Abstract
The association between vascular stiffening and blood pressure is likely bidirectional. The present study was designed to examine temporal relationships among vascular stiffness, blood pressure progression, and hypertension. The Asymptomatic Polyvascular Abnormalities Community study is a community‐based, prospective, long‐term follow‐up observational study. The present investigation is based on the baseline examinations (2010–2011) and the first follow‐up measurements (2012–2013) included in the study. A total of 4025 participants were followed for an average of 27 months. Of 2153 participants free of hypertension at the baseline examination, 432 (20.07%) had incident hypertension. The authors observed that brachial‐ankle pulse wave velocity (baPWV) was an independent predictor of incident hypertension. baPWV during baseline examination was positively associated with higher systolic blood pressure, diastolic blood pressure, pulse pressure, and mean arterial pressure during the first follow‐up examination. baPWV but not blood pressure during baseline examination was associated with baPWV during the first follow‐up examination. This study not only provides evidence that baPWV is an independent predictor of blood pressure progression and incident hypertension, but also provides evidence that blood pressure is not associated with baPWV after adjusting for baseline baPWV.
Arterial stiffness and blood pressure (BP), which increase with age, are associated with increased morbidity and mortality of cardiovascular diseases.1, 2 It is traditionally believed that elevated BP may cause structural and functional alterations in the walls of the central elastic arteries and accelerated conduit artery stiffening.3, 4 Conversely, conduit artery stiffening may increase pressure pulsatility and thereby may increase systolic BP (SBP). Temporal relationships between vascular stiffness and BP remain incompletely elucidated. Moreover, arterial stiffness is increasingly recognized as an important prognostic index and potential therapeutic target in patients with hypertension.5, 6 The 2013 guidelines for the management of arterial hypertension acknowledge the potential role of arterial stiffness measurement in clinical management.7
Several previous observational studies have revealed that aortic stiffness predicts progression to hypertension in normotensive individuals.8, 9, 10, 11, 12, 13, 14, 15 However, assessment of aortic stiffness using echocardiography is not the most suitable method since specialized equipment and expertise is required. Carotid‐femoral pulse wave velocity (cf‐PWV) is considered a “gold‐standard” method for assessing central artery stiffness, but the technique is hindered by the intimate nature of femoral pulse acquisition and is not the most suitable method for mass examination of individuals to identify those with increased risk of hypertension. Brachial‐ankle pulse wave velocity (baPWV) is an alternatively developed technique with high validity and reproducibility that is suitable for screening arterial stiffness in a large population.16, 17 The value of baPWV, reflecting a combination of elastic and muscular arterial stiffness, has been shown to be associated with intima‐media thickness of the carotid artery18 and cf‐PWV.17
Several studies have examined whether measures of baPWV are related to BP in future or incident hypertension13, 15 but are limited by size or lack of assessment on whether baseline BP were related to baPWV in the future. To examine temporal relationships between vascular stiffening and BP, we evaluated baPWV, incident hypertension, and BP longitudinally in the Asymptomatic Polyvascular Abnormalities Community (APAC) study cohort.
Methods
Study Population
The APAC trial was a community‐based, prospective, long‐term follow‐up observational study to investigate the epidemiology of asymptomatic polyvascular abnormalities in Chinese adults. Details and design of the study have been previously described.19, 20 From June 2010 to June 2011, a sample of 7000 participants older than 40 years was randomly selected from the Kailuan cohort using a stratified random‐sampling method by age and sex based on data of the Chinese National Census from 2010. The sample size was calculated based on detection of a 7% event rate with 0.7% precision and an α value of 0.05. The response rate was assumed to be >80%. A total of 5852 participants agreed to take part in the APAC study and 5816 people eventually completed the baseline data collection. Among these 5816 individuals, 376 did not meet the following inclusion criteria: (1) no history of stroke, transient ischemic attack, and coronary disease at baseline as assessed by a validated questionnaire; and (2) absence of neurologic deficits for stroke, which was assessed by experienced doctors. Finally, a total of 5440 participants were eligible and included in the APAC study. Participants underwent follow‐up examinations, including standardized questionnaires, physical examination, and assessment of standard cardiovascular risk factors, at the Heart Study clinic at regular intervals of approximately 2 years. The present investigation is based on baseline examinations (2010–2011) and first follow‐up measurements (2012–2013). The APAC study was performed according to the guidelines of the Helsinki Declaration and was approved by both the ethics committees of the Kailuan General Hospital and Beijing Tiantan Hospital. Written informed consent was obtained from all participants and approved by the above ethics committees.
BP Measurement
BP was measured on the left arm to the nearest 2 mm Hg using a mercury sphygmomanometer with an appropriately sized cuff following standard recommended procedures. Three readings each of SBP and DBP were taken at a 5‐minute interval after participants had rested in a chair for at least 5 minutes. The average of three BP readings served as the examination BP, the primary exposure for subsequent analyses. Pulse pressure (PP) was calculated as SBP minus DBP. Mean arterial pressure (MAP) was calculated as DBP plus PP divided by three. Presence of hypertension was defined as an SBP ≥140 mm Hg, DBP ≥90 mm Hg, or the use of antihypertensive medication. Participants with incident hypertension were defined as those who were free of hypertension at baseline examination and presence of hypertension during the first follow‐up examination.
baPWV Measurement
baPWV was assessed in a controlled environment at a room temperature of 22°C to 25°C. The study participants refrained from smoking and drinking coffee, tea, or alcohol for at least three hours before measurement and did not exercise for the 30 minutes prior to measurement. baPWV was measured twice using a volume‐plethysmographic device (PWV/ankle/brachial SBP index; BP‐203RPE III, Omron Colin Co, Tokyo, Japan) by specially trained physicians and nurses. All patients were in a supine position in light clothing without a pillow and were asked to stop moving and talking at the time of the examination. Electrocardiographic electrodes were placed on both wrists and a microphone for the phonocardiogram was attached to the left side of the chest. Electrocardiography and phonocardiography were used to provide timing markers for the device. Occlusion cuffs, which were connected to both the plethysmographic and oscillometric sensors, were wrapped on both arms and ankles. The lower edge of the arm cuff was placed 2 cm to 3 cm above the transverse striation of cubital‐fossa, while the lower edge of the ankle was placed 1 cm to 2 cm above the superior aspect of the medial malleolus. The second readings of each body side baPWV were recorded. Recordings performed on the right side of the body served as the examination baPWV and secondary exposure for subsequent analyses. Heart rate was simultaneously obtained during baPWV measurement.
