Abstract
The authors aimed to study the impact of a combined 9‐month lifestyle program (Mediterranean diet nutritional counselling, and high‐intensity interval training twice a week) on blood pressure (BP) in individuals with abdominal obesity, taking into account the regression‐to‐the‐mean phenomena. A total of 115 participants (53±9 years; 84 women; waist circumference [WC]: 111±13 cm; systolic/diastolic BP [SBP/DBP]: 133±13/82±8 mm Hg; 13% diabetics; 12% smokers; and 30% taking antihypertensive therapy) were retrospectively analyzed before and after the program. After 9 months, we observed an improvement in weight (−5.2±5.6 kg) and WC (−6.3±6.0 cm), and an average SBP/DBP net decrease of −5.1±13.7/−2.8±8.7 mm Hg. These changes were not uniform: 67 participants (58%) decreased their SBP by 2 mm Hg or more. The characteristics of responders included a higher baseline BP than nonresponders (SBP/DBP: 137.2±13.7/83.1±7.3 mm Hg vs 127.0±10.3/80.0±7.3 mm Hg, P<.05) and a higher proportion of participants with a baseline BP ≥130/85 mm Hg (81% vs 52%, P=.001) or with the metabolic syndrome (75% vs 54%, P=.02).
Abdominal obesity is a major public health problem.1 As a result of intra‐abdominal accumulation of visceral fat mass, abdominal obesity is associated with the occurrence of multiple cardiometabolic diseases, independently of body mass index (BMI).2 In other words, abdominal obesity can be present in individuals with normal BMI and has at times remained undiagnosed, resulting in missed opportunities for interventions. It contributes to the definition of the metabolic syndrome3 as well as systolic/diastolic blood pressure (SBP/DBP) above 130/85 mm Hg (high‐normal range according to European Society of Hypertension [ESH] guidelines).4 To a much greater extent than dietetic excesses, physical inactivity appears to be an independent and strong risk factor for this accumulation of visceral fat, and also for an overall group of diseases contributing to the concept of a “diseasome of physical inactivity.”5 Therefore, lifestyle interventions such as physical activity to improve both abdominal fat loss and BP levels are strongly promoted with the aim of preventing the development of these diseases.3, 6
Regarding the modalities of lifestyle intervention combining diet counseling and physical training that have demonstrated favorable effects, both the Mediterranean diet7 and high‐intensity interval training (HIIT) have been shown to improve the cardiometabolic parameters of individuals with abdominal obesity8, 9, 10 or the metabolic syndrome.11
Nevertheless, when studying the clinical or biological parameters likely to have high variability, as is the case in blood pressure (BP) measurements, part of the improvement may be the result of not only the intervention but also chance. And, so, in order to avoid assigning undue importance to an extreme variable, we need to weight our results by taking into account “regression to the mean” (RTM), particularly when describing changes in clinical BP records.12, 13 In other words, before drawing any conclusion as to whether improvement has occurred, we must determine the degree of net change, rather than jumping to conclusions based on gross change.
Therefore, the aim of our study was to report the results of BP change following 9 months of a lifestyle program including Mediterranean diet nutritional counseling and HIIT in individuals with abdominal obesity by assessing the net change of variables while taking RTM into consideration. Our hypothesis was that whatever the impact of RTM, the hypotensive effect of such a program would remain significant.
Methods
Participants
A total of 115 individuals with abdominal obesity (84 women, 53±9 years [24–81 years]) were retrospectively analyzed before and after a combined long‐term lifestyle and HIIT program twice a week, which has been described elsewhere.8, 10, 14 This clinical program was conducted at the Cardiovascular Prevention and Rehabilitation Center of the Montreal Heart Institute on a voluntary basis. Individuals visited the clinic because of their overweight and sedentary status, and they paid to participate in the 9‐month lifestyle program. They were included between 2009 and 2012. Inclusion criteria were: men or women aged 18 years or older and abdominal obesity defined by a waist circumference above 94 cm in men or 80 cm in women according to the consensus statement of the International Diabetes Federation.3 Exclusion criteria were: any relative or absolute contraindications to high‐intensity exercise, major cardiovascular event or procedure within the 12 months preceding enrollment, chronic atrial fibrillation, and pregnancy. The research protocol was approved by the Montreal Heart Institute's ethics committee.
