Abstract
The objective of this cross‐sectional study was to investigate risk markers indicating the presence of albuminuria in patients with hypertension in rural sub‐Saharan Africa (SSA). Urine albumin‐creatinine ratio, glycated hemoglobin (HbA1c), blood pressure, anthropometry, and other patient characteristics including medications were assessed. We identified 160 patients with hypertension, of whom 68 (42.5%) were co‐diagnosed with diabetes mellitus (DM). Among the included participants, 57 (35.6%) had albuminuria (microalbuminuria [n=43] and macroalbuminuria [n=14]). A backward multivariate logistic regression model identified age (per 10‐year increment) (odds ratio [OR], 1.42; 95% confidence interval [CI], 1.03–1.95), HbA1c >53 compared with <48 mmol/mol (OR, 3.81; 95% CI, 1.74–8.35), and treatment with dihydropyridine calcium channel blockers (OR, 2.59; 95% CI, 1.09–6.16) as the variables significantly associated with albuminuria. Only dysregulated DM and age were the conventional risk markers that seemed to suggest albuminuria among patients with hypertension in rural SSA.
Albuminuria (microalbuminuria and macroalbuminuria) has shown a strong association with adverse cardiovascular outcomes and end‐stage renal disease among patients with hypertension in the Western world and Asia.1 Recently, albuminuria has also been shown to be associated with all‐cause mortality and cardiovascular events among hypertensive Africans.2 Albuminuria can easily be assessed as albumin‐creatinine ratio (ACR) in urine samples even in simple clinical settings. Risk markers associated with presence of albuminuria in Caucasians and Asians with hypertension include age, diabetes mellitus (DM), obesity, smoking, and insufficient blood pressure (BP) control.3, 4 Knowledge on risk markers suggesting presence of albuminuria among patients with hypertension in sub‐Saharan Africa (SSA) has only been investigated in urban areas and is, in general, comparable with those of the Western world.5, 6 The lifestyle and living conditions in rural areas of SSA differ from urban environments in terms of physical activity, nutrition, and patterns of cardiovascular disease.7 Thus, risk markers indicating presence of albuminuria could diverge from urban settings. Comorbidity of DM has been shown to be strongly associated with albuminuria among patients with hypertension in rural SSA,8 but, otherwise, information on risk markers indicating albuminuria is virtually nonexistent. The objective of this study is to investigate the risk markers suggesting presence of albuminuria among patients with hypertension in a rural population of SSA.
Methods
We conducted a cross‐sectional study from February to April 2011 in patients with hypertension at St. Francis Hospital in Katéte, a rural area in the Eastern province of Zambia. We assessed anthropometry, glycemic control, albuminuria, and other patient characteristics including information on medication among patients with DM and hypertension.8, 9 All patients with known hypertension who came to the outpatient department of St. Francis Hospital for a clinical review of their hypertension treatment were invited to participate in this substudy. Exclusion criteria were positive urine dipstick for nitrites, leukocytes, blood, or fever (defined as an ear temperature ≥37.5°C). All procedures were performed during one clinical review between 8:30 am and 4:00 pm. BP was measured in a quiet room with the participant seated in a resting position for at least 10 minutes using the Omron M8 Comfort automatic BP monitor (Omron Corporation, Kyoto, Japan) and an appropriately sized cuff on the upper arm. BP was measured once in each arm. Thereafter, in the arm with the highest systolic BP, two measurements were performed with a 1‐minute interval. The mean systolic and diastolic BPs were then calculated from these two measurements. Pulse pressure (PP) was estimated as: PP=systolic BP−diastolic BP. Hypertension was defined as BP ≥140/90 mm Hg or ongoing therapy with antihypertensive medication. ACR in one urine sample was used to quantify albuminuria by use of the Afinion AS100 Analyzer (Alere‐Axis Shield PoC, Oslo, Norway). We defined cutoff values for microalbuminuria as ACR=2.5–25.0 mg/mmol and macroalbuminuria as ACR >25.0 mg/mmol for both women and men. Glycosylated haemoglobin (HbA1c) was also measured with the Afinion AS100 Analyzer. The validity of HbA1c and ACR measurements by the Afinion AS100 Analyzer has previously been documented.10, 11 Moreover, we ensured ongoing validity and exact precision of the Afinion using HbA1c and ACR control samples each day prior to any use in the participants. Neither ACR nor HbA1c had been measured in any of the participants prior to the study. DM was defined as HbA1c ≥48 mmol/mol12 or ongoing therapy with antidiabetes medication. Weight and height, wearing light clothing only, and waist circumference at the midpoint between the iliac crest and the lowest rib were also assessed. Information including age, smoking, medication use, and duration since diagnosis of hypertension was obtained in an interview conducted in English or with an interpreter in the local language, Chichewa. All participants were offered an HIV test prior to entering the study but all refused to participate in the study if it was conditioned by HIV testing.
