Abstract
Management of blood pressure in children with pheochromocytoma and other catecholamine‐secreting tumors (CSTs) is unique and challenging. The authors report a single‐center experience using sequential α‐adrenergic blockade (phenoxybenzamine), increased fluid intake, and β‐blockade for presurgical management of 10 CSTs in children. In this retrospective review, mean duration for blood pressure control in preparation for surgery was 4.5±2.6 weeks. Intraoperative hypertension was noted transiently (<2 hours) in eight patients (80%) and was treated with continuous infusion of short‐acting antihypertensive agents. Two (20%) patients required vasopressor medication infusion to manage intraoperative hypotension. Only two (20%) patients developed postoperative hypotension and required vasopressor medication infusion for <24 hours. All antihypertensive medications were discontinued in the immediate (≤4 days) postoperative period in 80% of patients. In conclusion, a systematic and multidisciplinary approach utilizing adrenergic blockade is effective in treating children with CSTs.
Pheochromocytoma (PH) is a catecholamine‐secreting neuroendocrine tumor that arises from chromaffin cells in the medulla of the adrenal gland. A total of 5% to 10% originate from the ganglia of the sympathetic or parasympathetic nervous system (extra adrenal) and are known as paraganglioma (PG).1, 2, 3, 4 Neuroblastoma (NB) is also a neuroendocrine tumor that originates from the adrenal gland or primitive neural crest elements in the sympathetic nervous system.5
Symptoms of these catecholamine‐secreting tumors (CSTs) are secondary to increased catecholamines (dopamine, norepinephrine, epinephrine, and others) that are released into the circulation. Most of the PHs secrete norepinephrine, resulting in hypertension, tachycardia, cardiac conduction anomalies, headaches, sweating, flushing, nausea, vomiting, and dizziness.1, 2, 5, 6, 7 Sustained, rather than paroxysmal, hypertension is seen in 60% to 90% of children with PH/PG.3, 6, 8 Hypertension can also be a result of renal artery compression or tumor effect, particularly in large tumors such as NB. Intraoperative hypertension associated with catecholamine secretion is present in 14.5% of patients with PH/PG as compared with 3% to 3.5% of patients with NB.5, 9, 10
Surgical resection is the mainstay of treatment of most CSTs.1 Manipulation during the intraoperative period can lead to massive catecholamine release resulting in severe hypertensive crisis, cardiac arrhythmias and cerebrovascular accidents. A sudden decrease in tumor burden and catecholamine effect can cause post‐resection hypotension requiring fluid resuscitation, in turn, leading to pulmonary edema. Goldstein and colleagues11 reported a reduction in perioperative complications from 69% to 3% with appropriate preoperative medical management. Several regimens in adult patients have used α‐adrenergic blockers (phenoxybenzamine or doxazosin), calcium channel blockers (CCBs) (amlodipine or nifedipine), a tyrosine hydroxylase inhibitor (α‐methyltyrosine), or a combined α‐ and β‐adrenergic blocker (labetalol).3, 5, 12, 13 The First International Symposium on PH also recommended preoperative management of hypertension but was not able to establish clear guidelines on management of blood pressure (BP) in pediatric patients.14
The aim of this study was to report our single‐center experience with sequential preoperative adrenergic blockade in children with CSTs. In the current case series, we also describe the intraoperative course and postoperative outcomes on α‐ and β‐adrenergic blockade regimens.
Methods
A retrospective review of medical records of children (6 months–18 years) from 2005–2013 with a diagnosis of CST undergoing surgical removal was conducted. CST diagnosis was confirmed by presence of elevated catecholamines (plasma and/or urine) and tumor on imaging (computed tomography or magnetic resonance imaging [MRI]). Cases were identified using The International Classification of Diseases, 9th Revision (ICD‐9) coding for PH, PG, and NB with help of a data analyst in the department of Health Information Management at Children's Hospital of Michigan. Excluded from the study were patients with CST with incomplete medical records or in whom BP was not treated with adrenergic blockade medication before surgical removal. Hypertension was defined as BP ≥95 percentile for age, sex, and height on at least three separate measurements. BP was measured with an oscillometric device and/or auscultation using an appropriately sized cuff. Once a diagnosis of CST was confirmed and surgical removal scheduled, adrenergic blockage was initiated and other antihypertensive medications were tapered.
