Abstract
Arterial stiffness and endothelial dysfunction are important determinants of cardiovascular events in patients with arterial hypertension. There are few data regarding the role of aliskiren on the central hemodynamics and endothelial function in patients with uncontrolled arterial hypertension. The aim of this study was to assess the addition of aliskiren to other antihypertensive drug treatment for arterial stiffness and endothelial function. Thirty uncontrolled hypertensive patients (mean age, 60.4±12.2 years), without any other cardiovascular risk factors, were enrolled. Augmentation index (AIx) and carotid‐femoral pulse wave velocity (cfPWV) by applanation tonometry and reactive hyperemia peripheral arterial tonometry (RH PAT) index using peripheral arterious tonometry at baseline and after 6 months of aliskiren titrated to 300 mg once a day was evaluated. The addition of aliskiren had no effect on values of central AIx (33.26±10.74% vs 28.86±10.74%; P=.36) but did significantly improve values of cfPWV (9.36±2.65 m/s vs 8.72±2.48 m/s; P=.04) and RH PAT index (1.64±0.57 vs 1.75±0.45; P=.05). In addition to improving systolic and diastolic blood pressure, the addition of aliskiren to concomitant antihypertensive drugs in uncontrolled hypertensive patients may be effective in improving aortic stiffness and endothelial function. These results encourage further studies to evaluate the use of aliskiren for cardiovascular prevention.
In essential hypertension, vascular alterations, including arterial stiffness and endothelial dysfunction, are important determinants of cardiovascular events. The relationship between uncontrolled blood pressure (BP) and arterial stiffness is not fully understood.1 It has been hypothesized that vascular changes in the structure of tissue components within the vascular wall through mechanical factors2 may lead to increased arterial stiffness. European Society of Hypertension/European Society of Cardiology guidelines include increased arterial stiffness among the established measures of target organ damage for the stratification of cardiovascular risk.3 In addition, endothelial dysfunction is accepted as a promoter of atherosclerosis4 and cardiovascular disease.5
Previous meta‐analyses also showed that augmentation index (AIx) and pulse wave velocity (PWV) are independent risk factors for cardiovascular disease and that these variables may reflect the different characteristics of the pathophysiologic abnormalities related to arterial stiffness.6 Several previous studies demonstrated that these variables are more closely related to organ damage than brachial BP7, 8, 9, 10 and the potential relevance of enhanced central aortic stiffness to the transition from hypertension to heart failure.11
Short‐test studies have shown that various classes of BP‐lowering drugs may have profoundly different effects on pulse wave morphology and thus central hemodynamic parameters despite similar effects on brachial artery pressures.12, 13, 14, 15
Experimental and clinical evidence has indicated that activation of the renin‐angiotensin system (RAS) is involved in the pathogenesis of hypertension and the related target organ damage. Multiple studies have proven the usefulness of RAS blockade induced by angiotensin‐converting enzyme (ACE) inhibitors and angiotensin 1 receptor antagonists (ARBs) for the management of hypertension.16 Although ACE inhibitors and ARBs are generally effective in reversing vascular alterations in essential hypertension, available evidence clearly indicates that these drug classes do not improve endothelium‐dependent vasodilation in the peripheral resistance arteries.17, 18, 19, 20, 21 It is conceivable that these negative results might be explained by the noncomplete RAS blockade exerted by both ACE inhibitors and ARBs in such vascular district.
Both ACE inhibitors and ARBs function downstream of the rate‐limiting step in the RAS cascade, which involves the renin‐catalyzed conversion of angiotensinogen to angiotensin (Ang) I. This leads to the promotion of renin release and to an increase in plasma renin activity (PRA) via the intrakidney short feedback loop due to lack of angiotensin II type 1 (AT1) receptor–mediated suppression of renin production in the juxtaglomerular cells of the kidney. Renin inhibition is a means for blocking the RAS.
Aliskiren is a direct renin inhibitor that acts at the rate‐limiting step of the RAS cascade, reducing renin activity and the circulating levels of Ang I, Ang II, and aldosterone. Therefore, unlike either ACE inhibitors or ARBs, aliskiren does not induce a compensatory increase in PRA, but rather reduces it.
Currently, limited data are available on the effect of aliskiren on vascular function in human hypertension. The aim of the present study was to assess the role of the addition of aliskiren in patients with hypertension, not controlled with conventional antihypertensive drugs treatment, in modifying endothelial function and arterial stiffness.