All participants gave oral and written informed consent prior to inclusion. The study was approved by the ethics committee of St. Francis’ Hospital and consultatively approved by the Developing Country Subcommittee of the National Committee on Biomedical Research Ethics in Denmark.
Statistical Analysis
Categorical variables were compared by chi‐square or Fisher exact test as appropriate. The t test was used to compare continuous variables if their residuals were normally distributed and had equal variances. Otherwise, Wilcoxon rank‐sum test was used. Mean values are presented with standard deviations (SDs) and medians (25th–75th percentiles). We used a backward multivariate stepwise logistic regression model to identify risk markers for the presence of albuminuria. All variables with a P value <.30 in the univariate logistic regression analyses (Table S1) were included in the backward multivariate model and remained in the model if the P value was <.20 and if they did not demonstrate interaction or multicollinearity in the final multivariate model. Assumption of linearity between each of the continuous variables and log‐odds for albuminuria was assessed. Overall goodness of fit was assessed with the Hosmer‐Lemeshow test and the model with estimates was analyzed for overdispersion. Odds ratios (ORs) are presented with 95% confidence intervals (95% CIs). A P value <.05 was considered statistically significant. Data were analyzed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
We invited 179 patients with hypertension to participate during the study period. Three patients refused to participate; seven were excluded because of repeated positive urine tests of blood, nitrites, or leucocytes despite relevant treatment and nine patients were excluded from statistical analyses because of unavailable data. Thus, 160 participants were included in the study. The majority of the participants were women (n=110, 68.8%), and the mean age was 60.6 (11.6) years. Among the participants, 68 (42.5%) were co‐diagnosed with DM and 95 (59.4%) had a body mass index (BMI) >25 kg/m2. Three (1.9%) participants were smokers and four (2.5%) were known to be HIV positive. Among the included participants, 57 (35.6%) had albuminuria (microalbuminuria [n=43] and macroalbuminuria [n=14]). ACR was 1.0 (0.7–1.2) mg/mmol among participants without albuminuria compared with 6.2 (3.8–22.0) mg/mmol among those with albuminuria. The characteristics of the participants with respect to albuminuria status are presented in the Table 1. The participants with albuminuria were significantly older, had higher HbA1c levels, had a longer duration since hypertension diagnosis, and were more likely to be treated with dihydropyridine calcium channel blockers (CCBs) (Figure, P<.01) than the participants who did not have albuminuria. In the logistic regression analyses, HbA1c was categorized into an ordinal variable (glycemic category) as the variable did not fulfill the assumption of linearity with the log odds for albuminuria. A backward multivariate logistic regression model identified age (per 10‐year increment) (OR, 1.42; 95% CI, 1.03–1.95), HbA1c >53 compared with <48 mmol/mol (OR, 3.81; 95% CI, 1.74–8.35), and treatment with CCBs (OR, 2.59; 95% CI, 1.09–6.16) as the variables significantly associated with albuminuria (Table 2).
Table 1.