Measurements
At baseline, all participants underwent anthropometric measurements, fasting blood test (glucose, lipid profile), and a maximal exercise test with an individualized ramp protocol. Resting BP was assessed by a nurse after 5 minutes of supine rest in a quiet room using a manual sphygmomanometer (Tycos 509 model; Welch Allyn Inc, Skaneateles Falls, NY) and a cuff and bladder adapted to arm circumference, with two consecutive measurements. After 9 months in the program, all participants underwent new anthropometric and resting BP measurements in the same conditions as at baseline.
Lifestyle Intervention Program
Participants had a combined lifestyle and HIIT program, which included: (1) Mediterranean diet nutritional counseling performed by a dietician, through five consultations and two group‐teaching sessions; (2) a resistance training program consisting of 20 minutes of strength exercises with free weights and elastic bands; and (3) HIIT supervised by a kinesiologist.8, 9, 10, 14 HIIT was based on the estimated maximal aerobic power (MAP, in W) from the metabolic equivalent (MET) value of the baseline maximal exercise test. The HIIT sessions performed on a stationary cycle ergometer (Precor model 846i; Precor, Woodinville, WA) consisted of the following protocol: a 5‐minute warm‐up at 50 W, followed by two sets of 10 minutes composed of repeated phases of 15 to 30 seconds at 80% of MAP interspersed by 15 to 30 seconds of passive recovery, 4 minutes of passive recovery between the two sets, and a 5‐minute cool‐down at 50 W after the last exercise phase; for a total exercise time of 34 minutes.8, 9, 10, 14
Statistical Analysis
Standard statistical methods were used for the calculation of means and standard deviations. Normal Gaussian distribution of the data was verified by the Shapiro‐Wilk test. One‐way analysis of variance was performed to test the null hypothesis that dependent variables will not be affected by training intervention. The magnitude of the difference was assessed by the Hedges' g (g), and the scale proposed by Cohen was used for interpretation, as presented elsewhere.15 The magnitude of the difference was considered either small (0.2<|g|≤0.5), moderate (0.5<|g|≤0.8), or large (|g|>0.8). The strength of the linear relationship between net change in BP and BP values at baseline was assessed using Pearson correlation coefficient (R).
The RTM was assessed using the corrections proposed by Shepard and Finison.12 We considered for the mean baseline BP values of “potential participants” the results observed in participants with an increased WC in the Quebec Health Survey16 ie, a mean SBP/DBP of 127/78 mm Hg in women and 129/81 mm Hg in men. Given the fact that we carried out two replications of BP measurement in a single visit, the coefficients of reliability of average BP were .808 and .759 for SBP and DBP, respectively.12 We consequently had the data allowing for calculation of net BP changes following the 9‐month intervention program.
We distinguished two groups (responders vs nonresponders) according to the magnitude of the response in BP change following the 9‐month program. We established a cutoff point of 2 mm Hg for SBP decrease because of its clinical interest in populations when the precision of office BP measurement was given at this threshold in individuals, and because its value was higher than the difference due to RTM. Between‐group comparisons were made using chi‐square test for categorical variables and Student t test for continuous variables.
Calculations were carried out with StatView version 5.0 software (SAS Institute Inc, Cary, NC). Statistical significance was set at P<.05.
Results
Our results pertained to participants who completed the program, with their general characteristics presented in Table 1. Of those who had begun the program, the dropout rate was 20%. Sixteen individuals had completed the program but dropped out before the last visit, and their baseline characteristics were not different from those of the studied participants (14 women [88%]; aged 51±8 years; BMI, 35.9±3.6 kg/m²; WC, 109.3±9.0 cm; baseline SBP/DBP, 130±11/83±8 mm Hg). We did not establish the characteristics of other “dropouts.” Their BP class at baseline included 11 participants with normotension (BP <120/80 mm Hg), 37 with prehypertension (120/80≤BP<140/90 mm Hg), 32 with untreated hypertension with a resting BP ≥140/90 mm Hg, and 35 taking antihypertensive treatment (29 took an angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker, 14 a thiazide diuretic, 9 a calcium channel blocker, and 8 a β‐blocker).
