Abstract
The kidney is an important regulator of blood pressure (BP). To determine whether BP response to lifestyle modification varies across normal ranges of kidney function, the authors examined the moderating role of estimated glomerular filtration rate (eGFR) on clinic and ambulatory systolic BP (SBP) response in overweight and obese adults with unmedicated high BP. Among 144 participants of the Exercise and Nutritional Interventions for Cardiovascular Health (ENCORE) trial, mean age was 52.0±9.6 years and median eGFR was 89.1 (53–146) mL/min/1.73m2. After multivariable regression, the interaction between eGFR and weight loss was significant for clinic (P=.023) and ambulatory SBP (P=.041). Similarly, the interaction between eGFR and improved fitness was significant for clinic (P=.041) and ambulatory SBP (P=.044). The relationship between reduced dietary sodium and SBP was not moderated by eGFR. SBP findings were inconsistent for adherence to the Dietary Approaches to Stop Hypertension (DASH) diet. These findings suggest that the effects of lifestyle modifications on SBP may be influenced by eGFR, even when kidney function is preserved.
Lifestyle modifications that have been established in several randomized controlled clinical trials to lower blood pressure (BP) include weight loss, the Dietary Approaches to Stop Hypertension (DASH) diet, reduced dietary sodium intake, and regular exercise.1, 2, 3, 4, 5 The effectiveness of these lifestyle modifications to lower BP are influenced by a number of patient factors. For example, the DASH diet was demonstrated to lower BP more effectively in adults with hypertension compared with those with prehypertension, and more in African Americans compared with whites.6, 7, 8 In addition, sodium reduction was demonstrated to lower BP more effectively in older adults (>45 years) compared with younger adults.9, 10, 11 Another important patient factor that has not been extensively studied that may impact BP response to lifestyle modifications is kidney function. The kidney influences BP by regulating urinary sodium excretion,12 renin‐angiotensin‐aldosterone system (RAAS) activity,13 and sympathetic nervous system activity14—all mechanisms that may be altered when kidney function declines. Therefore, lifestyle modifications may vary in their effectiveness at lowering BP depending on an individual's preexisting level of kidney function.
The Exercise and Nutritional Interventions for Cardiovascular Health (ENCORE) trial15 compared the effectiveness of the DASH diet alone and the DASH diet combined with a behavioral weight loss program to lower BP in sedentary, overweight, and obese adults with unmedicated high BP. Although the ENCORE trial excluded adults with chronic kidney disease (CKD), its enrollment of individuals with a wide range of what is considered relatively normal kidney function (ie, estimated glomerular filtration rate [eGFR] ≥60 mL/min/1.73m2) provided us with the opportunity to examine the role of normal or mildly reduced kidney function on BP response to lifestyle modifications. There are gradations of kidney function within the normal range of eGFR values, leading the Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guidelines to define eGFR ≥90 mL/min/1.73m2 as “normal or high” and eGFR 60 to 89 mL/min/1.73m2 as “mildly decreased.”16 Clinical and population‐based studies have demonstrated that these cutpoints within the normal range of eGFR are clinically relevant because they are associated with cardiovascular‐related outcomes, kidney‐related morbidity, and mortality.17, 18, 19, 20 For example, a secondary analysis of the Atherosclerosis Risk in Communities (ARIC) study, 19 which is a prospective population‐based longitudinal study to evaluate causes of atherosclerotic disease and its clinical outcomes in 15,350 US adults, demonstrated that adults with an eGFR of 60 to 89 mL/min/1.73m2 had a 16% higher adjusted risk for cardiovascular disease–related events than adults with an eGFR of 90 to 150 mL/min/1.73m2. The premise of the current study is based on our hypothesis that kidney‐related regulation of BP may also vary across normal ranges of eGFR and this variation may in turn influence BP response to lifestyle modification. Because the ENCORE trial design manipulated changes in aerobic fitness, body weight, and dietary habits, we were able to examine how each individual lifestyle change influenced BP and how normal or mildly reduced eGFR modified BP response to each of these lifestyle changes.
Methods
Design Overview
The ENCORE trial was a randomized trial conducted from October 2003 to July 2008 that compared the effects of the DASH diet alone and the DASH diet combined with a behavioral weight loss program with a usual diet control condition on BP and markers of cardiovascular disease risk among overweight and obese adults with unmedicated high BP. Detailed descriptions of ENCORE trial methods and main results have been previously published.8, 15, 21 The study protocol was approved by the institutional review boards at Duke University and the University of North Carolina at Chapel Hill. All participants provided written informed consent. The present study examines lifestyle modifications (changes in body weight, aerobic fitness, DASH diet adherence, dietary sodium) and how they relate to changes in clinic and ambulatory systolic BP (SBP) as a function of eGFR after 4 months of trial participation.