Materials and Methods
Study Participants and Design
Patients affected by arterial hypertension from the cardiology unit of Brescia's Spedali Civili were enrolled from January to April 2011. Hypertension was defined as an average clinic systolic BP (SBP) of at least 140 mm Hg or diastolic BP (DBP) of at least 90 mm Hg or both on two different occasions (with at least a 2‐week interval).
Inclusion criteria were age older than 40 years, mild to moderate essential hypertension (140 mm Hg≤SBP≤180 mm Hg or 90 mm Hg≤DBP≤110 mm Hg) not controlled by at least 2 antihypertensive agents, one from each of the following drug classes: thiazide‐type diuretic, ACE inhibitor or ARB, long‐acting calcium channel blocker, β‐blocker, centrally active agents and alpha‐receptor blockers. Exclusion criteria included patients who exhibited severe hypertension (clinic SBP >180 mm Hg and/or DBP >110 mm Hg), type 1 or type 2 diabetes mellitus, arrhythmia, clinically significant cardiovascular disease, cerebrovascular disease or neuropathy, renal dysfunction (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), overt proteinuria (urinary protein to creatinine ratio >300 mg/g creatinine), no hyperkalemia (defined as serum potassium >5 mEq/L), taking cyclosporine, or women who were nursing or pregnant. Importantly, patients were excluded if they had a history of angioedema with an ACE inhibitor or ARB.
Determination of the clinic BP and heart rate were performed at baseline and 3 and 6 months after the start of aliskiren‐based antihypertensive treatment using an automated BP monitor (model HEM‐907XL; Omron Healthcare Inc, Kyoto, Japan).
Determination of carotid‐femoral PWV (cfPWV), AIx, and reactive hyperemia peripheral arterial tonometry (RH PAT) index were performed at baseline and at 6 months after the start of aliskiren‐based antihypertensive treatment.
Venous blood for the hematological, biochemical, and renal parameters were drawn and collected at baseline and at 3 and 6 months of follow‐up 12 weeks after treatment. We calculated the eGFR using the Modification of Diet in Renal Disease formula.22
In addition to the usual therapy, a tablet of 150 mg of aliskiren once daily was administered in the morning and was titrated up to 300 mg daily 3 months after the treatment to reach the BP target if necessary.
Assessment of Arterial Stiffness
To evaluate vascular rigidity we used applanation tonometry (Vascular Explorer, Enverdis GmbH, Jena), and central hemodynamics including aortic AIx were calculated from brachial pressure curves in combination with automated transfer algorithms. At the same time and in addition to the measurement of aortic AIx, we evaluated 3 different central and central‐peripheral PWVs calculated by the software. Brachial‐ankle PWV was registered by means of simultaneous cuff measurements taken on the upper arm and ankle at diastolic pressure (foot – foot measurement of the time difference between both pressure waves), aortic PWV was measured using the reflection method under brachial stop/flow conditions (foot – foot measurement of the time difference for the direct pulse wave and the pulse wave reflected at the bifurcation), and cfPWV was calculated from brachial‐ankle PWV and aortic PWV.
Assessment of Endothelial Function
Peripheral arterial tonometry signals were obtained using the EndoPAT 2000 (Itamar Medical Inc, Caesarea, Israel), which has been validated and used previously to assess peripheral arterial tone in other populations.23, 24, 25, 26, 27 Endothelial function was measured via RH PAT index (reactive hyperemia index [RHI]) as previously described (normal value≥1.67).28 The ratio of the PAT signal after cuff release (after 5 minutes) compared with baseline (RHI) was calculated through a computer algorithm automatically normalizing for baseline signal and indexed to the contralateral arm.
Laboratory Assessment
For safety, serum creatinine and serum electrolytes were measured from plasma samples taken from patients at baseline, 3 months, and 6 months at the end of follow‐up.
Statistical Analysis
All data were expressed as an average±standard deviation. Differences between continuous variables were evaluated using Student's t test. A P≤.05 value was considered statistically significant.
Results
Patient Characteristics
Thirty patients with mild to moderate essential hypertension, not controlled by therapy, were enrolled. Baseline characteristics of the patients are listed in Table 1. The mean age was 60±12 years and there were 10 women and 20 men. Body mass index (BMI) was 29.2±4.5 kg⁄m2 and clinic SBP/DPB was 161±17.9/89.7±9.3. No patients had any impairment of renal function, including reduced eGFR and albuminuria or glucose and lipid metabolism. There were no detectable abnormalities on electrocardiography or chest x‐ray. Aliskiren‐based therapy was well tolerated in all of the patients without any significant adverse events. Only 1 patient discontinued therapy with aliskiren at the end of 6 months for significant hyperkalemia. A detailed follow‐up at 3 and 6 months was completed in all the patients.
