 | 5515/5468 | 22.50 | 18.0–26.9 | 6.30 | 4.1–8.5 |
| <5.3 months | 23/29 | 2050/2329 | 16.90 | 12.5–21.4 | 2.60 | 1.4–3.8 |
Abbreviations: CI, confidence interval; PFS, progression‐free survival.
To further understand whether the treatment duration and PFS were related to the risk of sorafenib‐induced hypertension, we stratified the trials according to the median treatment duration time (4.1 months) and median PFS (5.3 months) of included trials. The trials with median PFS longer than 5.3 months demonstrated a significantly higher incidence of high‐grade hypertension compared with trials with shorter PFS (6.3% [95% CI, 4.1%–8.5%] vs 2.6% [95% CI, 1.4%–3.8%], P<.05). No differences were recorded in the subgroup analysis based on treatment duration (Table 3). We did not perform a subgroup analysis based on race because most trials enrolled patients of multiple races.
Publication Bias
No evidence of publication bias was detected for the incidence or RR of hypertension of this study by either the Begg's or the Egger's test (P>.1). The shapes of the funnel plots did not reveal any evidence of obvious asymmetry visually (Figure 5).
Figure 5.
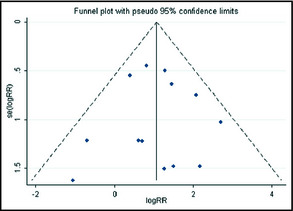
Funnel plot of the meta‐analysis (relative risk [RR] for high‐grade hypertension).
Discussion
In the present meta‐analysis, we not only showed a relatively more accurate incidence of hypertension associated with sorafenib, but also provided a better understanding of risk factors for sorafenib‐induced hypertension. In contrast to the previous study,7 our study showed a potentially higher incidence of both all‐grade and high‐grade hypertension in patients with RCC than those with other malignances. One possible explanation for this is the unique biology of RCC. Individuals with RCC usually have an inactivation of the von‐Hippel Lindau gene, which impairs the degradation of hypoxia‐inducible factor and, in turn, could increase vascular endothelial growth factor (VEGF).10 Therefore, blockade of the VEGF pathway in patients with RCC who have pre‐existing unregulated VEGF in the endothelial cell microenvironment may substantially impair endothelial function. In addition, patients with RCC can be more susceptible to developing hypertension because of previous nephrectomy and renal dysfunction.
One of the most remarkable outcomes of our meta‐analysis is that the incidence of sorafenib‐induced high‐grade hypertension was significantly higher in trials with a median PFS >5.3 months compared with those with a median PFS <5.3 months. Several studies have shown that early BP rise was associated with better anti‐tumor efficacy and improved prognosis.11, 12, 13 A retrospective analysis focusing on sunitinib, another angiogenesis inhibitor, demonstrated that systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg were associated with significantly better outcomes compared with those with lower systolic BP and diastolic BP (median overall survival of 30.5 months vs 7.8 months and 32.1 months vs 15 months, respectively).14 A more recent study evaluating the effect of sorafenib on patients with HCC showed that patients who had documented hypertension had significantly better overall survival (18.2 months vs 4.5 months; P=.016).11 The present study supported the hypothesis that the development of hypertension was correlated with improved outcomes.
Multiple mechanisms could be involved in the pathogenesis of sorafenib‐associated hypertension. The postulated mechanism includes abnormalities of nitric oxide (NO) pathway, endothelial dysfunction, and capillary rarefaction. The VEGF signaling pathway, as the primary target of multikinase inhibitor, is known to augment the transcription of endothelial NO synthase, leading to the increased production of NO.15 The inhibition of VEGF signaling pathway by sorafenib is, therefore, considered one of the major contributors to the development of hypertension, through vasoconstriction as a result of decreased NO. Furthermore, NO plays a direct role in controlling the vascular tone of glomerular arterioles, pressure natriuresis, and tubuloglomerular feedback.16 Therefore, reduction of NO synthesis can result in sodium retention, which may increase the severity of hypertension. Another postulated mechanism of hypertension induced by sorafenib therapy is called “rarefaction,” which means a reduction in capillary density. Since VEGF has been demonstrated to provide a survival signal to maintenance of endothelial viability,17 inhibition of the VEGF signaling pathway can induce endothelial cell apoptosis, reduction in capillary density and microvascular flow, and thus increase the afterload.18, 19 Platelet‐derived growth factor (PDGF), another target of sorafenib, functions mainly to enhance vascular smooth muscle cells or pericytes recruitment for maturation and stabilization of new vessels. Inhibition of PDGF/PDGF receptor may result in an inability to stabilize new vessels in the myocardium, which also contributes to the occurrence of hypertension.20 In addition, sorafenib may directly impact angiotensin II–mediated BP control by interfering with angiotensin II–dependent activation of tyrosine kinase receptors.21 Finally, none of the multikinase inhibitors are truly specific, and occurrence of hypertension may be correlated with lack of target specificity.