Characteristics of Hypertension Participants With Normoalbuminuria (Urine ACR <2.5 mmol/mol) and Albuminuria (Microalbuminuria and Macroalbuminuria)
| Normoalbuminuria | Albuminuria | P Value | |
|---|---|---|---|
| (n=103) | (n=57) | ||
| Women, No. (%) | 72 (69.9) | 38 (66.7) | .67 |
| Age, y | 59.2 (11.1) | 63.2 (12.1) | .04 |
| Systolic BP, mm Hg | 147 (24) | 150 (23) | .53 |
| Diastolic BP, mm Hg | 92 (11) | 92 (13) | .99 |
| Pulse pressure, mm Hg | 55 (19) | 58 (19) | .41 |
| Duration since diagnosis, y ● | 3.5 (1.0–8.0) | 5.0 (1.0–10.0) | .047 |
| HbA1c, mmol/mol ● | 41 (37–47) | 47 (41–66) | <.01 |
| DM, No. (%) | 36 (35.0) | 32 (56.1) | <.01 |
| BMI, kg/m2 | 26.9 (6.3) | 27.6 (5.4) | .44 |
| Waist circumference, cm | 93.6 (15.9) | 96.2 (13.5) | .30 |
Abbreviations: ACR, albumin‐creatinine ratio; BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; HbA1c, glycated hemoglobin. All continuous variables are given as mean (standard deviation) unless otherwise indicated. ●Median (25th–75th percentiles).
Figure 1.
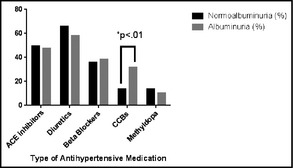
Antihypertensive therapy among 160 participants with hypertension with respect to albuminuria (n=57) and normoalbuminuria (n=103). There was a significant difference (*) between the groups in terms of dihydropyridine calcium channel blockers (CCBs) but not in any other medications. ACE indicates angiotensin‐converting enzyme.
Table 2.
Multivariate Backward Logistic Regression Model for Albuminuria (Micro‐ and Macroalbuminuria) Among Included Participants (n=160)
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Age (per 10 y increment) | 1.42 (1.03–1.95) | .03 |
| Glycemic category | ||
| HbA1c <48 mmol/mol | 1.00 | – |
| HbA1c 48–53 mmol/mol | 1.63 (0.39–6.80) | .50 |
| HbA1c >53 mmol/mol | 3.81 (1.74–8.35) | <.01 |
| Dihydropyridine calcium channel blockers | 2.59 (1.09–6.16) | .03 |
| Duration since diagnosis (per year since diagnosis) | 1.03 (0.99–1.07) | .16 |
Variables originally introduced all had a P value <.30 in a univariate logistic regression model for albuminuria: age, dihydropyridine calcium channel blockers, glycemic category, duration since diagnosis, and waist circumference. The variables were tested for multicollinearity and interaction. The Hosmer‐Lemeshow test showed appropriate overall goodness of fit (P=.75).
Sensitivity and Subgroup Analyses
Age, HbA1c, and CCB use were also significantly associated with albuminuria in a non‐stepwise logistic regression model that introduced the same original variables as the backward multivariate model (data not shown). Further, the association of the three variables with albuminuria in the backward logistic regression withstood the exclusion of all patients with macroalbuminuria (n=14) (data not shown). Age and CCB withstood the exclusion of all participants with DM (n=68). The participants who were treated with CCBs did not differ significantly from the others in terms of age, BP, sex ratio, and HbA1c (Table S2).