Table 1.
Characteristics of the Population
| Patient Chararcteristics | Patients, No. (n=30) |
|---|---|
| Age, y | 60.4±12.2 |
| Female:male ratio | 1:2 |
| Systolic blood pressure, mm Hg | 161±17.9 |
| Diastolic blood pressure, mm Hg | 89.7±9.3 |
| Heart rate | 76±10 |
| Concomitant antihypertensive drugs, % | |
| Angiotensin‐converting enzyme inhibitor or Angiotensin receptor blocker | 95 |
| Calcium antagonist | 45 |
| Others | |
| Diuretics | 65.7 |
| Alpha lytics | 24.8 |
| β‐Blockers | 34 |
Effects of Aliskiren‐Based Therapy on BP Profile
From baseline to month 3, aliskiren had significantly improved SBP at 3 months (−8% from baseline; P=.02) and at 6 months (−14%; P=.0002) and DBP at 3 months(−10%; P=.006) and at 6 months (14%; P=.0006).
Effects of Aliskiren on Aortic Stiffness
All the patients had altered values of AIx at baseline while only 10 patients in the population had altered values of cfPWV.
However, aliskiren had no effect on values of central aortic AIx (−33.26±10.74% vs −28.86±10.74%; P=.36, Figure 1) but significantly improved values of cfPWV (9.36±2.65 m/s vs 8.72±2.48 m/s; P=.04 (Figure 2) in the entire population and in the subgroup with altered cfPWV at baseline (11.61±1.77 m/s vs 10.23±1.78 m/s; P=.02).
Figure 1.
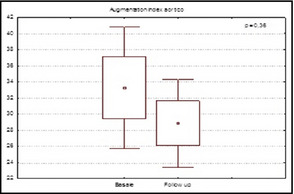
Values of central augmentation index in patients at baseline and at 6 months of follow‐up.
Figure 2.
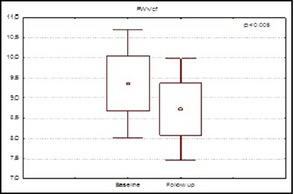
Values of carotid‐femoral pulse wave velocity in patients at baseline and at 6 months of follow‐up.
Effects of Aliskiren on Endothelial Function
In the entire group, 16 patients had values of RHI path index compatible with endothelial dysfunction and in 12 (75%) it was improved at the follow‐up.
In the entire population, RHI path index was significantly improved at 6‐month follow‐up (1.64±0.57 vs 1.7 5±0.45; P=.05) (Figure 3).
Figure 3.
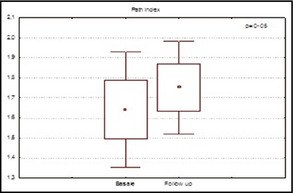
Values of reactive hyperemia peripheral arterial tonometry index in patients at baseline and at 6 months of follow‐up.
Effects of Aliskiren on Kidney's Function and on Potassium
The use of aliskiren significantly worsened renal function, expressed as GFR primarily in the first 3 months (86.4±7.5 vs 80±8.8; P=.04), and significantly increased levels of potassium both at 3 and 6 months (3.84±0.32 vs 4.45±0.32 vs 4.98±0.35; P<.0001).