Potentially devastating consequences such as arterial thromboembolic events may be related to the hypertension.22, 23 Therefore, the prevention and treatment of hypertension are critically important. Since patients with RCC have a significantly higher incidence of hypertension, more attention should be paid to the occurrence of hypertension in these patients. According to the National Cancer Institute Investigational Drug Steering Committee,24 patients should be advised to seek treatment to control preexisting hypertension before the start of sorafenib therapy. BP should be monitored weekly during the first cycle of angiogenesis inhibitor therapy and then at least every 2 to 3 weeks for the duration of treatment. Patients who develop stage 1 hypertension (>140/90 mm Hg) or an increase in diastolic BP ≥20 mm Hg from baseline should initiate antihypertensive therapy. The goal for hypertension control in patients receiving angiogenesis inhibitors therapy is a maximum BP of 140/90 mm Hg, and efforts to reach this goal should begin before initiation of therapy. While there is still no clear recommendation regarding the pharmacologic management of sorafenib‐induced hypertension, several preclinical and retrospective studies may give us some direction. An animal experiment demonstrated that treatment with captopril not only attenuated sorafenib‐induced hypertension but also decreased glomerular injury.25 A retrospective review found that bevacizumab‐induced hypertension could be controlled by a single antihypertensive drug, but higher starting doses were required (eg, median dose, 20 mg of quinapril).26 More recently, another retrospective study showed that amlodipine 5 mg daily controlled BP in a majority of patients with mild toxicity.27 In addition, the nondihydropyridine calcium channel blockers verapamil and diltiazem are CYP3A4 inhibitors and nifedipine has been shown to induce VEGF secretion.28 Hence, these drugs are not recommended for the treatment of angiogenesis inhibitor–induced hypertension.
Study Limitations
Our study has the following limitations. First, the prevalence of baseline hypertension and information on pretreatment of hypertension was not described in the included trials, which may have led to an inaccurate estimation of the incidence of sorafenib‐associated hypertension. Second, there was heterogeneity in a number of relevant aspects (such as patient clinical profiles and length of follow‐up) among clinical trials, and the incidences of hypertension showed significant heterogeneity among the included studies. Third, the studies included in this meta‐analysis were conducted at various institutions by different investigators with patients of different nationalities/ethnicities, and these differences may have biased the reported incidences. Fourth, there was no standardization of the methods for measuring BP, and the definition or grading of hypertension varied between studies, which are potentially confounding factors. Finally, although we concluded that higher BP may predict a longer PFS, various confounding factors cannot be assessed properly and incorporated into the analysis.
Conclusions
Despite the limitations of our meta‐analysis, we conclude that the widely used agent sorafenib is associated with a high risk of hypertension in patients with cancer. This study will enable physicians to more accurately advise patients on the risk of hypertension associated with sorafenib therapy. For patients with RCC receiving sorafenib, physicians should be highly vigilant in the prevention and treatment of high‐grade hypertension. Further studies are recommended to investigate the RR and proper management of sorafenib‐induced hypertension.
Acknowledgments and disclosures
Yan Li and Shun Li contributed equally to this study. We declare that we have no conflict of interest.
J Clin Hypertens (Greenwich).2014;16:177–185. ©2014 Wiley Periodicals, Inc.