Discussion
This study is, to our knowledge, the first to investigate risk markers suggesting albuminuria among rural Africans with hypertension. The key finding was that well‐known risk markers indicating presence of albuminuria such as obesity, BP level, and duration since diagnosis were not associated with albuminuria. Information on albuminuria in patients with hypertension in SSA is sparse, but the participants in this study had approximately the same BMI and BP level as urban Ghanaian patients with hypertension5 and more adequate BP control than newly diagnosed Nigerian hypertension patients living in urban areas.6 Thus, we speculate whether the rural vs urban difference in living conditions and lifestyle could modify the effect of some of the conventional risk markers for albuminuria in rural SSA. A recent review suggested that living in an urban SSA environment caused more physical and psychic stress in general than living in a rural setting.13 It is a possibility that ongoing exposure to physical and psychic stress could aggravate the chronic endothelial inflammation usually found in patients with hypertension. HbA1c level >53 mmol/mol corresponding to suboptimal or poor glycemic control was strongly associated with albuminuria. Thus, suggesting that the synergistic combination of dysregulated DM and hypertension may be the driving factor for albuminuria in rural SSA,8 whereas roles of obesity, duration of hypertension, and BP control may be secondary. Access to adequate treatment of both conditions may be more limited in rural areas. On the other hand, another finding in this study was that treatment with CCBs was associated with albuminuria. A recent study has demonstrated that the CCB, amlodipine, increased albuminuria by decreasing the resistance of the afferent arteriole in the glomerulus, leading to increased intraglomerular pressure.14 Even though the CCB used among the participants in this study was predominantly nifedipine, the mechanism that explains the association with albuminuria may be similar. Agodoa and colleagues15 showed that the angiotensin‐converting enzyme (ACE) inhibitor, ramipril, was superior to the CCB, amlodipine, in terms of limiting proteinuria among African Americans with hypertensive nephrosclerosis. Moreover, Guasch and colleagues16 demonstrated that isradipine and captopril had similar effects in terms of BP reduction among African Americans with diabetic nephropathy, but isradipine increased proteinuria whereas captopril reduced it. ACR had not been measured in any participant prior to the study. Thus, it can be excluded that CCBs had been preferred in participants with existing albuminuria. Furthermore, we could not demonstrate any significant difference between the participants who were treated with CCBs compared with those who were not. Hence, our results suggest that the association between CCBs and albuminuria was not an expression of confounding by indication and, perhaps, that CCBs should not be the first choice of antihypertensive treatment among rural Africans with albuminuria. Further investigation in larger‐scale and longitudinal studies are needed.
Study Limitations
This study has some limitations. The study population was small, which caused large 95% CIs and could have led to statistical type II errors. We had limited information on comorbidity and doses of antihypertensive medication among participants. We assessed albuminuria only once in each of the participants, which could have overestimated or underestimated the true frequency of albuminuria. Further, since the participants were not systematically HIV tested before the study we cannot exclude that albuminuria may have been caused by HIV nephropathy.17 The hospital did not have laboratory facilities to measure markers of kidney function such as plasma creatinine or estimated glomerular filtration rate nor to assess other conventional bio risk markers such as dyslipidemia. Approximately 40% of the included participants had DM. Albuminuria among these participants was most likely caused by DM nephropathy and could weaken the link between other risk markers and albuminuria in the study population.
Conclusions
Conventional risk markers suggesting albuminuria other than dysregulated DM and age were rather weak among rural SSA patients with hypertension. Larger and longer studies are needed to verify our results, including whether CCBs may increase the risk for presence of albuminuria in rural SSA.
Disclosures
This work was supported by a grant from Tove Birte Jensens Mindelegat. The Afinion AS100 Analyzer and test cartridges, used to measure albuminuria and HbA1c, were all donated by Alere Axis Shield PoC (Oslo, Norway). None of the financial sources took any part in the preparation, data collection, analyses, writing process of the study, or the decision to publish the final version of the manuscript. The authors report no conflicts of interest to disclose in relation to this research.
Supporting information
Table S1. Univariate logistic regression for albuminuria among included participants with hypertension (n=160).
Table S2. Characteristics of hypertension participants treated with dihydropyridine calcium channel blockers (CCBs) compared with the other participants in the study.
Acknowledgments
The authors are most grateful to all the participants and staff of St. Francis’ Hospital for their contributions to this work. The authors are indebted to the foundation Tove Birte Jensens Mindelegat for its financial support and to Alere Axis Shield PoC (Oslo, Norway) for the equipment donated.