Participants
Participants were sedentary adults aged 35 years or older with a body mass index (BMI) of 25.0 to 39.9 kg/m2, SBP 130 to 159 mm Hg or diastolic BP (DBP) 85 to 99 mm Hg, and normal kidney function (eGFR ≥60 mL/min/1.73m2) who were not taking antihypertensive medications.
Assessments
Kidney Function
Serum creatinine was measured at baseline prior to randomization. The Chronic Kidney Disease Epidemiology Collaboration equation22 was used to calculate eGFR. Based on the CKD staging system,23 participants were dichotomized into groups by eGFR. An eGFR level ≥90 mL/min/1.73m2 was defined as “normal‐high” and an eGFR level <90 mL/min/1.73m2 was defined as “mildly decreased.”
Blood Pressure
Clinic and 24‐hour ambulatory BP (ABP) were assessed at baseline and 4 months postintervention. Clinic BP was measured using a mercury sphygmomanometer and stethoscope. BP was determined by averaging four seated measurements obtained 2 minutes apart on four separate days over a 3‐week period. ABP was obtained using the Accutracker II device (Suntech Medical Inc, Raleigh, NC).24 ABP was measured four times per hour during waking hours and two times per hour during sleeping hours. All values obtained during a 24‐hour period were averaged to determine mean ABP, which was adjusted for posture. All BP assessments were performed by trained research personnel blinded to study group assignment.
Weight
Body weight was measured using a calibrated digital scale (Detecto; Cardinal Scale Manufacturing Co, Webb City, MO) with participants wearing light indoor clothes and no shoes.
Diet
Four‐day food diaries were obtained to determine participants' daily macronutrient and energy intake. Data were analyzed using Food Processor SQL Edition software, version 10.3 (ESHA Research, Salem, OR).25 Self‐administered food frequency questionnaires26 (NutritionQuest, Berkeley, CA) were completed to assess participants' average monthly consumption of food items.27, 28 Adherence to the DASH diet was assessed using a composite score derived from 10 food and nutrition components: grains; fruits; vegetables; nuts, seeds, and legumes; dairy; meat; fat; saturated fat; sweets; and sodium.21
Aerobic Fitness
A maximal graded exercise stress test with increased workloads of one metabolic equivalent per minute29 was performed to determine aerobic fitness (peak oxygen uptake [VO2] in mL/kg/min). Participants exercised to exhaustion under continuous electrographic monitoring, during which metabolic measurements were obtained using the ParvoMedics TrueOne measurement System (Sandy, UT).
Interventions
Participants were randomized to one of the following three interventions: the DASH diet alone (DASH‐A), the DASH diet plus weight management (DASH+WM), or a usual care control (UC) condition. The DASH‐A and DASH+WM groups received education and counseling on the DASH diet and received feedback to facilitate adherence during 14 weekly nutritionist‐led small group sessions. In addition, the DASH+WM group participated in cognitive‐behavioral weight management counseling and thrice‐weekly supervised exercise sessions. The UC group was asked to continue their usual diet and exercise habits and to maintain a stable body weight.
Statistical Analysis
All statistical analyses were performed using SAS 9.2 (SAS Institute Inc, Cary, NC). Standard descriptive statistics were performed to characterize the cohort. Separate regression models were used to determine the relationship between each lifestyle modification (ie, changes in weight, dietary sodium, DASH adherence score, and aerobic fitness) and clinic and ambulatory BP. Because our primary outcome was change in SBP, data for clinic and ambulatory SBP are included in this report. Data for DBP are included as supporting information (Table S1 and Item S1). Models were adjusted for age, baseline BMI, sex, race (African American or other), preintervention BP, and baseline eGFR. In addition, ABP analyses were adjusted for percent of measurements in the seated or supine position. In order to test for treatment moderation and avoid multiple testing and an increased type‐I error rate, we examined the interaction term between baseline eGFR as a continuous variable and each lifestyle modification simultaneously. For descriptive purposes only, we also dichotomized participants by eGFR ≥90 mL/min/1.73m2 (normal‐high for eGFR ≥90 mL/min/1.73m2 and mildly decreased for eGFR <90 mL/min/1.73m2) per KDIGO 2012 Clinical Practice Guideline descriptors of eGFR16 and performed a set of secondary, explanatory analyses. Because weight change and improvements in fitness were confounded (ie, patients randomized to the DASH+WM condition exercised, improved their fitness, and lost weight), we examined changes in weight and aerobic fitness in separate multivariable models controlling for the above covariates. We evaluated the extent to which models met assumptions, including additivity, linearity, and distribution of residuals and found no evidence of significant violations of these assumptions. Missing data were imputed for ≤6% of participants (ie, postintervention clinic BP measurements for four participants, postintervention ambulatory BP measurements for 11 participants, and dietary data for nine participants).