Other Covariates
Information on potential confounders, such as age, sex, smoking, alcohol intake, salt intake, and physical activity was collected using questionnaires. Physical exercise was evaluated from responses to questions regarding the frequency of physical activity (≥30 minutes per activity) during leisure time, with possible responses including never, sometimes, and frequent (≥3 times per week). Both smoking status and alcohol intake were classified using self‐reported information as “never,” “past,” and “current.” Salt intake was classified into three categories “low,” “medium,” or “high,” with a definition of <6 g/d, 6–10 g/d, or >10 g/d, respectively, which was roughly estimated according to standard salt spoon. Information on medical history, such as hypertension, diabetes, and active treatment (eg, hypoglycemic agents, antihypertensive agent, lipid‐lowering agents) was also collected via questionnaires at baseline examination and updated at the follow‐up examinations. Weight and height were measured during the interview and body mass index was calculated as weight (kg)/height (m2).
Blood samples were collected from the antecubital vein in the morning after an overnight fast (8 hours) and transfused into vacuum tubes containing ethylene diamine tetra acetic acid (EDTA). An autoanalyzer (Hitachi 747; Hitachi, Tokyo, Japan) was used to measure fasting blood glucose (hexokinase/glucose‐6‐phosphate dehydrogenates), uric acid (enzymatic), serum creatinine (enzymatic), total cholesterol (endpoint method), triglycerides (enzymatic), high‐density lipoprotein cholesterol (direct test method), low‐density lipoprotein cholesterol (direct test method), C‐reactive protein (commercial, high‐sensitivity nephelometry assay), and homocysteic acid (immunoturbidimetry) at the central laboratory of Kailuan Hospital. Diabetes mellitus was defined as a self‐reported history, currently treated with insulin or oral hypoglycemic agents, or fasting blood glucose level ≥7.0 mmol/L.
Statistical Analysis
Statistical analysis was performed using a commercially available software program (SAS software version 9.3; SAS Institute Inc, Cary, NC). Because we observed a significant interaction between BP and sex in relation to baPWV (P interaction<.05), men and women were analyzed separately. Continuous variables are expressed as mean±standard deviation and categorical variables as number (percentage). Student t test or analysis of variance was applied for comparison of normally distributed parameters and chi‐square test was applied for the comparison of categorical variables.
Correlates of BP and baPWV were explored using multivariable regression analysis. The primary outcomes (dependent variables) assessed were continuous SBP, DBP, MAP, and PP during the first follow‐up examination as well as incident hypertension in participants free of hypertension during baseline examination. The secondary outcome was baPWV during the first follow‐up examination. We used a stepwise procedure to identify BP (SBP, DBP, PP, and MAP), baPWV, and potential clinical covariates (age; sex; height; body mass index; postmenopausal; current smoker; current alcohol drinker; exercise; high salt intake; use of hypoglycemic agents, antihypertensive agents, or lipid‐lowering agents; heart rate; HDL‐C; LDL‐C; total cholesterol; triglycerides; serum creatinine; fasting glucose; C‐reactive protein; homocysteic acid; uric acid; and time to first follow‐up) collected during the baseline examination that were associated with the assessed continuous outcome in multivariable models. Significance level for entry into and staying in the model was 0.15.
To avoid colinearity effects among BP components, models for each BP prediction model considered only the corresponding baseline BP; baPWV was included in all models. Correlates of incident hypertension were assessed using multivariable logistic regression modeling. Quintiles of baseline BP and baPWV were used as predictors of incident hypertension. Subanalyses on newly diagnosed nondrug‐treated hypertensive patients were also performed. In all analyses, a two‐sided P value <.05 was considered significant.
Results
Characteristics of the Study Cohort
Of the 5440 participants eligible and included into the APAC study, 236 (4.34%) were excluded because they either had no baPWV assessment (n=223) or had a missing covariate (n=13). Of the 5204 participants who met criteria during the baseline examination, 1179 (22.66%) were excluded because they either died (n=65) by the first follow‐up examination or did not have baPWV (n=1072) or BP (n=42) taken during the first follow‐up examination. A total of 4025 patients (77.34%) represent the primary sample for this study, with an average follow‐up of 27 months.
In contrast, the subset of 1179 participants who did not meet criteria during the first follow‐up examination were older (58.84 vs 54.06 years), included fewer women (31.89% vs 42.39%), and had more prevalent hypertension (53.27% vs 46.51%) (Appendix Table AI). Clinical and biochemical characteristics of the study sample during the baseline examination appear in Table 1 (Figure).
Table 1.