Table 1.
Baseline Characteristics
| Participants (n=115) | |
|---|---|
| Age, y | 53±9 |
| Female, No. (%) | 84 (73) |
| SBP/DBP ≥130/85 mm Hg | 79 (69) |
| Metabolic syndrome (IDF) | 76 (66) |
| History of hypertension | 39 (34) |
| Antihypertensive drug | 35 (30) |
| Diabetes | 15 (13) |
| Current smokers | 14 (12) |
| Glycemia, mmol/L | 5.40±0.88 |
| Total cholesterol, mmol/L | 4.95±1.03 |
| HDL cholesterol, mmol/L | 1.30±0.33 |
| LDL cholesterol, mmol/L | 3.03±0.91 |
| Triglycerides, mmol/L | 1.39±0.60 |
Abbreviations: DBP, diastolic blood pressure; HDL, high‐density lipoprotein; IDF, International Diabetes Federation; LDL, low‐density lipoprotein; SBP, systolic blood pressure. Values are expressed as mean±standard deviation or number of participants (percentage).
After 9 months we observed improvement in both the morphometric (Table 2) and hemodynamic parameters (Table 3). When including the changes caused by RTM (taking into account sex differences in results of the mean values of the potential population),16 we observed a significant net decrease of both SBP and DBP following the 9‐month program. As expected, the amplitude of net change in SBP/DBP depended on BP values at baseline (Figure). The magnitude of these net changes was either “moderate” or “small” in terms of the effect size assessed by Hedges' g (Table 3). Despite a higher baseline SBP in men than women (P=.003), the sex difference of net change following 9‐month training was not significant (P=.2).
Table 2.
Morphometric Characteristics at Baseline and Following a 9‐Month Lifestyle and High‐Intensity Interval Training Intervention
| Baseline | Post‐Training Changes | Hedges' g | P Value | |
|---|---|---|---|---|
| Weight, kg | 96.5±18.0 | −5.2±5.6 | −0.29 | <.0001 |
| Male (n=31) | 110.9±18.1 | −6.9±6.1 | −0.30 | <.0001 |
| Female (n=84) | 91.2±14.8 | −4.5±5.3 | −0.38 | <.0001 |
| Body mass index, kg/m² | 35.9±4.7 | −1.87±1.98 | −0.37 | <.0001 |
| Waist circumference, cm | 110.9±13.2 | −6.3±6.0 | −0.48 | <.0001 |
| Male (n=31) | 120.8±11.8 | −8.5±5.9 | −0.70 | <.0001 |
| Female (n=84) | 107.3±11.8 | −5.5±5.9 | −0.46 | <.0001 |
| Resting heart rate, beats per min | 75.2±13.1 | −5.7±9.7 | −0.46 | <.0001 |
Values are expressed as mean±standard deviation. Magnitudes of the difference assessed by the Hedges' g are considered either small (0.2<|g|≤0.5), moderate (0.5<|g|≤0.8), or large (|g|>0.8). Bold values indicate significance.
Table 3.
BP at Baseline and Net Changes Following a 9‐Month Lifestyle and High‐Intensity Interval Training Intervention
| Baseline | Post‐Training Changes | Changes Due to RTM | Net Changes | Hedges' g | P Value | |
|---|---|---|---|---|---|---|
| Systolic BP, mm Hg: | 132.9±13.3 | −6.1±13.6 | −5.1±13.7 | −0.48 | <.0001 | |
| Male (n=31) | 138.9±12.6 | −7.8±13.2 | −0.2 | −7.6±13.2 | −0.62 | .0025 |
| Female (n=84) | 130.7±13.0 | −5.5±13.8 | −1.3 | −4.2±13.8 | −0.44 | .0005 |
| Diastolic BP, mm Hg | 81.8±7.5 | −3.6±8.6 | −2.8±8.7 | −0.52 | <.0001 | |
| Male (n=31) | 83.2±6.9 | −2.9±6.9 | −1.2 | −1.7±6.9 | −0.43 | .0266 |
| Female (n=84) | 81.3±7.6 | −3.8±9.2 | −0.6 | −3.2±9.2 | −0.55 | .0003 |
| Pulse pressure, mm Hg | 51.1±11.9 | −2.6±12.1 | −2.3±12.2 | −0.23 | .0244 |
Abbreviations: BP, blood pressure; RTM, regression to the mean. Values are expressed as mean±standard deviation. Magnitudes of the difference assessed by the Hedges' g are considered either small (0.2<|g|≤0.5), moderate (0.5<|g|≤0.8), or large (|g|>0.8). Bold values indicate significance.