Results
Participant Characteristics
Demographic and baseline clinical characteristics are shown in Table 1. The cohort was predominantly women (65%) and white (60%), with an average age of 52.0±9.6 years. The median eGFR was 89.1 (range 53–146) mL/min/1.73m2. Eight participants (5%) had an eGFR outside of normal range (ie, 60–120 mL/min/1.73m2), five (3%) had an eGFR <60 mL/min/1.73m2, and three (2%) had an eGFR >120 mL/min/1.73m2.
Table 1.
Demographic and Baseline Characteristics of ENCORE Trial Participants by eGFR Group
| All (N=144) | eGFR ≥90 (n=74) | eGFR <90 (n=70) | |
|---|---|---|---|
| Age, y | 52.0 (9.6) | 47.9 (7.0) | 56.3 (10.2) |
| Female sex, No. (%) | 94 (65) | 56 (79) | 32 (46) |
| Race, No. (%) | |||
| White | 86 (60) | 40 (54) | 46 (66) |
| African American | 56 (39) | 32 (43) | 24 (34) |
| Other | 2 (1) | 2 (3) | 0 (0) |
| Years of education | 15.2 (2.6) | 15.3 (2.5) | 15.0 (2.7) |
| Annual household income, No. (%) | |||
| <$25,000 | 21 (17) | 13 (21) | 8 (13) |
| $25,000–50,000 | 31 (25) | 17 (27) | 14 (23) |
| $50,000–100,000 | 44 (36) | 21 (33) | 23 (38) |
| ≥$100,000 | 27 (22) | 12 (19) | 15 (25) |
| Weight, kg | 94.0 (14.1) | 94.2 (14.7) | 93.9 (13.6) |
| BMI, kg/m2 | 33.2 (3.9) | 33.9 (4.1) | 32.3 (3.5) |
| BP, mm Hg | |||
| Clinic SBP | 137.7 (8.8) | 136.5 (8.1) | 139.0 (9.3) |
| Clinic DBP | 85.5 (6.4) | 87.0 (5.9) | 84.0 (6.5) |
| Ambulatory SBP | 136.9 (12.1) | 136.0 (12.0) | 137.7 (12.4) |
| Ambulatory DBP | 81.3 (8.3) | 82.1 (7.8) | 80.4 (8.8) |
| Serum creatinine, mg/dL | 0.94 (0.17) | 0.85 (0.12) | 1.04 (0.16) |
| eGFR, mL/min/m2 | 89.1 (15.0) | 99.9 (9.5) | 77.3 (9.6) |
| Dietary sodium intake | 3231 (1051) | 3183 (986) | 3280 (1119) |
| DASH adherence score | 3.7 (1.3) | 3.6 (1.3) | 3.9 (1.3) |
| Aerobic fitness (peak VO2, mL/kg/min) | 23.4 (6.2) | 23.1 (5.3) | 23.8 (7.0) |
| Treatment group | |||
| DASH+WM, No. (%) | 49 | 26 (35) | 23 (33) |
| DASH‐A, No. (%)0 | 46 | 22 (30) | 24 (34) |
| UC, No. (%) | 49 | 26 (35) | 23 (33) |
Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; DASH, Dietary Approaches to Stop Hypertension; DASH+WM, DASH plus weight management; DASH‐A, DASH alone; eGFR, estimated glomerular filtration rate; ENCORE, the Exercise and Nutritional Interventions for Cardiovascular Health trial; SBP, systolic blood pressure; UC, usual care; VO2, peak oxygen uptake. eGFR reported in mL/min/1.73m2. Values are expressed as mean (standard deviation) unless otherwise indicated.
Changes in Lifestyle Modification: Body Weight, Dietary Habits, and Fitness
As previously reported, clinic and ABP were reduced in the DASH‐A and DASH+WM groups.15 The cohort lost weight (−3.36 kg [−4.2 to −2.5], P<.001), reduced dietary sodium intake (−260 mg/1000 calories [0.18–0.33], P<.001), increased DASH diet adherence (+1.6 DASH score [1.3–1.9], P<.001), and increased aerobic fitness (+1.19 mL/kg/min [0.4–2.0], P<.001).