Discussion
The main finding of this study is that aliskiren exerted a beneficial effect on values of cfPWV, a measure of arterial stiffness, and on RHI PAT index, a measure of endothelial function. The pro‐renin receptor is an emerging renin‐angiotensin‐aldosterone (RAA) system component and is expressed in the kidney and cardiovascular system. Pro‐renin receptor–bound renin and pro‐renin display enzymatic cleavage of angiotensinogen to Ang I, and the subsequently produced Ang II activates AT1 receptor signaling at local tissue sites, including the kidney.29, 30, 31, 32
Renin activates the pro‐renin receptor, thus exerting a direct (receptor‐mediated, Ang II‐independent) proinflammatory effect augmenting profibrotic pathways (transforming growth factor‐b1) through the formation of tumor growth factor (TGR) β, API‐1, collagen type 1, fibronectin, substances capable of promoting vasoconstriction, hypertrophy, fibrosis, and apoptosis.33 Huang and colleagues confirm this using human renin and rat pro‐renin34 and they found that these proteins increased the synthesis of TGF in mesangial cells that were previously found to contain renin/pro‐renin receptors.35 The increase in TGF mRNA that was induced by renin could not be inhibited by losartan or enalaprilat (the active form of enalapril). Therefore, the pro‐renin would be able to act as an effector via the interaction with its receptor, resulting in a series of RAA‐independent actions, potentially negatives.36
It was also demonstrated that renin enhanced synthesis of fibronectin, collagen I, and plasminogen activator inhibitor‐1, and, where tested, these effects were partially blocked by an inhibiting antibody against TGF.36
Aliskiren is the first direct renin inhibitor capable of interacting with the active site of renin preventing the binding with the specific substrate, angiotensinogen, and its consequent fragmentation enzyme with the formation of Ang I.
In a pilot study on the effect of aliskiren 300 mg daily in 10 patients with uncomplicated type 1 diabetes mellitus, aliskiren improved the parameters of systemic vascular function, including arterial stiffness and endothelial function.37 Most recently, a significantly greater reduction in central BP was observed with aliskiren ⁄hydrochlorothiazide combination therapy than amlodipine monotherapy.38 Results of the study by Kanaoka and colleagues demonstrated that aliskiren exerted a beneficial effect on central hemodynamics and arterial stiffness.39 Guo and associates demonstrated that aliskiren (150 mg/d) could ameliorate arterial stiffness and its effect was similar to ramipril (5 mg/d) in mild to moderate hypertensive patients, indicating that in addition to lowering BP, aliskiren had a beneficial effect on vascular protection.40
A previous study conducted leukocyte adhesion assays in vivo and in vitro using a novel real‐time imaging system and showed that treatment with aliskiren significantly suppressed the leukocyte‐endothelial interaction, a crucial step in vascular inflammation.41 In another previous study, treatment with aliskiren had protective effects on endothelial function via improvement in nitric oxide bioavailability and protected against atherosclerotic changes in watanabe heritable hyperlipidemic rabbits.42
Other animal studies have also revealed a prominent role for macrophage‐derived renin in the development of atherosclerotic vascular changes43 and showed that renin inhibition by aliskiren resulted in striking reductions of pathological vascular change and atherosclerotic lesion size in genetically atherosclerosis‐susceptible mice.44, 45
We also demonstrate a beneficial effect of aliskiren on endothelial function, measured by RHI index; these results are in line with a study by Imanishi and colleagues42 that suggested that treatment with a direct renin inhibitor has protective effects on endothelial function and atherosclerotic changes. In a pilot study of 10 patients with type 1 diabetes, aliskiren improved flow‐mediated vasodilatation of the brachial arteries.37 More recently, Virdis and associates showed that treatment with aliskiren improved endothelium‐dependent vasodilation and nitric oxide availability in the peripheral resistance arterioles of hypertensive patients.46 However, Flammer and coworkers demonstrated that renin inhibition with aliskiren did not improve endothelial function47 even if patients recruited by Virdis and colleagues were markedly hypertensive.46
Study Limitations
This study has important limitations. First, the limited number of recruited patients affects the robustness of the data. In particular, the AIx did not reach statistical significance that partly affects the strength of the conclusions. Second, we did not collect additional data regarding the anti‐oxidant activity and the regulation of nitric oxide availability by aliskiren. Despite those limits, we think our work adds important information to the current literature focusing on a subset of patients with uncontrolled hypertension. We hope that beneficial effects of aliskiren on aortic stiffness and endothelial function may be evaluated in trials designed to evaluate the prognostic impact on prevention of cardiovascular diseases.
Conclusions
Aliskiren yielded encouraging results in patients with hypertension not controlled with usual therapy. It is well tolerated and has significant beneficial hemodynamic effects in systemic circulation, leading to reductions in peripheral BP and augmented arterial compliance and endothelial function in patients with uncontrolled hypertension. This work highlights the need for future physiological and clinical studies that examine the effect of RAA blockade using aliskiren.
J Clin Hypertens (Greenwich). 2014;16:202–206. ©2014 Wiley Periodicals, Inc.