Characteristics of the Study Sample During Baseline Examination
| Variable | Overall (N=4025) | Men (n=2319) | Women (n=1706) | P Value |
|---|---|---|---|---|
| Age, y | 54±11 | 56±12 | 52±10 | <.0001 |
| Heart rate, beats per min | 70±10 | 70±11 | 71±10 | <.0001 |
| HDL‐C, mmol/L | 1.64±0.42 | 1.60±0.42 | 1.70±0.43 | <.0001 |
| LDL‐C, mmol/L | 2.60±0.74 | 2.62±0.74 | 2.59±0.74 | .218 |
| Total cholesterol, mmol/L | 5.06±0.99 | 5.06±0.99 | 5.07±1.00 | .764 |
| Triglycerides, mmol/L | 1.67±1.41 | 1.74±1.51 | 1.56±1.25 | <.0001 |
| Serum creatinine, mmol/L | 73.95±22.20 | 81.38±22.46 | 63.85±17.33 | <.0001 |
| Fasting glucose, mmol/L | 5.53±1.43 | 5.64±1.57 | 5.38±1.21 | <.0001 |
| C‐reactive protein, mg/mL | 1.96±3.57 | 1.96±3.50 | 1.96±3.67 | .968 |
| Homocysteic acid, mmol/L | 15.34±9.15 | 18.19±9.82 | 11.47±6.36 | <.0001 |
| Uric acid, mmol/L | 288.27±89.57 | 315.11±92.45 | 251.56±70.56 | <.0001 |
| Height, cm | 166.2±7.3 | 170.4±5.5 | 160.4±5.1 | <.0001 |
| Body mass index kg/m2 | 24.93±3.24 | 25.13±3.16 | 24.65±3.32 | <.0001 |
| Current smoker | 1256 (31.20) | 1226 (52.87) | 30 (1.76) | <.0001 |
| Current alcohol drinkers | 1296 (32.20) | 1261 (54.38) | 35 (2.05) | <.0001 |
| Frequent exercise | 1391 (34.56) | 840 (36.22) | 551 (32.30) | .010 |
| High salt intake | 797 (19.80) | 537 (23.16) | 260 (15.24) | <.0001 |
| Diabetes | 456 (11.33) | 302 (13.02) | 154 (9.03) | <.0001 |
| Hypoglycemic agents | 227 (5.65) | 137 (5.91) | 90 (5.29) | .396 |
| Hypertension | 1872 (46.51) | 1273 (54.89) | 599 (35.11) | <.0001 |
| Antihypertensive agents | 770 (19.14) | 453 (19.54) | 317 (18.59) | .449 |
| Lipid‐lowering agents | 52 (1.29) | 23 (0.99) | 29 (1.70) | .049 |
| Postmenopausal | 838 (20.85) | 0 (0.00) | 838 (49.29) | <.0001 |
Abbreviations: HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol. Values are expressed as mean±standard deviation or number (percentage).
Figure 1.
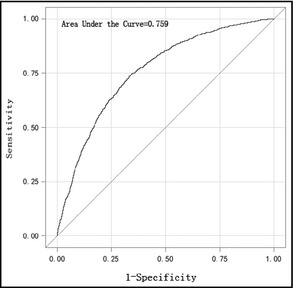
The receiver operator curve of right brachial‐ankle pulse wave velocity (age‐adjusted) for hypertension.
Correlates of Incident Hypertension and BP During First Follow‐Up Examination
Values of BP and baPWV during baseline examination and first follow‐up examination are shown in Table 2. In contrast, the subset of 807 (20.5%) participants who were on treatment for hypertension during the first follow‐up examination had significantly lower values of baPWV, SBP, DBP, PP, and MAP. In a multivariable‐adjusted regression model, higher baPWV during baseline examination was also associated with higher values of SBP, DBP, PP, and MAP during the first follow‐up examination (Table 3).
Table 2.
Blood Pressure and baPWV During Baseline and First Follow‐Up Examination
| Variable | Baseline Examination | First Follow‐Up Examination | P Value a | ||
|---|---|---|---|---|---|
| Total | Drug | Nondrug | |||
| Total, No. (%) | 4025 | 4025 | 807 (20.05) | 3218 (79.95) | |
| SBP, mm Hg | 130±20 | 130±17 | 126±16 b | 142±17 | .049 |
| DBP, mm Hg | 83±11 | 83±9 | 81±9 b | 87±10 | .914 |
| PP, mm Hg | 47±15 | 47±13 | 45±11 b | 55±15 | .01 |
| MAP, mm Hg | 98±13 | 98±11 | 96±10 b | 106±11 | .356 |
| baPWV, m/s | 15.2±3.5 | 16.0±5.9 | 15.5±5.5 b | 18.2±6.8 | <.0001 |
| Male, No. (%) | 2319 | 2319 | 498 (21.47) | 1821 (78.53) | |
| SBP, mm Hg | 134±18 | 132±17 | 129±15 b | 143±17 | <.0001 |
| DBP, mm Hg | 85±11 | 84±9 | 83±9 b | 89±10 | .001 |
| PP, mm Hg | 49±15 | 48±13 | 46±12 b | 54±15 | .005 |
| MAP, mm Hg | 101±12 | 100±11 | 98±10 b | 107±11 | <.0001 |
| baPWV, m/s | 15.8±3.5 | 16.7±5.4 | 16.2±5.1 b | 18.4±6.0 | <.0001 |
| Female, No. (%) | 1706 | 1706 | 309 (18.11) | 1397 (81.89) | |
| SBP, mm Hg | 125±20 | 126±17 | 122±16 b | 141±17 | .038 |
| DBP, mm Hg | 80±11 | 81±9 | 79±9 b | 85±10 | <.0001 |
| PP, mm Hg | 45±14 | 45±13 | 43±11 b | 56±15 | .599 |
| MAP, mm Hg | 95±13 | 96±11 | 94±10 b | 104±10 | .001 |
| baPWV, m/s | 14.4±3.3 | 15.2±6.4 | 14.6±5.8 b | 18.0±8.0 | <.0001 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure. Values are expressed as mean±standard deviation unless otherwise indicated. aPaired t test was used to test mean change during baseline and first follow‐up examination. bTwo‐independent t test was used to compare patients taking medication and those not (nondrug) (P<.05).
Table 3.