Impact of Lifestyle Modifications on BP
For separate regression models, improvements in each individual lifestyle modification were associated with reductions in clinic SBP and ambulatory SBP (Table 2). In a multivariable model that included changes in weight, sodium intake, and DASH adherence score as predictors, weight loss was associated with reductions in clinic SBP (b=−1.27 [−2.33 to −0.20], P=.021) and ambulatory SBP (b=−1.29 [−2.32 to −0.27], P=.014). In the same model, improved DASH adherence was associated with reductions in clinic SBP (b=−3.41 [−5.88 to −0.95], P=.007), and reduced dietary sodium intake was associated with reductions in ambulatory SBP (b=−3.19 [−5.43 to −0.94], P=.006). In a parallel multivariable regression model that substituted changes in weight with changes in aerobic fitness, improved aerobic fitness was also associated with reductions in clinic SBP (b=−2.90 [−3.96 to −1.84], P<.001) and ambulatory SBP (b=−1.65 [−2.74 to −0.55], P=.003). DBP results are shown in Table S1.
Table 2.
Regression Models of the Relationship Between Changes in Each Individual Lifestyle Component and SBP Measured in the Clinic and by Ambulatory Blood Pressure Monitoring
| Changes in targeted lifestyle modifications | Clinic SBP | Ambulatory SBP | ||
|---|---|---|---|---|
| b | 95% CI | b | 95% CI | |
| Change in weight, 2.5 kg | 2.04a | 1.06–3.02 | 2.01a | 1.00–3.03 |
| Change in fitness, 10% peak VO2 | 3.14a | 2.12–4.17 | 2.35a | 1.26–3.45 |
| Change in DASH score, two points | 4.93a | 2.68–7.18 | 4.06a | 1.72–6.40 |
| Change in dietary sodium, 1000 mg/d | 3.08b | 0.67–5.48 | 4.54a | 2.21–6.87 |
Abbreviations: b, beta coefficient; CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure. Models adjusted for age, race (African American or other), sex, preintervention blood pressure, baseline estimated glomerular filtration rate, and the preintervention level of the predictor. Ambulatory models also adjusted for percent of measurements in the seated or supine position. Changes in intervention components were scaled using the following increments: weight by 2.5 kg, fitness by 10% of peak oxygen uptake (VO2), Dietary Approaches to Stop Hypertension (DASH) score by two‐point increments, and dietary sodium by 1000 mg/d. a P<.001. b P<.02.
Interaction Between Kidney Function and Lifestyle Modifications on BP
Change in Weight
In the multivariable model, the interaction between eGFR and change in weight was significant for changes in clinic SBP (P=.023) and ambulatory SBP (P=.041) (Figure 1 and Figure 2). Weight loss was associated with larger reductions in SBP for the mildly decreased eGFR group (eGFR <90 mL/min/1.73m2) than the normal‐high eGFR group (eGFR ≥90 mL/min/1.73m2). In the mildly decreased eGFR group, every 2.5 kg reduction in weight was associated with reductions of 2.5 (95% confidence interval [CI], 2.0–3.0) mm Hg in clinic SBP and 1.7 (95% CI, 0.9–2.5) mm Hg in ambulatory SBP. In contrast, in the normal‐high eGFR group, every 2.5 kg reduction in weight was associated with reductions of 1.7 (95% CI, 1.1–2.4) mm Hg in clinic SBP and 1.0 (95% CI, 0.2–1.8) mm Hg in ambulatory SBP.
Figure 1.
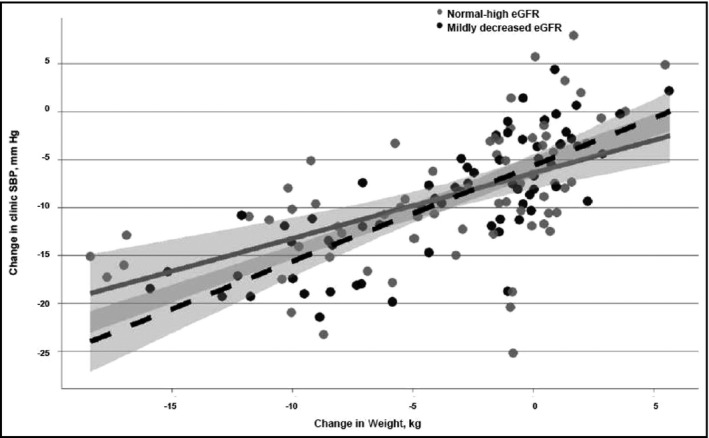
The relationship between change in weight and changes in clinic systolic blood pressure (SBP) stratified by estimated glomerular filtration rate (eGFR) group. Change in SBP derived from SBP value at baseline minus SBP value at 4‐month follow‐up visit. The dotted line represents the mildly decreased eGFR group and the solid line represents the normal‐high eGFR group.