References
- 1. Wilhelm SM, Carter C, Tang L, et al. BAY 43‐9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. [DOI] [PubMed] [Google Scholar]
- 2. Verslype C, Rosmorduc O, Rougier P. Hepatocellular carcinoma: ESMO‐ESDO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2012;23 (Suppl 7):vii41–vii48. [DOI] [PubMed] [Google Scholar]
- 3. Escudier B, Eisen T, Porta C, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2012;23 (Suppl 7):vii65–vii71. [DOI] [PubMed] [Google Scholar]
- 4. Gupta‐Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ott PA, Hamilton A, Min C, et al. A phase II trial of sorafenib in metastatic melanoma with tissue correlates. PLoS ONE. 2010;5:e15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Safarinejad MR. Safety and efficacy of sorafenib in patients with castrate resistant prostate cancer: a Phase II study. Urol Oncol. 2010;28:21–27. [DOI] [PubMed] [Google Scholar]
- 7. Wu S, Chen JJ, Kudelka A, et al. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta‐analysis. Lancet Oncol. 2008;9:117–123. [DOI] [PubMed] [Google Scholar]
- 8. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 9. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. [DOI] [PubMed] [Google Scholar]
- 10. Choueiri TK, Vaziri SA, Jaeger E, et al. von Hippel‐Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol. 2008;180:860–865. [DOI] [PubMed] [Google Scholar]
- 11. Estfan B, Byrne M, Kim R. Sorafenib in advanced hepatocellular carcinoma: hypertension as a potential surrogate marker for efficacy. Am J Clin Oncol. 2013;36:319–324. [DOI] [PubMed] [Google Scholar]
- 12. Jain L, Sissung TM, Danesi R, et al. Hypertension and hand‐foot skin reactions related to VEGFR2 genotype and improved clinical outcome following bevacizumab and sorafenib. J Exp Clin Cancer Res. 2010;29:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravaud A, Sire M. Arterial hypertension and clinical benefit of sunitinib, sorafenib and bevacizumab in first and second‐line treatment of metastatic renal cell cancer. Ann Oncol. 2009;20:966–967. [DOI] [PubMed] [Google Scholar]
- 14. Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274(3 Pt 2):H1054–H1058. [DOI] [PubMed] [Google Scholar]
- 16. Zou AP, Cowley AW Jr. Role of nitric oxide in the control of renal function and salt sensitivity. Curr Hypertens Rep. 1999;1:178–186. [DOI] [PubMed] [Google Scholar]
- 17. Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lacouture ME, Reilly LM, Gerami P, Guitart J. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1955–1961. [DOI] [PubMed] [Google Scholar]
- 19. Steeghs N, Gelderblom H, Roodt JO, et al. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14:3470–3476. [DOI] [PubMed] [Google Scholar]
- 20. Rees ML, Khakoo AY. Molecular mechanisms of hypertension and heart failure due to antiangiogenic cancer therapies. Heart Fail Clin. 2011;7:299–311. [DOI] [PubMed] [Google Scholar]
- 21. Nakashima H, Suzuki H, Ohtsu H, et al. Angiotensin II regulates vascular and endothelial dysfunction: recent topics of angiotensin II type‐1 receptor signaling in the vasculature. Curr Vasc Pharmacol. 2006;4:67–78. [DOI] [PubMed] [Google Scholar]
- 22. Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. [DOI] [PubMed] [Google Scholar]
- 23. Steingart RM, Bakris GL, Chen HX, et al. Management of cardiac toxicity in patients receiving vascular endothelial growth factor signaling pathway inhibitors. Am Heart J. 2012;163:156–163. [DOI] [PubMed] [Google Scholar]
- 24. Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagasawa T, Hye Khan MA, Imig JD. Captopril attenuates hypertension and renal injury induced by the vascular endothelial growth factor inhibitor sorafenib. Clin Exp Pharmacol Physiol. 2012;39:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pande A, Lombardo J, Spangenthal E, Javle M. Hypertension secondary to anti‐angiogenic therapy: experience with bevacizumab. Anticancer Res. 2007;27(5B):3465–3470. [PubMed] [Google Scholar]
- 27. Mir O, Coriat R, Ropert S, et al. Treatment of bevacizumab‐induced hypertension by amlodipine. Invest New Drugs. 2012;30:702–707. [DOI] [PubMed] [Google Scholar]
- 28. Gomo C, Coriat R, Faivre L, et al. Pharmacokinetic interaction involving sorafenib and the calcium‐channel blocker felodipine in a patient with hepatocellular carcinoma. Invest New Drugs. 2011;29:1511–1514. [DOI] [PubMed] [Google Scholar]
- 29. McDermott DF, Sosman JA, Gonzalez R, et al. Double‐blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008;26:2178–2185. [DOI] [PubMed] [Google Scholar]