Figure 1.
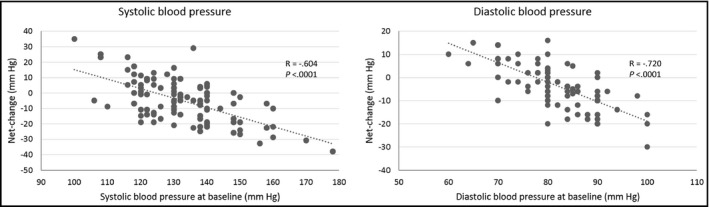
Net change in systolic blood pressure (left) and diastolic blood pressure (right) according to blood pressure values at baseline.
Therefore, not all of the participants showed improvement in their resting BP following the programmed intervention. By comparing the characteristics of participants who decreased their SBP by 2 mm Hg or more (responders, n=67) with those who did not (nonresponders, n=48), we observed that the parameters which differentiated them were their initial resting BP, with a proportion of participants with a baseline BP ≥130/85 mm Hg or with metabolic syndrome that was higher in responders (Table 4). Post‐training body weight was available for 112 participants. For the 97 participants (87%) who experienced reduction in body weight, the net change in SBP/DBP was −5.4±13.6/−2.5±8.8 mm Hg, while the SBP/DBP change was −5.4±14.1/−5.2±8.5 mm Hg for the 15 participants who did not.
Table 4.
Baseline Characteristics in Subgroups of BP Responders and Nonresponders
| Nonresponders (n=48) | Responders (n=67) | P Value | |
|---|---|---|---|
| Age, y | 52±9 | 54±10 | .39 |
| Female, No. (%) | 34 (71) | 50 (75) | .65 |
| Weight, kg | 98.3±19.1 | 95.2±17.2 | .37 |
| Body mass index, kg/m² | 35.9±4.7 | 34.7±5.3 | .25 |
| Waist circumference, cm | 112.6±14.3 | 109.8±12.4 | .26 |
| Systolic BP, mm Hg | 127.0±10.3 | 137.2±13.7 | <.0001 |
| Diastolic BP, mm Hg | 80.0±7.3 | 83.1±7.3 | .0282 |
| Pulse pressure, mm Hg | 46.9±8.7 | 54.1±12.9 | .0011 |
| Heart rate, beats per min | 77.4±14.7 | 73.6±11.8 | .13 |
| Systolic BP/diastolic BP ≥130/85 mm Hg, No. (%) | 25 (52) | 54 (81) | .0011 |
| Metabolic syndrome (IDF), No. (%) | 26 (54) | 50 (75) | .0223 |
| History of hypertension, No. (%) | 16 (33) | 23 (34) | .91 |
| Antihypertensive drug, No. (%) | 16 (33) | 19 (28) | .57 |
| Diabetes, No. (%) | 8 (17) | 7 (10) | .33 |
Abbreviations: BP, blood pressure; IDF, International Diabetes Federation. Values are expressed as mean±standard deviation or number of participants (percentage). Bold values indicate significance.