Figure 2.
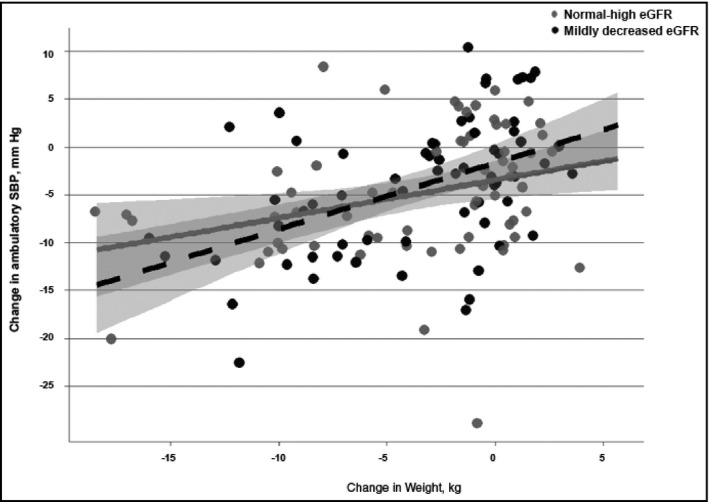
The relationship between change in weight and changes in ambulatory systolic blood pressure (SBP) stratified by estimated glomerular filtration rate (eGFR) group. Change in SBP derived from SBP value at baseline minus SBP value at 4‐month follow‐up visit. The dotted line represents the mildly decreased eGFR group and the solid line represents the normal‐high eGFR group.
Change in DASH Adherence Score
In the multivariable model, the interaction between baseline eGFR and change in DASH adherence score was significant for clinic SBP (P=.032) but not ambulatory SBP (P=.116). Improved DASH adherence was associated with larger reductions in clinic SBP for the normal‐high eGFR group than the mildly decreased eGFR group. Every two‐point increase in DASH adherence score was associated with a 6.1 (95% CI, 5.2–7.1) mm Hg in clinic SBP for the normal‐high eGFR group compared with a 3.7 (95% CI, 2.4–5.0) mm Hg reduction in clinic SBP for the mildly decreased eGFR group.
Change in Sodium Intake
In the multivariable model, the interaction between baseline eGFR and change in dietary sodium intake was not significant for clinic SBP (P=.834) or ambulatory SBP (P=.424).
Change in Aerobic Fitness
In a parallel analysis in which changes in aerobic fitness were modeled instead of weight loss, a pattern similar to that for weight loss was observed. There were significant interactions between baseline eGFR and change in fitness for clinic SBP (P=.041) and ambulatory SBP (P=.044) (Figure 3 and Figure 4). Improvements in aerobic fitness were associated with larger SBP reductions for the mildly decreased eGFR group than the normal‐high eGFR group. In the mildly decreased eGFR group, every 10% increase in peak VO2 was associated with reductions of 4.4 (2.9–5.9) mm Hg in clinic SBP and 2.7 (0.7–4.8) mm Hg in ambulatory SBP. In contrast, for the normal‐high eGFR group, every 10% increase in peak VO2 was associated with reductions of 2.8 (1.4–4.3) mm Hg in clinic SBP and 1.1 (−0.7 to 2.8) mm Hg in ambulatory SBP.
Figure 3.
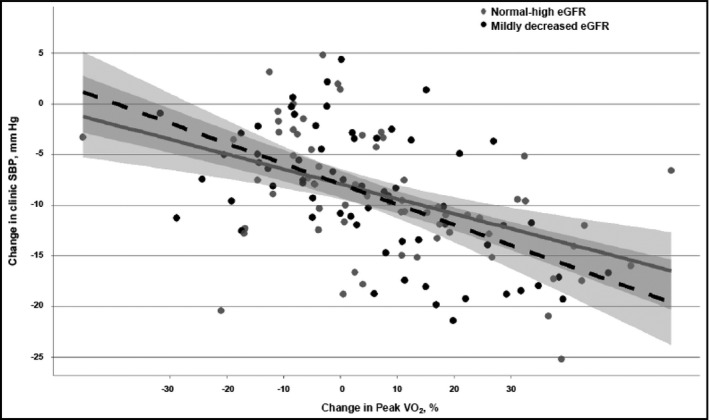
The relationship between percent change in peak oxygen uptake (VO2) and changes in clinic systolic blood pressure (SBP) stratified by estimated glomerular filtration rate (eGFR) group. Change in SBP derived from SBP value at baseline minus SBP value at 4‐month follow‐up visit. The dotted line represents the mildly decreased eGFR group and the solid line represents the normal‐high eGFR group.