Blood Pressure During First Follow‐Up Correlates With baPWV During Baseline
| Population | Dependent Variables During First Follow‐up | Estimated Regression Coefficient | Standard Error | F Value | P Value |
|---|---|---|---|---|---|
| Total | SBP | 0.75 | 0.09 | 63.7 | <.0001 |
| Male | SBP | 0.52 | 0.12 | 19.15 | <.0001 |
| Female | SBP | 1.08 | 0.17 | 42.85 | <.0001 |
| Total | DBP | 0.33 | 0.05 | 36.94 | <.0001 |
| Male | DBP | 0.24 | 0.07 | 12.55 | <.0001 |
| Female | DBP | 0.47 | 0.07 | 41.88 | <.0001 |
| Total | PP | 0.53 | 0.07 | 60.85 | <.0001 |
| Male | PP | 0.41 | 0.09 | 22.23 | <.0001 |
| Female | PP | 0.75 | 0.13 | 35.7 | <.0001 |
| Total | MAP | 0.48 | 0.05 | 91.43 | <.0001 |
| Male | MAP | 0.34 | 0.06 | 27.64 | <.0001 |
| Female | MAP | 0.78 | 0.09 | 78.22 | <.0001 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure. The initial variables for stepwise regression include baPWV, corresponding baseline blood pressure (eg, baseline SBP and first follow‐up SBP), age, sex, height, body mass index, postmenopausal, current smoker, current alcohol drinkers, exercise, high salt intake, hypoglycemic agents, antihypertensive agents, lipid‐lowering agents, heart rate, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, total cholesterol, triglyceride, serum creatinine, fasting glucose, C‐reactive protein, homocysteic acid, uric acid, and time to first follow‐up.
For the analysis of incident hypertension, we excluded 1872 participants (46.51%) with prevalent hypertension during baseline examination, resulting in 2153 participants who experienced 432 cases (20.07%) of incident hypertension during the first follow‐up examination. Table 4 shows the incidence of hypertension according to groupings defined by quintiles of BP and baPWV at baseline examination. The incidence of nondrug‐treated hypertension is shown in Appendix Table AII. We observed that baPWV was associated with incident hypertension during the first follow‐up examination. After excluding 67 participants who were taking antihypertensive treatment, subanalysis on patients with newly diagnosed nondrug‐treated hypertension showed the same trends (Table 5). Furthermore, we observed that baPWV could predict the onset of isolated systolic hypertension, systolic and diastolic hypertension, and hypertension with normal BP, but could not predict isolated diastolic hypertension (Appendix Table AIII and Appendix Table AIV).
Table 4.
Hypertension Incidence at First Follow‐Up According to Groupings Defined by Quintiles of Blood Pressure and baPWV at Baseline Examination
| Population | Variable | Q1 | Q2 | Q3 | Q4 | Q5 | Trend for P |
|---|---|---|---|---|---|---|---|
| Total | baPWV | 35 (8.14) | 49 (11.34) | 83 (19.21) | 105 (24.48) | 160 (37.21) | <.0001 |
| SBP | 30 (6.68) | 40 (10.93) | 99 (21.90) | 136 (25.81) | 127 (35.38) | <.0001 | |
| DBP | 46 (11.03) | 60 (14.15) | 110 (21.65) | 84 (22.64) | 132 (30.48) | <.0001 | |
| PP | 45 (10.69) | 60 (15.46) | 86 (17.55) | 116 (24.63) | 125 (32.64) | <.0001 | |
| MAP | 31 (7.06) | 61 (14.81) | 84 (18.92) | 107 (24.71) | 149 (35.06) | <.0001 | |
| Male | baPWV | 24 (11.48) | 32 (15.31) | 57 (27.14) | 66 (31.58) | 84 (40.19) | <.0001 |
| SBP | 24 (10.30) | 20 (15.27) | 71 (26.39) | 98 (35.77) | 50 (35.97) | <.0001 | |
| DBP | 35 (16.36) | 38 (20.54) | 74 (27.01) | 39 (25.66) | 77 (34.84) | 0.002 | |
| PP | 31 (14.76) | 37 (22.16) | 58 (22.57) | 82 (32.41) | 55 (34.59) | <.0001 | |
| MAP | 25 (11.57) | 40 (19.80) | 50 (23.81) | 72 (33.96) | 76 (36.89) | <.0001 | |
| Female | baPWV | 11 (4.98) | 17 (7.62) | 26 (11.71) | 39 (17.73) | 76 (34.39) | <.0001 |
| SBP | 6 (2.78) | 20 (8.51) | 28 (15.30) | 38 (15.02) | 77 (35.00) | <.0001 | |
| DBP | 11 (5.42) | 22 (9.21) | 36 (15.38) | 45 (20.55) | 55 (25.94) | <.0001 | |
| PP | 14 (6.64) | 23 (10.41) | 28 (12.02) | 34 (15.60) | 70 (31.25) | <.0001 | |
| MAP | 6 (2.69) | 21 (10.00) | 34 (14.53) | 35 (15.84) | 73 (33.33) | <.0001 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; Q, quintile; SBP, systolic blood pressure. Values are expressed as number (percentage).
Table 5.
Odds Ratios and 95% Confidence Intervals of Quintiles of baPWV to Developing Hypertension During First Follow‐Up
| Model | Variable | Total | Male | Female |
|---|---|---|---|---|
| Model1 | baPWV_Q1 | 1.00 | 1.00 | 1.00 |
| baPWV_Q2 | 1.08 (0.68–1.72) | 1.22 (0.68–2.19) | 1.07 (0.51–2.25) | |
| baPWV_Q3 | 2.11 (1.37–3.24) | 2.3 (1.34–3.95) | 1.59 (0.79–3.19) | |
| baPWV_Q4 | 2.34 (1.52–3.6) | 2.81 (1.63–4.83) | 1.81 (0.92–3.58) | |
| baPWV_Q5 | 3.66 (2.31–5.8) | 4.26 (2.48–7.31) | 3.5 (1.8–6.84) | |
| Model2 | baPWV_Q1 | 1.00 | 1.00 | 1.00 |
| baPWV_Q2 | 1.24 (0.75–2.05) | 1.43 (0.77–2.67) | 0.94 (0.41–2.18) | |
| baPWV_Q3 | 2.13 (1.33–3.41) | 2.64 (1.48–4.71) | 1.21 (0.54–2.72) | |
| baPWV_Q4 | 2.58 (1.63–4.08) | 3.12 (1.75–5.57) | 1.6 (0.74–3.44) | |
| baPWV_Q5 | 4.41 (2.82–6.89) | 4.17 (2.36–7.38) | 3.32 (1.59–6.93) |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; Q, quartile. The initial variables for stepwise logistic regression include pulse wave velocity (Q1–Q5), systolic blood pressure (Q1–Q5), diastolic blood pressure (Q1–Q5), mean arterial pressure (Q1–Q5), pulse pressure (Q1–Q5), age, sex, height, body mass index, postmenopausal, current smoker, current alcohol drinkers, exercise, high salt iintake, hypoglycemic agents, antihypertensive agents, lipid‐lowering agents, heart rate, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, total cholesterol, triglyceride, serum creatinine, fasting glucose, C‐reactive protein, homocysteic acid, uric acid, and time to first follow‐up. Model 1: analysis on 432 newly diagnosed hypertensive patients. Model 2: analysis on 365 newly diagnosed nondrug‐treated hypertensive patients.