Preoperative preparation was done as per the proposed algorithm (Figure). Phenoxybenzamine (α‐adrenergic blockade) was started at least 7 to 10 days preoperatively with a goal to decrease BP to <95 percentile for age, sex, and height. BP was recommended to be monitored twice daily at home in the seated position. Orthostasis was also monitored at clinic visits or during hospital admission. Heart rate was recorded and pulse volume assessed by palpation at each clinic visit. Patients were advised to increase oral fluid intake to at least 1.5 times of the daily maintenance fluid requirement, with an aim to increase pulse volume. Clinic visits were scheduled at least once a week. At least 3 days prior to surgery, a β‐adrenergic blocker was administered with a goal to control tachycardia and decrease heart rate to a normal range for age. All patients were admitted a day before surgery to ensure BP control and receive intravenous normal saline prior to surgery.
Figure 1.
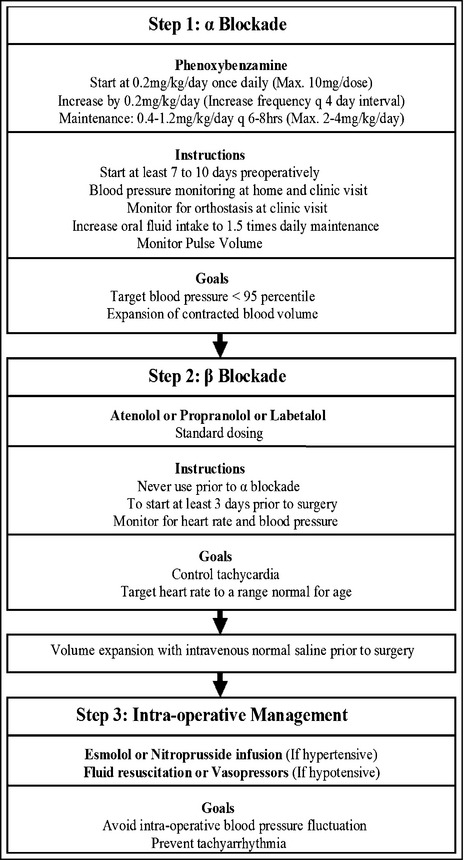
Management of hypertension in children with catecholamine‐secreting tumors undergoing surgical removal.
Intraoperative hypertension was defined as the need to use short‐acting antihypertensive medication as per anesthesiologist recommendations for persistent elevated BP readings. Hypotension in the intraoperative and postoperative periods was treated with crystalloid and/or colloid (albumin/packed red blood cells) replacement. Vasopressor medications were used in patients not responding to crystalloid and/or colloids. All patients were monitored in the Pediatric Intensive Care Unit after surgery, usually for 24 to 48 hours. BP medication was tapered according to the pediatric nephrologists' recommendation. A multidisciplinary approach involving the surgeon, anesthesiologist, hematologist‐oncologist, endocrinologist, and pediatric nephrologist was preferred in overall management.
Data on clinical presentation, diagnostic evaluation, genetic workup, treatment of hypertension, intraoperative BP control, and postoperative outcomes were recorded. The study was approved by the institutional review board of Wayne State University.
Results
Eight hypertensive children with CSTs underwent 10 surgical removal procedures (N=10) during the study period (Table 1). Two children with incomplete records were excluded from analysis. The median age was 8±6.5 years (range, 10 months to 18 years) and five were male. The primary diagnosis was PH in seven (70%), PG in one (10%), and NB in two (20%) patients. Bilateral PH was noted in three children and one patient had two recurrences (each required surgical intervention). Tachycardia was noted in five (50%), sweating in five (50%), headache in three (30%) patients. Sustained hypertension was seen in nine (90%) and paroxysmal hypertension in one (10%) patient. Only one (sibling of patient with recurrent PH) was asymptomatic and was found to be hypertensive on screening evaluation. Elevated catecholamines (plasma or urine) were present in all patients. Eight (80%) had elevated plasma catecholamines: normetanephrine in six (2–40 × normal range [N]), norepinephrine in three (1–20 × N), dopamine in three (1–6 × N), and metanephrine in one (3 × N). Nine (90%) had elevated 24‐hour urine catecholamines: vanillylmandelic acid in six (3–30 × N), normetanephrine in five (4–11 × N), metanephrines in three (1–6 × N), homovanillic acid (HVA) in two (3–4 × N), and norepinephrine in one. CST was confirmed by computed tomography scan in one, MRI in nine, and iodine‐131‐meta‐iodobenzylguanidine scan in nine (90%).