J Clin Hypertens (Greenwich). 2016;18: 27–30. DOI: 10.1111/jch.12662. © 2015 Wiley Periodicals, Inc.
References
- 1. Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end‐stage renal disease in individuals with and without hypertension: a meta‐analysis. Lancet. 2012;380:1649–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schutte R, Schmieder RE, Huisman HW, et al. Urinary albumin excretion from spot urine samples predict all‐cause and stroke mortality in Africans. Am J Hypertens. 2014;27:811–818. [DOI] [PubMed] [Google Scholar]
- 3. Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa investigation on complications. Hypertension. 1997;30:1135–1143. [DOI] [PubMed] [Google Scholar]
- 4. Liu X, Liu Y, Chen Y, et al. Body mass index (BMI) is associated with microalbuminuria in Chinese hypertensive patients. Int J Environ Res Public Health. 2015;12:1998–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Osafo C, Mate‐Kole M, Affram K, Adu D. Prevalence of chronic kidney disease in hypertensive patients in Ghana. Ren Fail. 2011;33:388–392. [DOI] [PubMed] [Google Scholar]
- 6. Busari OA, Opadijo OG, Olarewaju OT. Microalbuminuria and its relations with serum lipid abnormalities in adult Nigerians with newly diagnosed hypertension. Ann Afr Med. 2010;9:62–67. [DOI] [PubMed] [Google Scholar]
- 7. Minicuci N, Biritwum RB, Mensah G, et al. Sociodemographic and socioeconomic patterns of chronic non‐communicable disease among the older adult population in Ghana. Glob Health Action. 2014;7:21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rasmussen JB, Thomsen JA, Rossing P, et al. Diabetes mellitus, hypertension and albuminuria in rural Zambia: a hospital‐based survey. Trop Med Int Health. 2013;18:1080–1084. [DOI] [PubMed] [Google Scholar]
- 9. Rasmussen JB, Nordin LS, Rasmussen NS, et al. Random blood glucose may be used to assess long‐term glycaemic control among patients with type 2 diabetes mellitus in a rural African clinical setting. Trop Med Int Health. 2014;19:1515–1519. [DOI] [PubMed] [Google Scholar]
- 10. Kvam C, Dworsky E, Campbell A, et al. Development of an ACR assay for the Afinion TM AS100 Analyzer. AACC 2007 Congress; 15‐19 July 2007, 2007; San Diego.
- 11. Lenters‐Westra E, Slingerland RJ. Six of eight hemoglobin A1c point‐of‐care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010;56:44–52. [DOI] [PubMed] [Google Scholar]
- 12. International Diabetes Federation Guideline Development Group . Global Guideline for Type 2 Diabetes. Diabetes Res Clin Pract. 2014; 104:1–52. [DOI] [PubMed] [Google Scholar]
- 13. Iwelunmor J, Airhihenbuwa CO, Cooper R, et al. Prevalence, determinants and systems‐thinking approaches to optimal hypertension control in West Africa. Global Health. 2014;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ott C, Schneider MP, Raff U, et al. Effects of manidipine vs. amlodipine on intrarenal haemodynamics in patients with arterial hypertension. Br J Clin Pharmacol. 2013;75:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285:2719–2728. [DOI] [PubMed] [Google Scholar]
- 16. Guasch A, Parham M, Zayas CF, et al. Contrasting effects of calcium channel blockade versus converting enzyme inhibition on proteinuria in African Americans with non‐insulin‐dependent diabetes mellitus and nephropathy. J Am Soc Nephrol. 1997;8:793–798. [DOI] [PubMed] [Google Scholar]
- 17. Han TM, Naicker S, Ramdial PK, Assounga AG. A cross‐sectional study of HIV‐seropositive patients with varying degrees of proteinuria in South Africa. Kidney Int. 2006;69:2243–2250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate logistic regression for albuminuria among included participants with hypertension (n=160).
Table S2. Characteristics of hypertension participants treated with dihydropyridine calcium channel blockers (CCBs) compared with the other participants in the study.


