Abstract
To evaluate the effects of achieved systolic blood pressure (SBP) during treatment on cardiovascular (CV) outcomes, the authors measured event rates of a composite primary endpoint (CV death or nonfatal myocardial infarction or stroke) at on‐treatment SBPs of ≥140 mm Hg and the 10 mm Hg intervals of <140 mm Hg, <130 mm Hg, and <120 mm Hg in 6459 patients with diabetes (mean age, 67) and 4246 patients without diabetes (mean age, 69) from the Avoiding Cardiovascular Events in Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) trial. In the diabetic cohort, the primary endpoint was 49% lower (P<.001) at <140 mm Hg than at ≥140 mm Hg, and the separate components of this endpoint were also significantly reduced. Further SBP reductions did not improve outcomes, and at <120 mm Hg they were no longer different (except for stroke) from ≥140 mm Hg. In contrast, in the nondiabetic cohort, the primary endpoint event rate fell steadily (although not significantly) through the decreasing SBP categories until it was reduced by 45% (P=.0413) at <120 mm Hg. Total stroke rates for both the diabetic (−56%, P=.0120) and nondiabetic (−68%, P=.0067) cohorts were lowest at <120 mm Hg, and adverse renal events (serum creatinine increase ≥50%) were significantly lowest in the range of 130 mm Hg to 139 mm Hg for both cohorts. Diabetic patients (<140 mm Hg or <130 mm Hg) and nondiabetic patients (<120 mm Hg) may require different SBP targets for optimal CV protection, although stroke and renal considerations should also influence the selection of blood pressure targets.
There has been uncertainty regarding optimal blood pressure (BP) targets in patients with hypertension. For several years, guideline publications have recommended a systolic BP (SBP) of below 140 mm Hg for most patients,1, 2, 3 although one recent report asserted—for patients aged 60 years or older—that the available clinical trial evidence best supported a goal of below 150 mm Hg.4
Most information on BP targets has come from retrospective analyses of major clinical trials that were not originally designed to prospectively compare the effects of differing achieved SPB on cardiovascular (CV) outcomes. Still, these trials have been reasonably consistent in demonstrating that SBP values <140 mm Hg are associated with lower event rates than values ≥140 mm Hg.5, 6, 7, 8 Although interpretation of other hypertension trials9, 10 was thought to justify the less rigorous goal of <150 mm Hg in older people,4 this recommendation has been disputed.11, 12
Recently, two prospective trials have explored a more intensive target in predominantly older high‐risk hypertensive patients, comparing CV event rates in patients randomized to SBP targets of <140 mm Hg or <120 mm Hg. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial13 found that there was no greater overall CV benefit at <120 mm Hg than at <140 mm Hg, although stroke rates were significantly lower at the more intensive target.12 However, the Systolic Prevention Intervention Trial (SPRINT),14 conducted in high‐risk patients without diabetes, did show lower overall fatal and nonfatal CV event rates in patients treated to <120 mm Hg.
The Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) trial15 was a major outcomes study designed to compare the effects on CV outcomes of differing classes of drugs in both diabetic and nondiabetic hypertensive patients at high CV risk.14 Using the ACCOMPLISH database, we have previously analyzed the effects of achieving differing levels of SBPs in the full cohort of patients.8 We found that, compared with ≥140 mm Hg, achieving <140 mm Hg produced clear CV outcomes benefits, but that there was no further benefit at lower SBP levels, including <120 mm Hg.
In the present study, we have further analyzed CV outcomes according to achieved SBP categories, but studied patients with and without diabetes separately to enable a comparison of optimal treatment targets in these two patient groups.
Methods
This report is based on data obtained from the ACCOMPLISH trial.15 ACCOMPLISH was designed to compare the effects on CV outcomes of the combination of the angiotensin‐converting enzyme (ACE) inhibitor, benazepril, plus amlodipine with the combination of the same ACE inhibitor plus hydrochlorothiazide. The methods for the trial have been published previously.15, 16, 17 As part of the ACCOMPLISH research plan, the relationships of achieved BP levels to CV and other clinical endpoints was a prespecified analysis.
Management of the Trial
The ACCOMPLISH study was conducted under the supervision of an executive committee, all of whose members are among the authors of the present report (MW, GB, BP, EV, KJ). The roles of the committees involved in the trial and the role of the trial's sponsor (Novartis) have also been published previously.15, 16, 17 Institutional review boards at each participating site approved the study protocol. The trial was registered at ClinicalTrials.gov (number NCT00170950).