Table 1.
Clinical Profile of Children With CSTs (N=10)
| Case | Age | Sex | Symptoms | Elevated Catecholamine Plasma (P) ±Urine (U) | Imaging | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 16 y | F |
Tachycardia Headache |
Normetanephrine (P) Norepinephrine (P) Dopamine (P) Normetanephrine (U) |
MRI + MIBG | PH (Bilateral) |
| 2 | 15 y | F | Headache |
Normetanephrine (P) VMA (U) |
MRI+MIBG | PH |
| 3 | 12 y | M |
Sweating Headache |
Norepinephrine (P) Dopamine (P) VMA (U) |
MRI + MIBG | PH (Bilateral) |
| 4 | 18 y | M |
Tachycardia Sweating |
Norepinephrine (P) Dopamine (P) Metanephrine (U) |
CT + MIBG | PG |
| 5 | 11 months | F | Tachycardia |
Normetanephrine (P) VMA(U) HVA (U) |
MRI + MIBG | NB |
| 6 | 10 months | M | Tachycardia |
Normetanephrine (P) VMA(U) HVA (U) |
MRI + MIBG | NB |
| 7 | 5 y | M | Asymptomatic |
Normetanephrine (P) Normetanephrine (U) Metanephrine (U) |
MRI + MIBG | PH |
| 8 | 3 y | M |
Tachycardia Sweating |
Normetanephrine (U) Metanephrine (U) VMA(U) |
MRI | PH (Left) |
| 5 y | Sweating |
Normetanephrine (U) Norepinephrine (U) VMA (U) |
MRI + MIBG | PH (Right) | ||
| 5 y | Sweating |
Normetanephrine (P) Metanephrine (P) Normetanephrine (U) |
MRI + MIBG | PH (Recurrence) |
Abbreviations: CT, computed tomography; F, female; HVA, homovanillic acid; MIBG, iodine‐131‐meta‐iodobenzylguanidine; M, male; MRI, magnetic resonance imaging; PH, pheochromocytoma; VMA, vanillyl mandelic acid.
Genetic testing was performed in six of eight children. Von Hippel‐Lindau gene mutation was confirmed in two patients with PH. One patient was positive for succinate dehydrogenase subunit B gene mutation. Of two patients with NB who were screened for N‐myc mutations, one patient was positive. All patients with PH/PG were prepared for surgery using α‐adrenergic blockade (phenoxybenzamine) followed by β‐adrenergic blockade (atenolol or propranolol or labetalol). Two children with NB were initially treated with oral labetalol and preoperative preparation with phenoxybenzamine was performed once surgery was scheduled. Dosage and duration of each medication are shown in Table 2. The mean duration to achieve BP control in preparation for surgery was 4.5±2.6 weeks. The intraoperative and postoperative outcomes are shown in Table 3.
Table 2.
Preoperative Treatment of BP in Patients With CSTs (N=10)
| Study Variables | Frequency |
|---|---|
| Preoperative α blockade | |
| Phenoxybenzamine, (n) | 10 |
| Dose, (mg/kg/d) | 0.6 |
| Duration, (weeks) | 4.5±2.6 |
| Preoperative β blockade | |
| Atenolol, (n) | 4 |
| Dose, (mg/kg/d) | 0.4 |
| Duration, (days) | 34±14.8 |
| Propranolol, (n) | 2 |
| Dose, (mg/kg/d) | 0.8 |
| Duration, (days) | 3±0 |
| Labetalol, (n) | 4 |
| Dose, (mg/kg/d) | 2.3 |
| Duration, (days) | 11.5±9.2 |
| Preoperative volume expansion, (n) | 9 |
| Duration, (days) | 19.8±15.2 |
| Duration to achieve BP control in preparation for surgery, (weeks) | 4.5±2.6 |
Abbreviations: BP, blood pressure; CSTs, catecholamine‐secreting tumors.
Table 3.