For a practical application, we dichotomized the cohort according to the threshold of 130/85 mm Hg (between “normal” and “high‐normal” BP ranges of the ESH guidelines)4 group 1 with a baseline BP <130/85 mm Hg (n=36) and group 2 with a baseline BP ≥130/85 mm Hg (n=79). We observed no differences between groups in terms of age, resting heart rate, lipid analysis, diabetes, weight loss (−4.1±6.1 kg vs −5.7±5.3 kg, P=.2), and WC improvement (−5.4±4.8 cm vs −6.8±6.5 cm, P=.3). The proportion of women was higher in group 1 than in group 2 (86% vs 67%, P=.03), and antihypertensive therapies were less present in group 1 than in group 2 (17% vs 37%, P=.03). Furthermore, BP did not improve in group 1 and actually decreased in a higher proportion of responders in group 2 (Table 5). While distinguishing the 35 individuals taking antihypertensive medication, we observed a higher proportion with a baseline BP ≥130/85 mm Hg (Table 5), 19 (54%) of whom were responders. We found no significant differences between treated and untreated participants in terms of their average BP at baseline (SBP/DBP: 135.6±11.4/83.5±6.5 mm Hg vs 131.8±14.0/81.1±7.7 mm Hg, P=.2/P=.1, respectively) or their average net BP change following the program (SBP/DBP: −3.9±14.0/−2.8±8.4 mm Hg vs −5.6±13.6/−2.8±8.8 mm Hg, P=.5/P=.9, respectively).
Table 5.
Net Changes of BP Following a 9‐Month Lifestyle and High‐Intensity Interval Training Intervention, According to the Baseline BP Threshold of 130/85 mm Hg
| Net Changes | Overall (n=115) | Baseline BP <130/85 mm Hg (n=36) | Baseline BP ≥130/85 mm Hg (n=79) | P Value |
|---|---|---|---|---|
| Systolic BP, mm Hg | −5.1±13.7 | +2.9±13.2 | −8.7±12.3 | <.0001 |
| Diastolic BP, mm Hg | −2.8±8.7 | +0.1±9.4 | −4.1±8.0 | .0144 |
| Responders (systolic BP decrease ≥2 mm Hg), No. (%) | 67 (58) | 13 (36) | 54 (68) | .0011 |
| Participants under antihypertensive treatment, No. (%) | 35 (30) | 6 (17) | 29 (83) | .0303 |
Abbreviation: BP, blood pressure. Values are expressed as mean±standard deviation or number of participants (percentage). Bold values indicate significance.
Discussion
Following a 9‐month combined lifestyle and HIIT program in participants with abdominal obesity, we observed significant improvement in resting BP that persisted when taking into account RTM, with the net change reaching −5.1/−2.8 mm Hg for SBP/DBP, respectively. This was not modulated by sex difference or antihypertensive medication. Not all of the participants in the program were responders in terms of BP improvement; in fact, responders had higher baseline BP values (137/83 mm Hg in mean) than nonresponders. We also observed greater BP improvement and a higher proportion of responders in participants with a BP ≥130/85 mm Hg at baseline.
The mean values of SBP/DBP lowering found in our study (SBP/DBP: −5.1/−2.8 mm Hg) are close to the median values of the improvement as a result of physical exercise reported in a meta‐analysis of individuals presenting with prehypertension (SBP/DBP: −2.1/−1.7 mm Hg) or hypertension (SBP/DBP: −8.3/−5.2 mm Hg).17 The objective of our observational work was not to compare HIIT with other modalities of exercise, and there have been few comparative studies assessing BP changes.18 Our program also included Mediterranean diet nutritional counseling and induced weight loss. However, as we reported, weight loss and WC improvement were not different between BP responders and nonresponders. In other words, body weight reduction seemed to have no impact on BP in our study. There is no readily evident explanation, as we found no difference between baseline characteristics of individuals experiencing a decrease in body weight. While meta‐analysis suggested that, for 1 kg of weight loss, 1 mm Hg reduction should be possible for both SBP/DBP,19 linkage of weight to BP changes have been far from obvious in reviews focusing on long‐term programs.20 Some heterogeneity between the different studies and less responsive changes were reported in populations with moderate obesity.20 This the reason we tend to attribute the beneficial BP effect of the program to the HIIT component. Nevertheless, we did not assess the effect of HIIT alone, or Mediterranean diet alone, which could specifically affect arterial function independently of weight change.7