Patient Selection
This work was performed in hypertensive patients at high risk for CV events established by histories of documented CV events. These details have been described previously.17
Study Protocol
ACCOMPLISH was a prospective, randomized, controlled, double‐blind trial. Immediately upon entering the study, patients were randomized to one of two treatment arms: benazepril plus hydrochlorothiazide or benazepril plus amlodipine, although the data from those two arms have been pooled for the present analysis and report. Upon randomization, all previous antihypertensive therapies were discontinued and replaced immediately by one of the fixed combination therapies. Starting doses were benazepril 20 mg/d plus hydrochlorothiazide 12.5 mg/d or amlodipine 5 mg/d. There was a mandatory increase in benazepril to 40 mg/d in both study arms at the following study visit. Thereafter, hydrochlorothiazide could be increased to 25 mg/d or amlodipine to 10 mg/d as needed to achieve the target BP of <140/90 mm Hg. In patients with diabetes or chronic kidney disease, a target BP <130/80 mm Hg was recommended but not required. As necessary, investigators could add other agents (except ACE inhibitors, angiotensin receptor blockers, calcium channel blockers, or thiazide diuretics). After an initial 3‐month period during which these treatment intensifications could be made, patients returned for a visit after a further 3 months and then at 6‐month intervals until the trial ended. BPs were measured by trained study personnel as described previously.15
Major Endpoints
For the analyses in this report, we defined the primary endpoint as the composite of the first occurrence of CV death or nonfatal myocardial infarction or nonfatal stroke. Death from CV causes was defined as sudden death from cardiac events or death from myocardial infarction, stroke, coronary interventions, or heart failure. Only the first event in each patient was counted toward the primary endpoint. In addition, there were analyses of secondary endpoints that were counted without censoring for previous occurrences of other endpoints. The secondary endpoints in this report were CV death, total stroke (fatal or nonfatal), total myocardial infarction (fatal or nonfatal), and the presence of increased serum creatinine (increase from baseline of ≥50%).
Reporting of Data
Outcomes in ACCOMPLISH were adjudicated according to standard criteria by a clinical endpoints committee.15 Interim analyses were performed at 6‐month intervals from the study's Data Safety Monitoring Committee. This committee recommended early termination of the trial based on evidence that the criteria for satisfying the trial's stopping rules had been met.15
For this report, the entire study cohort was divided into two major groups: those with diabetes and those without diabetes. The principal observations for each of these cohorts were the measurement of event rates for the primary and secondary endpoints within each of the specified SBP categories: ≥140 mm Hg, 130 mm Hg to <140 mm Hg, 120 mm Hg to <130 mm Hg, and 110 mm Hg to <120 mm Hg. (We did not analyze data for systolic values <110 mm Hg because of small patient numbers). The achieved SBP category was derived using mean SBP starting from month 6 to the end of the trial (inclusive). Patients with no SBP measurements during this period were not included in the analysis. SBP measurements were based on the mean of three readings (per visit). For each endpoint, Cox regression survival analysis (univariate), hazard ratio (HR), 95% confidence interval (CI), P values (Cox and log‐rank), plus comparator group denominators (number) and number (percentage) for the number of events in the comparator group were calculated for each endpoint. HRs were based on a univariate Cox regression model including SBP category but no other factors or covariates. CIs are two‐sided 95% CIs for HRs. An HR <1 indicates a reduced risk of experiencing the indicated event for patients in the lower SBP group.
Funding Source
The original ACCOMPLISH trial was funded by Novartis. Their role in the conduct of the trial has been described.15 No additional support was provided for the present analysis.
Results
Duration of Treatment
The ACCOMPLISH trial was terminated early by the executive committee following a report from the Date Safety Monitoring Committee that the prespecified stopping rules for the study had been met on the basis of efficacy.15 The mean duration of treatment for patients in the trial was 35.7 months.
Patient Disposition
Figure 1 shows the disposition of the 11,506 patients enrolled in the trial. Post‐titration BPs were not available in 564 patients, and a further 237 with on‐treatment SBPs <110 mm Hg were excluded because there were too few patients to analyze. Of the 10,705 patients remaining, 6459 had diabetes and 4246 did not have diabetes. The disposition of these patients in the four categories of achieved SBPs are detailed separately in Figure 1 for patients with and without diabetes. The distribution (percentage) of patients in each of the four achieved BP categories was similar for the diabetic and nondiabetic cohorts.
Figure 1.