Correlates of baPWV During the First Follow‐Up Examination
In multivariable models, baPWV during baseline examination was associated with baPWV during the first follow‐up examination, but SBP, DBP, and MAP was not included in the final stepwise regression. The association between PP during the baseline examination and baPWV during the first follow‐up examination did not reach statistical significance in men (P=.068) (Table 6).
Table 6.
Right baPWV During First Follow‐Up Correlates With Blood Pressure During Baseline
| Population | Dependent Variables During First Follow‐Up | Predictor Variables During Baseline | Parameter Estimate | Standard Error | F Value | P Value |
|---|---|---|---|---|---|---|
| Total | baPWV1 | baPWV | 0.72 | 0.03 | 628.22 | <.0001 |
| Male | baPWV2 | baPWV | 0.72 | 0.03 | 451.36 | <.0001 |
| PP | 1.36 | 0.75 | 3.33 | .068 | ||
| Female | baPWV3 | baPWV | 0.67 | 0.05 | 169.02 | <.0001 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; PP, pulse pressure. The initial variables for stepwise regression: baPWV, systolic blood pressure, diastolic blood pressure, mean arterial pressure, PP, age, sex, height, body mass index, postmenopausal, current smoker, current alcohol drinkers, exercise, high salt intake, hypoglycemic agents, antihypertensive agents, lipid‐lowering agents, heart rate, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, total cholesterol, triglyceride, serum creatinine, fasting glucose, C‐reactive protein, homocysteic acid, uric acid, and time to first follow‐up.
Discussion
The main findings of this study are that baPWV, reflecting not only central arterial but also peripheral arterial stiffness, was predictive of incident hypertension, whereas higher initial BP was not predictive of an increase in arterial stiffness. We were also able to demonstrate that baPWV was positively related to future SBP, DBP, MAP, and PP by measuring BP and baPWV at two points in the APAC study.
Relationship of Vascular Stiffness With Incident Hypertension and Future BP
Using different techniques, it has been shown that higher vascular stiffness in normotensive individuals is associated with accelerated BP progression and increased risk for incident hypertension during follow‐up. Dernellis and colleagues12 reported that arterial stiffness markers, such as aortic strain, distensibility, and the stiff index (β), evaluated by M‐mode echocardiography could predict incident hypertension after adjustment for conventional risk factors. Najjar and colleagues11 reported that higher cf‐PWV was associated with an increase in SBP and incident hypertension. Kaess and colleagues14 also found that the initial value of cf‐PWV was strongly associated with subsequent BP progression.
The relationship between stiffness measures and future BP generally persisted in models that adjusted for initial values of BP and other known or suspected risk factors for hypertension. The significance of our study is that baPWV was found to be an independent predictor of hypertension. It should be noted that unlike the echocardiographic or cf‐PWV methods used in previous studies, baPWV could become the most widely used conventional index of arterial stiffness.
A recent observational study conducted in middle‐aged Japanese men reported that values of baPWV in the highest quartile were predictive of progression to higher BP categories in patients with high‐normal and normal BP.21 Similarly, Takase and colleagues13 observed that higher baPWV was associated with BP progression and incident hypertension. However, they failed to assess whether baseline BP was related to baPWV in the future. Our findings are in agreement with these previous studies. To our knowledge, our present investigation is the largest and most comprehensive longitudinal study that has assessed the relationships among baPWV and BP progression. We demonstrate prospectively that baPWV is predictive of incident hypertension and is positively related to future SBP, DBP, MAP, and PP, after adjustment for potential clinical covariates (age, sex, height, body mass index, postmenopausal, current smoker, current alcohol drinker, exercise, high salt intake, diabetes, use of hypoglycemic agents, hypertension, use of antihypertensive agents or lipid‐lowering agents, heart rate, HDL‐C, LDL‐C, total cholesterol, triglyceride, serum creatinine, fasting glucose, C‐reactive protein, homocysteic acid, and uric acid) during baseline. Hence, we were able to make the conclusion that baPWV predates rather than postdates hypertension.
Kaess and colleagues14 reported that lower cf‐PWV during examination cycle 7 was associated with higher PP during examination cycle 8. Conversely, the present results demonstrated that baPWV is positively related to future DBP. Takase13 also reported that longitudinal changes in DBP were positively correlated with baseline values of baPWV after adjustment for possible risk factors. A possible reason for the different conclusions is that the clinical significance of baPWV is different from that of aortic stiffness measured using cf‐PWV; baPWV is an indicator of the combination of central and peripheral arterial stiffness.
Several basic and clinical studies have demonstrated that disrupting elastin in the aortic wall is associated with subsequent development of hypertension. Salaymeh and colleagues22 reported that children with Williams syndrome (elastin haploinsufficiency) have increased arterial stiffness before the development of hypertension. Le and colleagues23 studied mouse models of impaired elastin expression and showed that aortic stiffness is increased before the increase in systolic pressure and that subsequent increments in systolic pressure are inversely proportional to elastin content in the aorta. Recently, alterations in diet alone were shown to increase aortic PWV early (1 month) after introduction of a high‐fat, high‐sucrose diet, before an increase in SBP, which occurred at 6 months.24 These results support the conclusion that arterial stiffness predates hypertension.