- 30. Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo‐controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. [DOI] [PubMed] [Google Scholar]
- 31. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear‐cell renal‐cell carcinoma. N Engl J Med. 2007;356:125–134. [DOI] [PubMed] [Google Scholar]
- 32. Stadler WM, Figlin RA, McDermott DF, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. 2010;116:1272–1280. [DOI] [PubMed] [Google Scholar]
- 33. Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first‐line treatment with sorafenib versus interferon Alfa‐2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–1289. [DOI] [PubMed] [Google Scholar]
- 34. Procopio G, Verzoni E, Bracarda S, et al. Sorafenib with interleukin‐2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial. Br J Cancer. 2011;104:1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 36. Di Lorenzo G, Carteni G, Autorino R, et al. Phase II study of sorafenib in patients with sunitinib‐refractory metastatic renal cell cancer. J Clin Oncol. 2009;27:4469–4474. [DOI] [PubMed] [Google Scholar]
- 37. Akaza H, Tsukamoto T, Murai M, et al. Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn J Clin Oncol. 2007;37:755–762. [DOI] [PubMed] [Google Scholar]
- 38. Beck J, Procopio G, Bajetta E, et al. Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU‐ARCCS) expanded‐access study: a large open‐label study in diverse community settings. Ann Oncol. 2011;22:1812–1823. [DOI] [PubMed] [Google Scholar]
- 39. Zhang H, Dong B, Lu JJ, et al. Efficacy of sorafenib on metastatic renal cell carcinoma in Asian patients: results from a multicenter study. BMC Cancer. 2009;9:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcia JA, Hutson TE, Elson P, et al. Sorafenib in patients with metastatic renal cell carcinoma refractory to either sunitinib or bevacizumab. Cancer. 2010;116:5383–5390. [DOI] [PubMed] [Google Scholar]
- 41. Motzer R, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: results from a Phase III randomized, open‐label, multicenter trial. ASCO. 2012;Abstract No. 4501.
- 42. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. [DOI] [PubMed] [Google Scholar]
- 43. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 44. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia‐Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double‐blind, placebo‐controlled trial. Lancet Oncol. 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- 45. Sansonno D, Lauletta G, Russi S, et al. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV‐related intermediate‐stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yau T, Chan P, Ng KK, et al. Phase 2 open‐label study of single‐agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B‐endemic Asian population: presence of lung metastasis predicts poor response. Cancer. 2009;115:428–436. [DOI] [PubMed] [Google Scholar]
- 47. Di Costanzo GG, Tortora R, Iodice L, et al. Safety and effectiveness of sorafenib in patients with hepatocellular carcinoma in clinical practice. Dig Liver Dis. 2012;44:788–792. [DOI] [PubMed] [Google Scholar]
- 48. Duan F, Wang MQ, Liu FY, et al. Sorafenib in combination with transarterial chemoembolization and bronchial arterial chemoinfusion in the treatment of hepatocellular carcinoma with pulmonary metastasis. Asia Pac J Clin Oncol. 2012;8:156–163. [DOI] [PubMed] [Google Scholar]
- 49. Steinbild S, Mross K, Frost A, et al. A clinical phase II study with sorafenib in patients with progressive hormone‐refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research‐EWIV. Br J Cancer. 2007;97:1480–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aragon‐Ching JB, Jain L, Gulley JL, et al. Final analysis of a phase II trial using sorafenib for metastatic castration‐resistant prostate cancer. BJU Int. 2009;103:1636–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dahut WL, Scripture C, Posadas E, et al. A phase II clinical trial of sorafenib in androgen‐independent prostate cancer. Clin Cancer Res. 2008;14:209–214. [DOI] [PubMed] [Google Scholar]
- 52. Chi KN, Ellard SL, Hotte SJ, et al. A phase II study of sorafenib in patients with chemo‐naive castration‐resistant prostate cancer. Ann Oncol. 2008;19:746–751. [DOI] [PubMed] [Google Scholar]
- 53. Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non‐small‐cell lung cancer. J Clin Oncol. 2010;28:1835–1842. [DOI] [PubMed] [Google Scholar]