Figure 4.
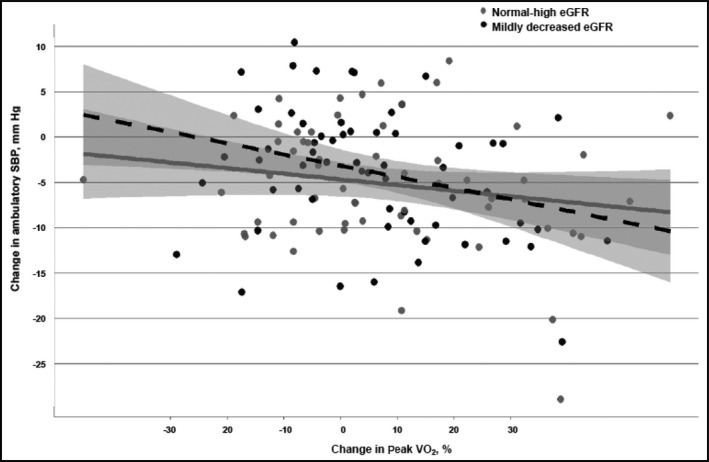
The relationship between percent change in peak oxygen uptake (VO2) and changes in ambulatory systolic blood pressure (SBP) stratified by estimated glomerular filtration rate (eGFR) group. Change in SBP derived from SBP value at baseline minus SBP value at 4‐month follow‐up visit. The dotted line represents the mildly decreased eGFR group and the solid line represents the normal‐high eGFR group.
Discussion
In this secondary analysis of the ENCORE trial, weight loss, DASH diet adherence, reduced dietary sodium intake, and improved aerobic fitness were each associated with reductions in SBP. For example, weight loss of 2.5 kg and 10% increase in peak VO2 were incrementally associated with a reduction in clinic SBP of 2 and 3 mm Hg, respectively. Furthermore, kidney function influenced the magnitude that clinic and ambulatory SBP were reduced by weight loss and improved aerobic fitness. BP was lowered to a greater extent among participants with lower eGFR, suggesting that individuals with lower levels of kidney function can benefit just as much, or perhaps more, from weight loss and improved fitness as those with higher levels of kidney function. Although somewhat inconsistent for clinic and ambulatory SBP outcomes, we also found that the effects of increased DASH diet adherence on BP was moderated by kidney function. In contrast to weight loss and aerobic fitness, individuals with higher eGFR experienced larger clinic SBP reductions in response to DASH diet adherence compared with those with lower eGFR. Taken together, these results indicate that kidney function may influence the degree by which lifestyle modifications lower SBP, even among adults with normal kidney function. It also suggests that certain lifestyle interventions may provide greater benefit to patient subgroups based on their level of eGFR.
The finding that kidney function may influence the impact of lifestyle modifications on BP is not unexpected considering declines in eGFR alter several physiological mechanisms linking kidney function and BP. Declines in eGFR are associated with reduced urinary sodium and water excretion, increased activity of the RAAS system, enhanced activation of the sympathetic nervous system, and impaired nitric oxide synthesis and endothelial function.12, 13, 14, 30 The time course for the onset of each of these adaptations varies by eGFR, raising the possibility that lifestyle modifications may have different effects on BP depending on kidney function. Weight loss and exercise are two lifestyle modifications that alter multiple kidney‐related determinants of BP. Weight loss achieved by caloric restriction and increased physical activity has been shown to lower BP by reducing plasma norepinephrine concentration, plasma renin activity, and serum aldosterone concentration and by increasing urinary sodium and water excretion.31, 32, 33 The multifactorial effect of weight loss and exercise on BP likely accounts for our findings that BP was consistently lowered to a larger extent in participants with mildly decreased eGFR compared with those with normal‐high eGFR. Randomized controlled trials have shown that BP is reduced by weight loss (5–20 mm Hg/10 kg) and exercise (4–9 mm Hg).4, 5 Our study suggests that eGFR may have an influential role on the magnitude that BP is reduced.