A common interpretation of known relationships between arterial stiffness and hypertension is that elevated BP, particularly PP, increases pulsatile aortic wall stress, which accelerates elastin degradation. Thus, hypertension is viewed as an accelerated form of vascular aging that leads to aortic stiffening. Several recent longitudinal studies evaluated the correlates of progressive aortic stiffening and found mixed results with respect to relationships between initial BP and progressive aortic stiffening.
In the Bogalusa Heart Study,25 the cumulative burden of SBP measured since childhood and over an average follow‐up of 26.5 years was an independent predictor of baPWV measured in young adulthood. However, this analysis was not adjusted for baseline baPWV because childhood measurements were not performed. McEniery and colleagues4 showed that midlife BP was associated with cf‐PWV 20 years later, but again these analyses were not adjusted for baseline vascular stiffness.
Conversely, BP during the baseline examination was not found to be associated with baPWV during the first follow‐up examination in our study. Benetos and colleagues26 found that SBP was not an independent predictor of cf‐PWV increase among normotensive or hypertensive patients. Wildman and colleagues27evaluated change in cf‐PWV during 2 years of follow‐up and found that SBP was not associated with the annual change in PWV, even by univariate analyses. The authors14 found that neither BP component (SBP, DBP, PP and MAP) entered the model for future stiffness, after accounting for the initial value of cf‐PWV.
The possible mechanism underlying the higher initial BP was not predictive of an increase in arterial stiffness because other factors rather than BP may contribute to the predictive value of baPWV. For example, many risk factors for hypertension generally impair the elastic properties of the arterial wall and the effect of such factors on arterial stiffness should be taken into consideration when interpreting the present data. The present results would imply that age, sex, fasting glucose, homocysteic acid, height, time to first follow‐up, postmenopause, current smoking, and lipid‐lowering treatment during the baseline examination were associated jointly with baPWV during the first follow‐up examination. Thus, increased values of baPWV may simply reflect clustering of factors that cause or accelerate the development of hypertension.
Study Limitations
There are several limitations to our study that should be acknowledged. First, baPWV is an indirect marker of increased arterial stiffness or decreased arterial compliance and we did not examine the structural changes of the arterial wall using ultrasound technology in our study. Second, the identification of hypertension was performed twice (at the beginning of the study and at the first follow‐up examination) and we were unable to examine detailed information on BP, such as variations in BP, during the follow‐up period. Third, there were some participants who were older, included fewer women, and had a high prevalence of hypertension who did not undergo measurement of baPWV during the first follow‐up examination; these features may have caused selection bias. Last, our participants were middle‐aged to older adults. Our findings may not be generalizable to younger individuals or ethnic/racial minorities.
Conclusions
A total of 4025 participants were followed an average of 27 months. Of the 2153 participants free of hypertension at baseline examination, 461 (21.41%) had incident hypertension. This study not only provides evidence that baPWV is an independent predictor of BP progression and incident hypertension, it also provides evidence that BP is not associated with baPWV after adjusting for baseline baPWV.
Disclosures
The authors declare no financial or other conflicts of interest.
Acknowledgments
The authors appreciate the enrolled participants and their relatives and the members of the survey teams in the 11 regional hospitals of Kailuan Medical Group, as well as the project development and management teams in the Kailuan Group and Beijing Tiantan Hospital. This study was performed as a collaborative study supported by the Ministry of Science and Technology, by the Ministry of Health of the People's Republic of China (2008BAI52B03), and by a grant from the National Natural Science Foundation of China (81202279).
Table Table AI.
Characteristics of the Follow‐Up Sample and those Lost to Follow‐Up
| Variable | Follow‐Up Sample (n=4025) | Lost to Follow‐Up Sample (n=1179) | T/x2 | P Value |
|---|---|---|---|---|
| Age, y | 54.06±11.27 | 58.84±12.75 | 12.43 | <.0001 |
| SBP, mm Hg | 130.04±19.52 | 135.43±21.05 | 8.19 | <.0001 |
| DBP, mm Hg | 82.66±10.88 | 83.58±11.49 | 2.52 | .012 |
| PP, mm Hg | 47.38±14.84 | 51.85±16.55 | 8.86 | <.0001 |
| MAP, mm Hg | 98.45±12.53 | 100.86±13.22 | 5.74 | <.0001 |
| baPWV, cm/s | 15.20±3.52 | 16.41±4.13 | 9.95 | <.0001 |
| Heart rate, beats per min | 70.25±10.60 | 71.28±12.35 | 2.79 | .005 |
| HDL‐C, mmol/L | 1.64±0.42 | 1.58±0.55 | −3.88 | <.0001 |
| LDL‐C, mmol/L | 2.60±0.74 | 2.72±0.86 | 4.56 | <.0001 |
| Total cholesterol, mmol/L | 5.06±0.99 | 5.08±1.06 | 0.48 | .634 |
| Triglycerides, mmol/L | 1.67±1.41 | 1.71±1.46 | 0.93 | .352 |
| Serum creatinine, mmol/L | 73.95±22.20 | 77.56±36.62 | 4.16 | <.0001 |
| Fasting glucose, mmol/L | 5.53±1.43 | 5.78±1.72 | 5.04 | <.0001 |
| C‐reactive protein, mg/mL | 1.96±3.57 | 2.69±5.77 | 5.18 | <.0001 |
| Homocysteic acid mmol/L | 15.34±9.15 | 17.84±10.79 | 7.86 | <.0001 |
| Uric acid, mmol/L | 288.27±89.57 | 288.67±90.02 | 0.14 | .892 |
| Height, cm | 166.18±7.28 | 166.55±7.24 | 1.55 | .121 |
| Body mass index | 24.93±3.24 | 24.99±3.35 | 0.59 | .558 |
| Male | 2319 (57.61) | 803 (68.11) | 41.84 | <.0001 |
| Female | 1706 (42.39) | 376 (31.89) | ||
| Postmenopausal | 838 (20.85) | 265 (22.61) | 1.68 | .195 |
| Current smoker | 1256 (31.20) | 414 (35.11) | 6.40 | .011 |
| Current alcohol drinkers | 1296 (32.20) | 418 (35.45) | 4.37 | .036 |
| Frequent exercise | 1391 (34.56) | 396 (33.59) | 0.38 | .537 |
| High salt intake | 797 (19.80) | 218 (18.49) | 1.00 | .318 |
| Diabetes | 456 (11.33) | 172 (14.59) | 9.13 | .003 |
| Hypoglycemic agents | 227 (5.65) | 87 (7.38) | 4.81 | .028 |
| Hypertension | 1872 (46.51) | 628 (53.27) | 16.68 | <.0001 |
| Antihypertensive agents | 770 (19.14) | 239 (20.27) | 0.75 | .388 |
| Lipid‐lowering agents | 52 (1.29) | 16 (1.36) | 0.03 | .862 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein‐cholesterol; LDL‐C, low‐density lipoprotein‐cholesterol; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure. Values are expressed as mean±standard deviation or number (percentage). T/x2, T Value or x2 Value
Table Table AII.