- 54. Paz‐Ares LG, Biesma B, Heigener D, et al. Phase III, randomized, double‐blind, placebo‐controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first‐line treatment of advanced, nonsquamous non‐small‐cell lung cancer. J Clin Oncol. 2012;30:3084–3092. [DOI] [PubMed] [Google Scholar]
- 55. Dingemans AM, Mellema WW, Groen HJ, et al. A phase II study of sorafenib in patients with platinum‐pretreated, advanced (Stage IIIb or IV) non‐small cell lung cancer with a KRAS mutation. Clin Cancer Res. 2013;19:743–751. [DOI] [PubMed] [Google Scholar]
- 56. Wakelee HA, Lee JW, Hanna NH, et al. A double‐blind randomized discontinuation phase‐II study of sorafenib (BAY 43‐9006) in previously treated non‐small‐cell lung cancer patients: eastern cooperative oncology group study E2501. J Thorac Oncol. 2012;7:1574–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Blumenschein GR Jr, Gatzemeier U, Fossella F, et al. Phase II, multicenter, uncontrolled trial of single‐agent sorafenib in patients with relapsed or refractory, advanced non‐small‐cell lung cancer. J Clin Oncol. 2009;27:4274–4280. [DOI] [PubMed] [Google Scholar]
- 58. Spigel DR, Burris HA 3rd, Greco FA, et al. Randomized, double‐blind, placebo‐controlled, phase II trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non‐small‐cell lung cancer. J Clin Oncol. 2011;29:2582–2589. [DOI] [PubMed] [Google Scholar]
- 59. Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol. 2013;31:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Savvides P, Nagaiah G, Lavertu PN, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moreno‐Aspitia A, Morton RF, Hillman DW, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Baselga J, Segalla JG, Roche H, et al. Sorafenib in combination with capecitabine: an oral regimen for patients with HER2‐negative locally advanced or metastatic breast cancer. J Clin Oncol. 2012;30:1484–1491. [DOI] [PubMed] [Google Scholar]
- 65. Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25:3766–3773. [DOI] [PubMed] [Google Scholar]
- 66. Williamson SK, Moon J, Huang CH, et al. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: Southwest Oncology Group Study S0420. J Clin Oncol. 2010;28:3330–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high‐grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study[J]. Ann Oncol. 2012;23:508–516. [DOI] [PubMed] [Google Scholar]
- 69. von Mehren M, Rankin C, Goldblum JR, et al. Phase 2 Southwest Oncology Group‐directed intergroup trial (S0505) of sorafenib in advanced soft tissue sarcomas. Cancer. 2012;118:770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Santoro A, Comandone A, Basso U, et al. Phase II prospective study with sorafenib in advanced soft tissue sarcomas after anthracycline‐based therapy. Ann Oncol. 2013;24:1093–1098. [DOI] [PubMed] [Google Scholar]
- 71. Hobday TJ, Rubin J, Holen K. et al. MC044h, a phase II trial of sorafenib in patients (pts) with metastatic neuroendocrine tumors (NET): A Phase II Consortium (P2C) study[J]. J Clin Oncol. 2007;25 (18S): 4504. [Google Scholar]
- 72. Nimeiri HS, Oza AM, Morgan RJ, et al. A phase II study of sorafenib in advanced uterine carcinoma/carcinosarcoma: a trial of the Chicago, PMH, and California Phase II Consortia. Gynecol Oncol. 2010;117:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. El‐Khoueiry AB, Rankin CJ, Ben‐Josef E, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs. 2012;30:1646–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dubey S, Janne PA, Krug L, et al. A phase II study of sorafenib in malignant mesothelioma: results of Cancer and Leukemia Group B 30307. J Thorac Oncol. 2010;5:1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Park SH, Ryu MH, Ryoo BY, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs. 2012;30:2377–2383. [DOI] [PubMed] [Google Scholar]
- 76. Guidetti A, Carlo‐Stella C, Locatelli SL, et al. Phase II study of sorafenib in patients with relapsed or refractory lymphoma. Br J Haematol. 2012;158:108–119. [DOI] [PubMed] [Google Scholar]
- 77. Serve H, Wagner R, Sauerland C, et al. Sorafenib in combination with standard induction and consolidation therapy in elderly AML patients: results from a randomized, placebo‐controlled phase II trial. Blood (ASH Annual Meeting Abstracts). 2010;116:333. [Google Scholar]
- 78. Goncalves A, Gilabert M, Francois E, et al. BAYPAN study: a double‐blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23:2799–2805. [DOI] [PubMed] [Google Scholar]