Our BP results are even more interesting inasmuch as we took into account the statistical phenomenon of RTM and calculated the net change of BP representing real BP improvement.12 The clinical relevance of this BP reduction reaching −5.1 mm Hg for SBP could be questionable. However, it has been established that a 5 mm Hg reduction in SBP could lead to a 14% reduction in stroke mortality, a 9% reduction in mortality from coronary heart disease, and a 7% reduction in all deaths.21 The clinical interest in the 2 mm Hg threshold we retained for the definition of responders vs nonresponders has been established in populations with a significant reduction of 6% for stroke death, 4% for coronary death, and 3% for all death.21
For a practical application, we assessed the post‐program BP net change function of baseline BP level. We observed that participants with a baseline BP ≥130/85 mm Hg had a greater likelihood to be responders with more pronounced BP net improvement. The particularly substantially favorable impact of physical exercise on BP with higher baseline BP is well‐known.17 For example, the above‐mentioned BP improvement did not occur in cases of baseline BP in the “optimal” range (<120/80 mm Hg) in overweight women22 and, on the other hand, it appeared much greater in hypertensive patients.23 Our results nevertheless underscore the interest of the threshold of 130/85 mm Hg, distinguishing “normal” and “high‐normal” ranges and not only “normotensive” vs “hypertensive” individuals.4 This threshold was missing in the North American guidelines postulating a wider range of “prehypertension” between 120/80 mm Hg and 140/90 mm Hg.24, 25 While, in our study, individuals with a BP between 120/80 mm Hg and 130/85 mm Hg clearly did not benefit from the same BP improvement following the 9‐month program. The use of antihypertensive medication was significantly higher in responders vs nonresponders, but we observed no significant differences between treated and untreated individuals in terms of either BP at baseline and BP response to the program. Thus, the benefit of the program was above all dependent on the BP level at baseline rather than the presence of antihypertensive drug.
Perspectives
Our study demonstrated the interest of a real‐life long‐term lifestyle program including Mediterranean diet nutritional counseling and HIIT on office BP. Few studies have assessed the effect of the manipulation of different lifestyles and exercise modalities on ambulatory BP. This kind of comparison should have practical interest in view of the successful development of recreational physical activities, such as water‐based exercises in swimming pools and fitness centers.14
STUDY LIMITATIONS
Our study was observational, without any control group to differentiate between the effects of diet and exercise. Our cohort included individuals who paid to participate in the lifestyle intervention program; therefore, they likely were of higher socioeconomic status and potentially more motivated to achieve the goals compared with the general population. The use of manual BP is less precise than automatic BP but the conditions of measurement were in accordance with the guidelines.26 We used office BP, which is less informative than ambulatory methods such as ambulatory BP monitoring or home BP measurement because of the white‐coat effect.27 In addition, the amplitude of RTM is likely to be blunted by the numerous measurements performed in case of ambulatory BP monitoring, which should be preferred in assessment of BP changes.
Conclusions
Long‐term lifestyle and HIIT interventions had a beneficial effect on the net change of BP levels in individuals with abdominal obesity, in particular in cases of baseline BP ≥130/85 mm Hg whatever the treatment. This threshold could be retained for future studies to assess BP improvement following physical exercise intervention.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
We wish to address our thanks to all of the participants, the team of the ÉPIC Centre, and to Jeffrey Arsham, who reviewed the paper. This work was supported by the ÉPIC Centre and Montreal Heart Institute Foundations, Quebec, Canada.
J Clin Hypertens (Greenwich). 2016;18:1128–1134. DOI: 10.1111/jch.12829. © 2016 Wiley Periodicals, Inc.
This work was performed at the Cardiovascular Prevention and Rehabilitation Center (ÉPIC), Montreal Heart Institute, Quebec, Canada.