Intraoperative and Postoperative Treatment of BP in Patients With CSTs (N=10)
| Study Variables | Frequency |
|---|---|
| Intra‐operative hypertension | 3 (30%) |
| Labetalol infusion, (n) | 1 |
| Duration, (hours) | 2 |
| Sodium nitroprusside infusion, (n) | 1 |
| Duration, (hours) | 1.5 |
| Esmolol infusion, (n) | 1 |
| Duration, (hours) | 1.5 |
| Intra‐operative hypotension | 2 (20%) |
| Albumin infusion, (n) | 2 |
| PRBC infusion, (n) | 2 |
| Vasopressor medication infusion, (n) | 1 |
| Duration, (hours) | 1 |
| Intra‐operative hypertension and hypotension | 5 (50%) |
| Sodium nitroprusside infusion, (n) | 3 |
| Duration, (hours) | 0.6 |
| Esmolol infusion, (n) | 2 |
| Duration, (hours) | 1 |
| Albumin infusion, (n) | 4 |
| PRBC infusion, (n) | 1 |
| Vasopressor medication infusion, (n) | 1 |
| Duration, (hours) | 0.5 |
| Postoperative hypertension | 2 (20%) |
| Oral anti‐hypertensives, (n) | 2 |
| Postoperative hypotension | 2 (20%) |
| Crystalloid infusion, (n) | 1 |
| Vasopressor medication infusion, (n) | 2 |
| Duration <24 hours | 2 |
| Discontinuation of all BP medications, (n) | 10 (100%) |
| Immediate post‐op period | 6 |
| 1–4 days | 2 |
| >4 days | 2 |
Abbreviations: BP, blood pressure; CSTs, catecholamine‐secreting tumors; PRBC, packed red blood cell.
Discussion
Our report highlights the importance of a systematic approach in treating hypertensive children with CST. No randomized trials or prospective studies are currently available to compare the different preoperative treatment regimens for adults or children with CSTs. Mostly, retrospective studies or case reports in the literature support the use of an adrenergic blockade regimen.1, 10, 11, 12 Weingarten and collegues15 report the largest retrospective series in adults, comparing the perioperative management algorithm at two different institutions. In children, Pham and colleagues1 retrospectively reviewed their medical and surgical management and used α‐ and β‐adrenergic blockers for control of hypertension in 76% of patients. However, intraoperative and postoperative outcomes on this sequential regimen were not described. We report our single‐center experience of a systematic approach that can be a useful tool for physicians and surgeons caring for children with CST.
The aim of preoperative α‐adrenergic blockade is to prevent the well‐known “catecholamine storm” during induction of anesthesia or at the time of surgical manipulation of the tumor. The goal is to avoid hypertensive crisis and/or cardiac arrhythmias by normalizing BP and heart rate before surgery.14, 15, 16 Restoration of the contracted blood volume occurs preoperatively with use of a combination of α‐adrenergic blockade and increased fluid intake. This “expanded” intravascular volume in the setting of aggressive α‐blockade reduces the likelihood of post‐resection hypotension.2
Phenoxybenzamine (a noncompetitive α‐adrenergic blocker) was used in all of our patients and has also been reported to be the preferred drug in adult regimens because of its longer half‐life.2, 14, 17 Intraoperative hypertension was transient (<2 hours) in our study and no patient required continuous infusion of short‐acting antihypertensive medication (nitroprusside, labetalol, or esmolol) in the postoperative period, thus showing adequate adrenergic blockade. Phenoxybenzamine is reported to be advantageous over competitive or short‐acting α1‐adrenergic blockers (doxazosin, prazosin), which have a risk of being displaced from the receptor by excessive catecholamine release during surgery.2, 12, 14 On the other hand, phenoxybenzamine is a long‐acting agent (half‐life of 24 hours)18 as compared with prazosin (half‐life of 3 hours)19 and has increased risk of postoperative hypotension. In our series, intraoperative hypotension was treated with colloid resuscitation in seven (70%) of the cases; one patient (10%) required vasopressor medication. Two (20%) patients had postoperative hypotension requiring vasopressor medication for <24 hours. Thus, phenoxybenzamine was a safe option for our patients and did not lead to persistent postoperative hypotension.
In adults, phenoxybenzamine is usually recommended to be started 1 to 2 weeks preoperatively.2, 3, 17, 20 In the large retrospective adult series from the Mayo Clinic, phenoxybenzamine was used for 1 to 4 weeks prior to surgery.15, 17 Interestingly, the mean duration of phenoxybenzamine use was 4.5 weeks in our series, reflecting a longer time to achieve target BP (<95 percentile) and pulse volume. We believe this could be the result of a lower starting dose of medication, slower dose increments, and increased sympathetic activity in children as compared with adults.