References
- 1. Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) . 2013 ESH/ESC Guidelines for the management of arterial hypertension. J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 4. Ghiadoni L, Taddei S, Virdis A, et al. Endothelial function and common carotid artery wall thickening in patients with essential hypertension. Hypertension. 1998;32:25–32. [DOI] [PubMed] [Google Scholar]
- 5. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. [DOI] [PubMed] [Google Scholar]
- 6. Vlachopoulos C, Aznaouridis K, O'Rourke MF, et al. Prediction of cardiovascular events and all‐cause mortality with central haemodynamics: a systematic review and meta‐analysis. Eur Heart J. 2010;31:1865–1871. [DOI] [PubMed] [Google Scholar]
- 7. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 8. Wang KL, Cheng HM, Chuang SY, et al. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang KL, Cheng HM, Sung SH, et al. Wave reflection and arterial stiffness in the prediction of 15‐year all‐cause and cardiovascular mortalities: a community‐based study. Hypertension. 2010;55:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gomez‐Marcos MA, Recio‐Rodriguez JI, Rodriguez‐Sanchez E, et al. Central blood pressure and pulse wave velocity: relationship to target organ damage and cardiovascular morbidity‐mortality in diabetic patients or metabolic syndrome. An observational prospective study. LOD‐DIABETES study protocol. BMC Public Health. 2010;10:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail. 2009;15:658–664. [DOI] [PubMed] [Google Scholar]
- 12. Kelly MP, Gibbs HH, O'Rourke M, et al. Nitroglycerine has more favourable effects on left ventricular after load than is apparent from measurement of pressure in a peripheral artery. Eur Heart J. 1990;11:138–144. [DOI] [PubMed] [Google Scholar]
- 13. Chen C‐H, Ting C‐T, Lin S‐J, et al. Different effects of fosinopril and atenolol on wave reflections in hypertensive patients. Hypertension. 1995;25:1034–1041. [DOI] [PubMed] [Google Scholar]
- 14. Pannier BM, Guerin AP, Marchais SJ, London G. Different aortic reflection wave responses following long‐term angiotensin‐converting enzyme inhibition and beta‐blocker in essential hypertension. Clin Exp Pharmacol Physiol. 2001;28:1074–1077. [DOI] [PubMed] [Google Scholar]
- 15. Kelly RP, Millasseau SC, Ritter JM, Chowienczyk PJ. Vasoactive drugs influence aortic augmentation index independently of pulse wave velocity in healthy men. Hypertension. 2001;37:1429–1433. [DOI] [PubMed] [Google Scholar]
- 16. Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004;17:1192–1200. [DOI] [PubMed] [Google Scholar]
- 17. Creager MA, Roddy MA. Effect of captopril and enalapril on endothelial function in hypertensive patients. Hypertension. 1994;24:499–505. [DOI] [PubMed] [Google Scholar]
- 18. Kiowski W, Linder L, Nuesch R, Martina B. Effects of cilazapril on vascular structure and function in essential hypertension. Hypertension. 1996;1:371–376. [DOI] [PubMed] [Google Scholar]
- 19. Taddei S, Virdis A, Ghiadoni L, et al. Effects of angiotensin converting enzyme inhibition on endothelium‐dependent vasodilatation in essential hypertensive patients. J Hypertens. 1998;16:447–456. [DOI] [PubMed] [Google Scholar]
- 20. Ghiadoni L, Virdis A, Magagna A, et al. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension. 2000;2:501–506. [DOI] [PubMed] [Google Scholar]
- 21. Klingbeil AU, John S, Schneider MP, et al. Effect of AT1 receptor blockade on endothelial function in essential hypertension. Am J Hypertens. 2003;16:123–128. [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Bosh JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 23. Goor DA, Sheffy J, Schnall RP, et al. Peripheral arterial tonometry: a diagnostic method for detection of myocardial ischemia induced during mental stress tests: a pilot study. Clin Cardiol. 2004;27:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. [DOI] [PubMed] [Google Scholar]
- 25. Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. [DOI] [PubMed] [Google Scholar]
- 26. Lavie P, Shlitner A, Sheffy J, Schnall RP. Peripheral arterial tonometry: a novel and sensitive non‐invasive monitor of brief arousals during sleep. Isr Med Assoc J. 2000;2:246–247. [PubMed] [Google Scholar]