The DASH diet has been shown to lower BP in both controlled feeding studies1, 6, 34 and among free‐living adults.2, 15 Although not as impactful as weight loss, the DASH diet also alters multiple kidney‐related determinants of BP. The exact mechanisms by which the DASH diet lowers BP have not been fully elucidated, but available evidence suggests that it enhances urinary sodium and water excretion,35 increases nitric oxide bioavailability, and improves endothelial function.36 In the present analysis, examination of whether kidney function moderates the effect of the DASH diet on BP yielded inconsistent results. We observed increased DASH diet adherence to be associated with larger improvements in clinic SBP for participants with normal‐high eGFR compared with those with mildly decreased eGFR. However, no difference was observed for ambulatory SBP. This limits our ability to draw a conclusion regarding the role of kidney function on BP reduction by the DASH diet. A secondary analysis of a subgroup of DASH‐Sodium trial participants, in which eGFR and presence of CKD did not influence the effects of the DASH diet on BP, contradicts our clinic SBP findings.37 Further studies are needed to better evaluate the role of kidney function on DASH diet–related BP changes.
In the present study, we did not observe the relationship between change in dietary sodium intake and change in SBP to be influenced by kidney function. Because salt‐sensitive hypertension is associated with altered renal hemodynamics, kidney function was expected to moderate this relationship.38, 39 It is possible that the eGFR values observed in our cohort were not low enough to influence BP response to sodium reduction. It is also possible that the degree that dietary sodium was reduced by our cohort (−260 mg/1000 calories) was not sufficient to observe a BP difference based on kidney function. Whether larger reductions in dietary sodium intake or more severe levels of kidney function are necessary to observe a kidney‐dependent benefit requires further investigation.
Study Limitations
The present study has several limitations. First, although serum creatinine measured at baseline permitted us to estimate GFR, urine albumin was not concurrently measured, thereby limiting our ability to determine the presence of CKD. Second, we did not measure serum creatinine following the ENCORE intervention and it is possible that changes in eGFR may have affected BP. Third, because participants who were randomized to the weight management arm of the trial lost weight and also improved their aerobic fitness, we have limited ability to determine the unique contribution of weight loss and aerobic fitness on BP.
Conclusions
Our study findings provide evidence that even among adults who have normal kidney function, variations in eGFR may influence the effectiveness of lifestyle modifications on BP reduction. Our observations have two significant implications with respect to future research. First, the role of kidney function should be considered when determining the impact of lifestyle modifications on BP, even among relatively healthy individuals. Identifying differences in BP response to lifestyle interventions for individuals with normal kidney function may inform studies that examine which interventions will be more effective in lowering BP for those with more advanced stages of CKD. Second, identifying which lifestyle modifications more effectively lower BP based on level of kidney function may help clinicians individualize treatment to better advise patients to adopt those lifestyle behaviors that may yield the greatest benefit. Future studies are needed to better define how kidney function may affect BP response to lifestyle modifications.
Supporting information
Table S1. Regression models of the relationship between changes in lifestyle modifications and diastolic blood pressure.
Item S1. Impact of lifestyle modification on diastolic blood pressure.
Acknowledgments and Disclosures
This work was supported, in part, by grants HL074103, HL122836, and 3R01HL122836‐01A1S1 from the National Heart, Lung, and Blood Institute, and grant M01‐RR‐30 from the General Clinical Research Center, National Institutes of Health. CCT also was supported by grant HL122836‐01A1S1 from the National Heart, Lung, and Blood Institute. Financial disclosures: None.
J Clin Hypertens (Greenwich). 2016;18:1260–1267. DOI: 10.1111/jch.12853. © 2016 Wiley Periodicals, Inc.
References
- 1. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 2. Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. [DOI] [PubMed] [Google Scholar]
- 3. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high‐normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 4. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta‐analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. [DOI] [PubMed] [Google Scholar]
- 5. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 6. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH‐Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 7. Svetkey LP, Simons‐Morton D, Vollmer WM, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159:285–293. [DOI] [PubMed] [Google Scholar]
- 8. Prather AA, Blumenthal JA, Hinderliter AL, Sherwood A. Ethnic differences in the effects of the DASH diet on nocturnal blood pressure dipping in individuals with high blood pressure. Am J Hypertens. 2011;24:1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vollmer WM, Sacks FM, Ard J, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH‐sodium trial. Ann Intern Med. 2001;135:1019–1028. [DOI] [PubMed] [Google Scholar]