Incidence of Nondrug‐Treated Hypertension at First Follow‐Up According to Groupings Defined by Quintiles of Blood Pressure and baPWV at Baseline Examination a
| Population | Variable | Q1 | Q2 | Q3 | Q4 | Q5 | Trend for P |
|---|---|---|---|---|---|---|---|
| Total | baPWV | 32 (7.49) | 45 (10.51) | 70 (16.71) | 90 (21.74) | 128 (32.16) | <.0001 |
| SBP | 29 (6.47) | 36 (9.94) | 82 (18.85) | 121 (23.63) | 97 (29.48) | <.0001 | |
| DBP | 37 (9.07) | 54 (12.92) | 100 (20.08) | 67 (18.93) | 107 (26.23) | <.0001 | |
| PP | 42 (10.05) | 49 (13.00) | 77 (16.01) | 102 (22.32) | 95 (26.91) | <.0001 | |
| MAP | 27 (6.21) | 53 (13.12) | 72 (16.67) | 92 (22.01) | 121 (30.48) | <.0001 | |
| Male | baPWV | 21 (10.19) | 31 (14.90) | 51 (25.00) | 59 (29.21) | 70 (35.90) | <.0001 |
| SBP | 23 (9.91) | 19 (14.62) | 60 (23.26) | 90 (33.83) | 40 (31.01) | <.0001 | |
| DBP | 29 (13.94) | 34 (18.78) | 69 (25.65) | 33 (22.60) | 67 (31.75) | .002 | |
| PP | 30 (14.35) | 31 (19.25) | 52 (20.72) | 75 (30.49) | 44 (29.73) | <.0001 | |
| MAP | 22 (10.33) | 34 (17.35) | 45 (21.95) | 65 (31.71) | 66 (33.67) | <.0001 | |
| Female | baPWV | 11 (4.98) | 14 (6.36) | 19 (8.84) | 31 (14.62) | 58 (28.57) | <.0001 |
| SBP | 6 (2.78) | 17 (7.33) | 22 (12.43) | 31 (12.60) | 57 (28.50) | <.0001 | |
| DBP | 8 (4.00) | 20 (8.44) | 31 (13.54) | 34 (16.35) | 40 (20.30) | <.0001 | |
| PP | 12 (5.74) | 18 (8.33) | 25 (10.87) | 27 (12.80) | 51 (24.88) | <.0001 | |
| MAP | 5 (2.25) | 19 (9.13) | 27 (11.89) | 27 (12.68) | 55 (27.36) | <.0001 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure. Values are expressed as number (percentage). aA total of 67 participants taking antihypertensive agents were excluded.
Table Table AIII.
Specific Hypertension Incidence at First Follow‐Up According to Groupings Defined by Quintiles of baPWV at Baseline Examination
| Population | Variable | Q1 | Q2 | Q3 | Q4 | Q5 | Trend for P |
|---|---|---|---|---|---|---|---|
| Total | ISH | 3 (0.70) | 6 (1.39) | 5 (1.16) | 31 (7.23) | 56 (13.02) | .000 |
| IDH | 22 (5.12) | 25 (5.79) | 27 (6.25) | 18 (4.20) | 22 (5.12) | .721 | |
| SDH | 7 (1.63) | 8 (1.85) | 37 (8.56) | 37 (8.62) | 56 (13.02) | .000 | |
| HNP | 3 (0.70) | 10 (2.31) | 14 (3.24) | 19 (4.43) | 26 (6.05) | .000 | |
| Male | ISH | 0 (0.00) | 3 (1.44) | 3 (1.43) | 14 (6.70) | 35 (16.75) | .000 |
| IDH | 15 (7.18) | 20 (9.57) | 17 (8.10) | 11 (5.26) | 10 (4.78) | .280 | |
| SDH | 6 (2.87) | 5 (2.39) | 28 (13.33) | 29 (13.88) | 25 (11.96) | .000 | |
| HNP | 3 (1.44) | 4 (1.91) | 9 (4.29) | 12 (5.74) | 14 (6.70) | .021 | |
| Female | ISH | 3 (1.36) | 3 (1.35) | 2 (0.90) | 17 (7.73) | 21 (9.50) | .000 |
| IDH | 7 (3.17) | 5 (2.24) | 10 (4.50) | 7 (3.18) | 12 (5.43) | .416 | |
| SDH | 1 (0.45) | 3 (1.35) | 9 (4.05) | 8 (3.64) | 31 (14.03) | .000 | |
| HNP | 0 (0.00) | 6 (2.69) | 5 (2.25) | 7 (3.18) | 12 (5.43) | .013 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; HNP, hypertension with normal blood pressure; IDH, isolated diastolic hypertension; ISH, isolated systolic hypertension; Q, quintile; SDH, systolic and diastolic hypertension. Values are expressed as number (percentage).
Table Table AIV.