- 27. Halligan SC, Murtagh B, Lennon RJ, et al. Effect of long‐term hormone replacement therapy on coronary endothelial function in postmenopausal women. Mayo Clin Proc. 2004;79:1514–1520. [DOI] [PubMed] [Google Scholar]
- 28. Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 29. Funke‐Kaiser H, Zollmann FS, Schefe JH, et al. Signal transduction of the (pro)renin receptor as a novel therapeutic target for preventing end‐organ damage. Hypertens Res. 2010;33:98–104. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen G. Renin and prorenin receptor in hypertension: what's new? Curr Hypertens Rep. 2011;13:79–85. [DOI] [PubMed] [Google Scholar]
- 31. Batenburg WW, Krop M, Garrelds IM, et al. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro) renin receptor. J Hypertens. 2007;25:2441–2453. [DOI] [PubMed] [Google Scholar]
- 32. Feldman DL, Jin L, Xuan H, et al. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen‐2)27 rats. Hypertension. 2008;52:130–136. [DOI] [PubMed] [Google Scholar]
- 33. Oliver JA. Receptor‐mediated actions of renin and prorenin. Kidney Int. 2006;69:13–15. [DOI] [PubMed] [Google Scholar]
- 34. Huang Y, Wongamornthan S, Kasting J, et al. Renin increases mesangial cell TGF‐1 and matrix proteins through receptor‐mediated, Ang II‐independent mechanisms. Kidney Int. 2006;69:105–113. [DOI] [PubMed] [Google Scholar]
- 35. Nguyen G, Delarue F, Berrou J, et al. Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor‐1 antigen. Kidney Int. 1996;50:1897–1903. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen G, Delarue F, Burckle C, et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cherney DZ, Lai V, Scholey JW, et al. Effect of direct renin inhibition on renal hemodynamic function, arterial stiffness, and endothelial function in humans with uncomplicated type 1 diabetes: a pilot study. Diabetes Care. 2010;33:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferdinand KC, Pool J, Weitzman R, et al. Peripheral and central blood pressure responses of combination aliskiren ⁄ hydrochlorothiazide and amlodipine monotherapy in African American patients with stage 2 hypertension: the ATLAAST trial. J Clin Hypertens (Greenwich). 2011;13:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanaoka T, Tamura K, Ohsawa M, et al. Effects of aliskiren‐based therapy on ambulatory blood pressure profile, central hemodynamics, and arterial stiffness in nondiabetic mild to moderate hypertensive patients. J Clin Hypertens (Greenwich). 2012;14:522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo JQ, Wang HY, SUN NL. Effect of aliskiren on arterial stiffness, compared with ramipril in patients with mild to moderate essential hypertension. Chin Med J. 2013;126:1242–1246. [PubMed] [Google Scholar]
- 41. Ino J, Kojima C, Osaka M, et al. Dynamic observation of mechanically‐injured mouse femoral artery reveals an anti‐inflammatory effect of renin inhibitor. Arterioscler Thromb Vasc Biol. 2009;29:1858–1863. [DOI] [PubMed] [Google Scholar]
- 42. Imanishi T, Tsujioka H, Ikejima H, et al. Renin inhibitor aliskiren improves impaired nitric oxide bioavailability and protects against atherosclerotic changes. Hypertension. 2008;52:563–572. [DOI] [PubMed] [Google Scholar]
- 43. Lu H, Rateri DL, Feldman DL, et al. Renin inhibition reduces hypercholesterolemia‐induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nussberger J, Aubert JF, Bouzourene K, et al. Renin inhibition by aliskiren prevents atherosclerosis progression: comparison with irbesartan, atenolol, and amlodipine. Hypertension. 2008;51:1306–1311. [DOI] [PubMed] [Google Scholar]
- 45. Kuhnast S, van der Hoorn JW, van den Hoek AM, et al. Aliskiren inhibits atherosclerosis development and improves plaque stability in APOE*3Leiden.CETP transgenic mice with or without treatment with atorvastatin. J Hypertens. 2012;30:107–116. [DOI] [PubMed] [Google Scholar]
- 46. Virdis A, Ghiadoni L, Qasem AA, et al. Effect of aliskiren treatment on endothelium‐dependent vasodilation and aortic stiffness in essential hypertensive patients. Eur Heart J. 2012;33:1530–1538. [DOI] [PubMed] [Google Scholar]
- 47. Flammer AJ, Gossla M, LiaJ J, et al. Renin inhibition with aliskiren lowers circulating endothelial progenitor cells in patients with early atherosclerosis. J Hypertens. 2013;31:630–635. [DOI] [PubMed] [Google Scholar]