References
- 1. Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. [DOI] [PubMed] [Google Scholar]
- 2. Després J‐P. Abdominal obesity: the most prevalent cause of the metabolic syndrome and related cardiometabolic risk. Eur Heart J Suppl. 2006;8:B4–B12. [Google Scholar]
- 3. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world‐wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. [DOI] [PubMed] [Google Scholar]
- 4. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 5. Pedersen BK. The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol. 2009;587:5559–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pescatello LS, MacDonald HV, Ash GI, et al. Assessing the existing professional exercise recommendations for hypertension: a review and recommendations for future research priorities. Mayo Clin Proc. 2015;90:801–812. [DOI] [PubMed] [Google Scholar]
- 7. Estruch R, Ros E, Salas‐Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 8. Gremeaux V, Drigny J, Nigam A, et al. Long‐term lifestyle intervention with optimized high‐intensity interval training improves body composition, cardiometabolic risk, and exercise parameters in patients with abdominal obesity. Am J Phys Med Rehabil. 2012;91:941–950. [DOI] [PubMed] [Google Scholar]
- 9. Drigny J, Gremeaux V, Guiraud T, et al. Long‐term high‐intensity interval training associated with lifestyle modifications improves QT dispersion parameters in metabolic syndrome patients. Ann Phys Rehabil Med. 2013;56:356–370. [DOI] [PubMed] [Google Scholar]
- 10. Dalzill C, Nigam A, Juneau M, et al. Intensive lifestyle intervention improves cardiometabolic and exercise parameters in metabolically healthy obese and metabolically unhealthy obese individuals. Can J Cardiol. 2014;30:434–440. [DOI] [PubMed] [Google Scholar]
- 11. Landaeta‐Diaz L, Fernandez JM, Da Silva‐Grigoletto M, et al. Mediterranean diet, moderate‐to‐high intensity training, and health‐related quality of life in adults with metabolic syndrome. Eur J Prev Cardiol. 2013;20:555–564. [DOI] [PubMed] [Google Scholar]
- 12. Shepard DS, Finison LJ. Blood pressure reductions: correcting for regression to the mean. Prev Med. 1983;12:304–317. [DOI] [PubMed] [Google Scholar]
- 13. Watson RD, Lumb R, Young MA, et al. Variation in cuff blood pressure in untreated outpatients with mild hypertension–implications for initiating antihypertensive treatment. J Hypertens. 1987;5:207–211. [DOI] [PubMed] [Google Scholar]
- 14. Boidin M, Lapierre G, Paquette Tanir L, et al. Effect of aquatic interval training with Mediterranean diet counseling in obese patients: results of a preliminary study. Ann Phys Rehabil Med. 2015;58:269–275. [DOI] [PubMed] [Google Scholar]
- 15. Dupuy O, Lussier M, Fraser S, et al. Effect of overreaching on cognitive performance and related cardiac autonomic control. Scand J Med Sci Sports. 2014;24:234–242. [DOI] [PubMed] [Google Scholar]
- 16. Poirier P, Lemieux I, Mauriege P, et al. Impact of waist circumference on the relationship between blood pressure and insulin: the Quebec Health survey. Hypertension. 2005;45:363–367. [DOI] [PubMed] [Google Scholar]
- 17. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharman JE, La Gerche A, Coombes JS. Exercise and cardiovascular risk in patients with hypertension. Am J Hypertens. 2015;28:147–158. [DOI] [PubMed] [Google Scholar]
- 19. Neter JE, Stam BE, Kok FJ, et al. Influence of weight reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension. 2003;42:878–884. [DOI] [PubMed] [Google Scholar]
- 20. Aucott L, Rothnie H, McIntyre L, et al. Long‐term weight loss from lifestyle intervention benefits blood pressure?: a systematic review. Hypertension. 2009;54:756–762. [DOI] [PubMed] [Google Scholar]
- 21. Stamler R. Implications of the Intersalt study. Hypertension. 1991;17:I16–I20. [DOI] [PubMed] [Google Scholar]
- 22. Sijie T, Hainai Y, Fengying Y, Jianxiong W. High intensity interval exercise training in overweight young women. J Sports Med Phys Fitness. 2012;52:255–262. [PubMed] [Google Scholar]
- 23. Molmen‐Hansen HE, Stolen T, Tjonna AE, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;19:151–160. [DOI] [PubMed] [Google Scholar]
- 24. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 25. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daskalopoulou SS, Rabi DM, Zarnke KB, et al. The 2015 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2015;31:549–568. [DOI] [PubMed] [Google Scholar]
- 27. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]