- 10. Bray GA, Vollmer WM, Sacks FM, et al. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH‐Sodium Trial. Am J Cardiol. 2004;94:222–227. [DOI] [PubMed] [Google Scholar]
- 11. Midgley JP, Matthew AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium on blood pressure: a meta‐analysis of randomized controlled trials. JAMA. 1996;275:1590–1597. [DOI] [PubMed] [Google Scholar]
- 12. Koomans HA, Roos JC, Boer P, et al. Salt sensitivity of blood pressure in chronic renal failure. Evidence for renal control of body fluid distribution in man. Hypertension. 1982;4:190–197. [DOI] [PubMed] [Google Scholar]
- 13. Weidmann P, Maxwell MH, Lupu AN, et al. Plasma renin activity and blood pressure in terminal renal failure. N Engl J Med. 1971;285:757–762. [DOI] [PubMed] [Google Scholar]
- 14. Neumann J, Ligtenberg G, Klein II, et al. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. 2004;65:1568–1576. [DOI] [PubMed] [Google Scholar]
- 15. Blumenthal JA, Babyak MA, Hinderliter A, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Group KDIGO . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(Suppl.):1–150. [DOI] [PubMed] [Google Scholar]
- 17. Vanholder R, Massy Z, Argiles A, et al. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048–1056. [DOI] [PubMed] [Google Scholar]
- 18. Van Biesen W, De Bacquer D, Verbeke F, et al. The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur Heart J. 2007;28:478–483. [DOI] [PubMed] [Google Scholar]
- 19. Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. [DOI] [PubMed] [Google Scholar]
- 20. Moranne O, Froissart M, Rossert J, et al. Timing of onset of CKD‐related metabolic complications. J Am Soc Nephrol. 2009;20:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Epstein DE, Sherwood A, Smith PJ, et al. Determinants and consequences of adherence to the dietary approaches to stop hypertension diet in African‐American and white adults with high blood pressure: results from the ENCORE trial. J Acad Nutr Diet. 2012;112:1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 24. White WB, Lund‐Johansen P, McCabe EJ, Omvik P. Clinical evaluation of the Accutracker II ambulatory blood pressure monitor: assessment of performance in two countries and comparison with sphygmomanometry and intra‐arterial blood pressure at rest and during exercise. J Hypertens. 1989;7:967–975. [DOI] [PubMed] [Google Scholar]
- 25. Bazzano LA, He J, Ogden LG, et al. Agreement on nutrient intake between the databases of the First National Health and Nutrition Examination Survey and the ESHA Food Processor. Am J Epidemiol. 2002;156:78–85. [DOI] [PubMed] [Google Scholar]
- 26. Eck LH, Klesges RC, Hanson CL, et al. Measuring short‐term dietary intake: development and testing of a 1‐week food frequency questionnaire. J Am Diet Assoc. 1991;91:940–945. [PubMed] [Google Scholar]
- 27. Block G, Hartman AM, Dresser CM, et al. A data‐based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. [DOI] [PubMed] [Google Scholar]
- 28. Boucher B, Cotterchio M, Kreiger N, et al. Validity and reliability of the Block98 food‐frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9:84–93. [DOI] [PubMed] [Google Scholar]
- 29. Blumenthal JA, Rejeski WJ, Walsh‐Riddle M, et al. Comparison of high‐ and low‐intensity exercise training early after acute myocardial infarction. Am J Cardiol. 1988;61:26–30. [DOI] [PubMed] [Google Scholar]
- 30. Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol. 2008;294:F1–F9. [DOI] [PubMed] [Google Scholar]
- 31. Tuck ML, Sowers J, Dornfeld L, et al. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–933. [DOI] [PubMed] [Google Scholar]
- 32. DeHaven J, Sherwin R, Hendler R, Felig P. Nitrogen and sodium balance and sympathetic‐nervous‐system activity in obese subjects treated with a low‐calorie protein or mixed diet. N Engl J Med. 1980;302:477–482. [DOI] [PubMed] [Google Scholar]
- 33. Masuo K, Rakugi H, Ogihara T, Lambert GW. Different mechanisms in weight loss‐induced blood pressure reduction between a calorie‐restricted diet and exercise. Hypertens Res. 2012;35:41–47. [DOI] [PubMed] [Google Scholar]
- 34. Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. [DOI] [PubMed] [Google Scholar]
- 35. Akita S, Sacks FM, Svetkey LP, et al. Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on the pressure‐natriuresis relationship. Hypertension. 2003;42:8–13. [DOI] [PubMed] [Google Scholar]
- 36. Lin PH, Allen JD, Li YJ, et al. Blood pressure‐lowering mechanisms of the DASH dietary pattern. J Nutr Metab. 2012;2012:472396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tyson CCKM, Patel UD, Pun PH, et al. Impact of kidney function on effects of the dietary approaches to stop hypertension (DASH) diet. J Hypertens (Los Angel). 2014;3:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guyton AC, Langston JB, Navar G. Theory for renal autoregulation by feedback at the juxtaglomerular apparatus. Circ Res. 1964;15(Suppl):187–197. [PubMed] [Google Scholar]
- 39. Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531–550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Regression models of the relationship between changes in lifestyle modifications and diastolic blood pressure.
Item S1. Impact of lifestyle modification on diastolic blood pressure.