Odds Ratios and 95% Confidence Intervals of Quintiles of baPWV to Developing Specific Phenotypes Hypertension During the First Follow‐Up
| Variable | ISH | SDH | HNP |
|---|---|---|---|
| baPWV_Q1 | 1.00 | 1.00 | 1.00 |
| baPWV_Q2 | 1.39 (0.33–5.93) | 1.04 (0.35–3.06) | 3.11 (0.84–11.6) |
| baPWV_Q3 | 0.93 (0.22–4.00) | 5.56 (2.26–13.65) | 5.49 (1.56–19.3) |
| baPWV_Q4 | 4.61 (1.35–15.75) | 4.63 (1.87–11.46) | 7.03 (2.04–24.26) |
| baPWV_Q5 | 3.89 (1.11–13.62) | 8.16 (3.33–19.97) | 10.37 (3.05–35.27) |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; HNP, hypertension with normal blood pressure; ISH, isolated systolic hypertension; Q, quartile; SDH, systolic and diastolic hypertension. The initial variables for stepwise logistic regression were pulse wave velocity (Q1–Q5), systolic blood pressure (Q1–Q5), diastolic blood pressure (Q1–Q5), mean arterial pressure (Q1–Q5), pulse pressure (Q1–Q5), age, sex, height, body mass index, postmenopausal, current smoker, current alcohol drinkers, exercise, high salt intake, hypoglycemic agents, antihypertensive agents, lipid‐lowering agents, heart rate, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, total cholesterol, triglycerides, serum creatinine, fasting glucose, C‐reactive protein, homocysteic acid, uric acid, and time to first follow‐up.
J Clin Hypertens (Greenwich). 2015;17:582–591. DOI: 10.1111/jch.12556 © 2015. Wiley Periodicals, Inc.
References
- 1. Cavalcante JL, Lima JA, Redheuil A, Al‐Mallah MH. Aortic stiffnesscurrent understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. [DOI] [PubMed] [Google Scholar]
- 2. Vasan RS, Larson MG, Leip EP, et al. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 3. O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–444. [DOI] [PubMed] [Google Scholar]
- 4. McEniery CM, Spratt M, Munnery M, et al. An analysis of prospective risk factors for aortic stiffness in men 20‐year follow‐up from the caerphilly prospective study. Hypertension. 2010;56:36–43. [DOI] [PubMed] [Google Scholar]
- 5. McEniery CM, McDonnell BJ, So A, et al. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53:524–531. [DOI] [PubMed] [Google Scholar]
- 6. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the american heart association professional education committee of the council for high blood pressure research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219 [DOI] [PubMed] [Google Scholar]
- 8. Arnett DK, Glasser SP, McVeigh G, et al. Blood pressure and arterial compliance in young adults: the Minnesota children's blood pressure study. Am J Hypertens. 2001;14:200–205. [DOI] [PubMed] [Google Scholar]
- 9. Femia R, Kozakova M, Nannipieri M, et al. Carotid intima‐media thickness in confirmed prehypertensive subjects predictors and progression. Arterioscler Thromb Vasc Biol. 2007;27:2244–2249. [DOI] [PubMed] [Google Scholar]
- 10. Liao D, Arnett DK, Tyroler HA, et al. Arterial stiffness and the development of hypertension: the ARIC study. Hypertension. 1999;34:201–206. [DOI] [PubMed] [Google Scholar]
- 11. Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore longitudinal study of aging. J Am Coll Cardiol. 2008;51:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stefanadis C, Dernellis J, Tsiamis E, et al. Assessment of aortic line of elasticity using polynomial regression analysis. Circulation. 2000;101:1819–1825. [DOI] [PubMed] [Google Scholar]
- 13. Takase H, Dohi Y, Toriyama T, et al. Brachial‐ankle pulse wave velocity predicts increase in blood pressure and onset of hypertension. Am J Hypertens. 2011;24:667–673. [DOI] [PubMed] [Google Scholar]
- 14. Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satoh H, Saijo Y, Kishi R, Tsutsui H. Brachial‐ankle pulse wave velocity is an independent predictor of incident hypertension in Japanese normotensive male subjects. Environ Health Prev Med. 2011;16:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. [DOI] [PubMed] [Google Scholar]
- 17. Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid‐femoral and brachial‐ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–2027. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi K, Akishita M, Yu W, et al. Interrelationship between non‐invasive measurements of atherosclerosis: flow‐mediated dilation of brachial artery, carotid intima‐media thickness and pulse wave velocity. Atherosclerosis. 2004;173:13–18. [DOI] [PubMed] [Google Scholar]
- 19. Zhou Y, Li Y, Xu L, et al. Asymptomatic polyvascular abnormalities in community (apac) study in China: objectives, design and baseline characteristics. PLoS One. 2013;8:e84685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Q, Zhang S, Wang C, et al. Ideal cardiovascular health metrics on the prevalence of asymptomatic intracranial artery stenosis: a cross‐sectional study. PLoS One. 2013;8:e58923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yambe M, Tomiyama H, Yamada J, et al. Arterial stiffness and progression to hypertension in japanese male subjects with high normal blood pressure. J Hypertens. 2007;25:87–93. [DOI] [PubMed] [Google Scholar]
- 22. Salaymeh KJ, Banerjee A. Evaluation of arterial stiffness in children with Williams syndrome: does it play a role in evolving hypertension? Am Heart J. 2001;142:549–555. [DOI] [PubMed] [Google Scholar]
- 23. Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin‐insufficient mice. Am J Physiol Heart Circ Physiol. 2011;301:H221–H229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weisbrod RM, Shiang T, Al Sayah L, et al. Arterial stiffening precedes systolic hypertension in diet‐induced obesity. Hypertension. 2013;62:1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li S, Chen W, Srinivasan SR, Berenson GS. Childhood blood pressure as a predictor of arterial stiffness in young adults: the Bogalusa heart study. Hypertension. 2004;43:541–546. [DOI] [PubMed] [Google Scholar]
- 26. Benetos A, Adamopoulos C, Bureau J‐M, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6‐year period. Circulation. 2002;105:1202–1207. [DOI] [PubMed] [Google Scholar]
- 27. Wildman RP, Farhat GN, Patel AS, et al. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension. 2005;45:187–192. [DOI] [PubMed] [Google Scholar]