An unusual and unique aspect in the management of hypertension in CST is increasing water intake.17 The rationale is to increase the blood volume, reverse chronic vasoconstriction, and ultimately prevent post‐resection hypotension. In our patients, we clinically monitored pulse volume to assess the adequacy of restoration of blood volume. Only two (20%) patients required vasopressor support in the postoperative period as compared with 56% adults.15 With a combination of phenoxybenzamine and increased fluid intake, we were able to decrease the risk of post‐resection hypotension.
The use of β‐adrenergic blockade is recommended to avoid tachyarrhythmias and achieve better BP control. However, β‐adrenergic blockade should never precede α‐adrenergic blockade since unopposed α‐receptor stimulation can cause severe hypertensive crisis.12, 14, 16 In our series, the choice of β‐adrenergic blockade was as per physician preference. Four patients received oral labetalol, which is known to provide a 1:3 α‐ to β‐adrenergic block ratio, as compared with a 1:7 ratio for the intravenous infusion.21 Patients were on β‐adrenergic blockade for a mean duration of approximately 2 weeks before surgery. As noticed with phenoxybenzamine, the time to achieve target heart rate and BP was longer. More importantly, none of our patients experienced cardiac arrhythmias during or after surgery.
In our series, 50% of patients had both intraoperative hypertension and hypotension. However, none of these patients had persistent hypertension or hypotension. BP was stabilized using continuous antihypertensive infusion (50%) of nitroprusside or esmolol and use of vasopressor medication infusion (10%) for <1 hour during the intraoperative period. Other medications such as magnesium sulfate or dexmedetomidine or nicardipine have also been reported as safe alternatives in pediatric patients for intraoperative treatment but were not used in our patients.7, 13 The role of anesthesiologists is crucial during the intraoperative period because of rapid fluctuations in BP with induction, manipulation, and removal of CSTs. As such, a preoperative anesthesia consultation is recommended routinely in these patients.
Different treatment regimens have recommended CCBs and/or a tyrosine hydroxylase inhibitor (metyrosine) in combination with adrenergic blockade.14 However, without use of additional drugs we were able to demonstrate good BP control, as evidenced by discontinuation of all BP medications in 80% of patients within 4 days of surgery.
Catecholamine‐secreting NB is also reported to cause severe intraoperative hypertension during induction of anesthesia.22 In this series, an adrenergic blockade regimen was successfully utilized in managing two infants with NB.
Study Limitations
The major limitation of our case series is its retrospective nature. We acknowledge that we are reporting on a small number of children with a rare diagnosis of CST. Our results are based on a single‐center experience, and large prospective studies are required to formulate treatment guidelines for children with CSTs.
Conclusion
A systematic multidisciplinary approach of BP control with α‐blockade, volume expansion, and β‐blockade is necessary in the perioperative period. The preparation for surgery may take longer (2–4 weeks) in children as compared with adults. However, goal‐directed therapy to normalize BP, heart rate, and restoration of blood volume ensures excellent outcomes in children with PH, PG, and catecholamine‐secreting NB.
Financial Disclosure
There were no sponsors as well as no honorariums, grants, or other forms of payments given to anyone while preparing the manuscript.
Conflict of Interests
There is no conflict of interest on behalf of any author.
Acknowledgments
None.
J Clin Hypertens (Greenwich). 2015:720–725. DOI: 10.1111/jch.12571. © 2015 Wiley Periodicals, Inc.
References
- 1. Pham TH, Moir C, Thompson GB, et al. Pheochromocytoma and paraganglioma in children: a review of medical and surgical management at a tertiary care center. Pediatrics. 2006;118:1109–1117. [DOI] [PubMed] [Google Scholar]
- 2. Hack HA. The perioperative management of children with phaeochromocytoma. Paediatr Anaesth. 2000;10:463–476. [DOI] [PubMed] [Google Scholar]
- 3. Mazza A, Armigliato M, Marzola MC, et al. Antihypertensive treatment in pheochromocytoma and paraganglioma: current management and therapeutic features. Endocrine. 2014;45:469–478. [DOI] [PubMed] [Google Scholar]
- 4. Joynt KE, Moslehi JJ, Baughman KL. Paragangliomas: etiology, presentation, and management. Cardiol Rev. 2009;17:159–164. [DOI] [PubMed] [Google Scholar]
- 5. Haberkern CM, Coles PG, Morray JP, et al. Intraoperative hypertension during surgical excision of neuroblastoma. Case report and review of 20 years' experience. Anesth Analg. 1992;75:854–858. [DOI] [PubMed] [Google Scholar]
- 6. Holwitt D, Neifeld J, Massey G, Lanning D. Case report of an 11‐year‐old child with a nonfunctionalmalignant pheochromocytoma. J Pediatr Surg. 2007;42:E13–E15. [DOI] [PubMed] [Google Scholar]
- 7. Kalra Y, Agarwal HS, Smith AH. Perioperative management of pheochromocytoma and catecholamine‐induced dilated cardiomyopathy in a pediatric patient. Pediatr Cardiol. 2013;34:2013–2016. [DOI] [PubMed] [Google Scholar]
- 8. Vaganovs P, Bokums K, Miklasevics E, et al. Von Hippel‐Lindau syndrome: diagnosis and management of hemangioblastoma and pheochromocytoma. Case Rep Urol. 2013;2013:624096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kain ZN, Shamberger RS, Holzman RS. Anesthetic management of children with neuroblastoma. J Clin Anesth. 1993;5:486–491. [DOI] [PubMed] [Google Scholar]
- 10. Conzo G, Musella M, Corcione F, et al. Role of preoperative adrenergic blockade with doxazosin on hemodynamic control during the surgical treatment of pheochromocytoma: a retrospective study of 48 cases. Am Surg. 2013;79:1196–1202. [PubMed] [Google Scholar]
- 11. Goldstein RE, O'Neill JA Jr, Holcomb GW 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg 1999;229:755–764 discussion 764–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fishbein L, Orlowski R, Cohen D. Pheochromocytoma/paraganglioma: review of perioperative management of blood pressure and update on genetic mutations associated with pheochromocytoma. J Clin Hypertens (Greenwich). 2013;15:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bryskin R, Weldon BC. Dexmedetomidine and magnesium sulfate in the perioperative management of a child undergoing laparoscopic resection of bilateral pheochromocytomas. J Clin Anesth. 2010;22:126–129. [DOI] [PubMed] [Google Scholar]
- 14. Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab. 2007;3:92–102. [DOI] [PubMed] [Google Scholar]
- 15. Weingarten TN, Cata JP, O'Hara JF, et al. Comparison of two preoperative medical management strategies for laparoscopic resection of pheochromocytoma. Urology 2010;76:508.e6–508.e11. [DOI] [PubMed] [Google Scholar]
- 16. Waguespack SG, Rich T, Grubbs E, et al. A current review of the etiology, diagnosis, and treatment of pediatric pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2010;95:2023–2037. [DOI] [PubMed] [Google Scholar]
- 17. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069–4079. [DOI] [PubMed] [Google Scholar]
- 18. Gerald K. McEvoy, PharmD, ed. AHFS Drug Information®. 56th ed. Bethesda, MD. American Society of Health‐System Pharmacists. ISBN‐10: 1‐58528‐380‐0, ISBN‐13: 978‐1‐58528‐380‐4. STAT!Ref Online Electronic Medical Library. http://online.statref.com/Document.aspx?fxId=1&docId=409. Accessed March 6, 2015. [Google Scholar]
- 19. Gerald K. McEvoy, PharmD, ed. AHFS Drug Information®. 56th ed. Bethesda, MD. American Society of Health‐System Pharmacists; ISBN‐10: 1‐58528‐380‐0, ISBN‐13: 978‐1‐58528‐380‐4. STAT!Ref Online Electronic Medical Library. http://online.statref.com/Document.aspx?fxId=1&docId=589. Accessed March 6, 2015. [Google Scholar]
- 20. Young WF Jr. Pheochromocytoma: issues in diagnosis & treatment. Compr Ther. 1997;23:319–326. [PubMed] [Google Scholar]
- 21. Gerald K. McEvoy, Pharm.D., ed. 2015. AHFS Drug Information® – 56th Ed. Bethesda, MD, American Society of Health‐System Pharmacists. ISBN‐10: 1‐58528‐380‐0, ISBN‐13: 978‐1‐58528‐380‐4. STAT!Ref Online Electronic Medical Library. http://online.statref.com/Document.aspx?fxId=1&docId=598. Accessed March 6, 2015. [Google Scholar]
- 22. Kako H, Taghon T, Veneziano G, et al. Severe intraoperative hypertension after induction of anesthesia in a child with a neuroblastoma. J Anesth. 2013;27:464–467. [DOI] [PubMed] [Google Scholar]