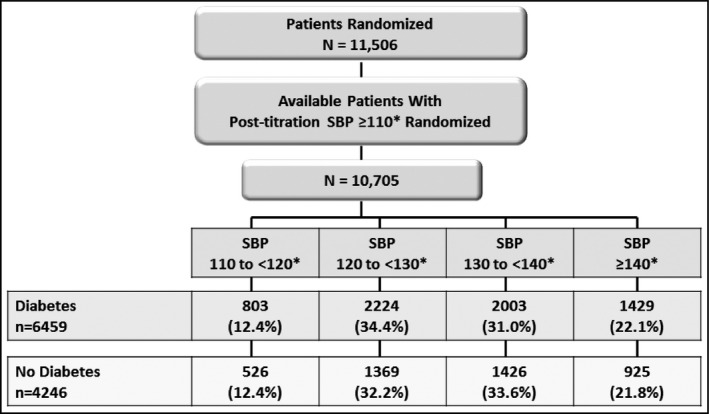
Patient disposition. The patients with on‐treatment systolic blood pressures (SBPs) >110 mm Hg were divided into diabetic and nondiabetic cohorts and then further divided into four subgroup categories based on achieved SBPs. * indicates mmHg.
Patient Characteristics
The characteristics of the study patients at baseline are shown in Table 1. Because of the large cohort sizes, even small differences between the diabetic and nondiabetic patients were statistically significant. However, the prevalence of myocardial infarction, coronary disease, and stroke were all clearly higher in the nondiabetic group, whereas fasting blood glucose and body mass index were higher in the diabetes group. The baseline BPs in the two study cohorts were closely similar.
Table 1.
Baseline Characteristics of Patients With and Without Diabetes
| Category | Patients With Diabetes | Patients Without Diabetes |
|---|---|---|
| Number | 6459 | 4246 |
| Age, mean (SD), y | 67.4 (6.6)a | 69.7 (6.9) |
| Men, No. (%) | 3712 (57.5)a | 2817 (66.3) |
| Previous coronary disease, No. (%) | 1849 (28.6)a | 3068 (72.3) |
| Previous myocardial infarction, No. (%) | 943 (14.6)a | 1560 (36.7) |
| History of stroke, No. (%) | 514 (8.0)a | 878 (20.7) |
| Chronic kidney disease (eGFR <60 mL/min), No. (%) | 1105 (17.1)a | 801 (18.9) |
| eGFR, mean (SD), mL/min | 80.7 (22.1)a | 76.5 (19.5) |
| Fasting blood glucose, mean (SD), mg/dL | 144.7 (57.2)a | 101.1 (17.1) |
| Body mass index, mean (SD), kg/m2 | 32.2 (6.4)a | 29.0 (5.3) |
| Systolic BP, mean (SD), mm Hg | 145.7 (17.8) | 146.1 (18.4) |
| Diastolic BP, mean (SD), mm Hg | 79.5 (10.6)a | 81.3 (10.8) |
Abbreviations: BP, blood pressure; eGFR, estimated glomerular filtration rate; SD, standard deviation. aSignificant difference between the two groups.
Achieved BPs
The mean systolic and diastolic BPs in the uncontrolled BP group (≥140mm Hg) and for each of the 10 mm Hg SBP strata below 140 mm Hg are shown in Table 2. The BP values in the diabetes and nondiabetes cohorts were similar within each of the BP categories.
Table 2.
Systolic and Diastolic BPs in Each of the Four Categories of Increased Systolic BP in Patients With or Without Diabetes
| Systolic BP Groups, mm Hg | ||||
|---|---|---|---|---|
| 110 to <120 | 120 to <130 | 130 to <140 | >140 | |
| Patients with diabetes | ||||
| Systolic BP | 116.3 (2.7) | 125.5 (2.8) | 134.4 (2.8) | 150.4 (9.7) |
| Diastolic BP | 67.8 (5.8) | 71.5 (6.3) | 74.1 (6.8) | 78.1 (8.7) |
| Patients without diabetes | ||||
| Systolic BP | 116.3 (2.8) | 125.5 (2.8) | 134.6 (2.8) | 149.8 (9.3) |
| Diastolic BP | 69.5 (6.1) | 73.1 (6.3) | 76.1 (6.9) | 79.8 (8.7) |
Abbreviation: BP, blood pressure. Values are expressed as means (standard deviations).
Achieved BPs and Clinical Outcomes
Comparisons of event rates for CV, stroke, and renal outcomes between patients with SBP ≥140 mm Hg and each of the three achieved 10 mm Hg SBP strata below 140 mm Hg (<140 mm Hg, <130 mm Hg, and <120 mm Hg) are shown in Figure 2 for diabetic patients and Figure 3 for nondiabetic patients.
Figure 2.
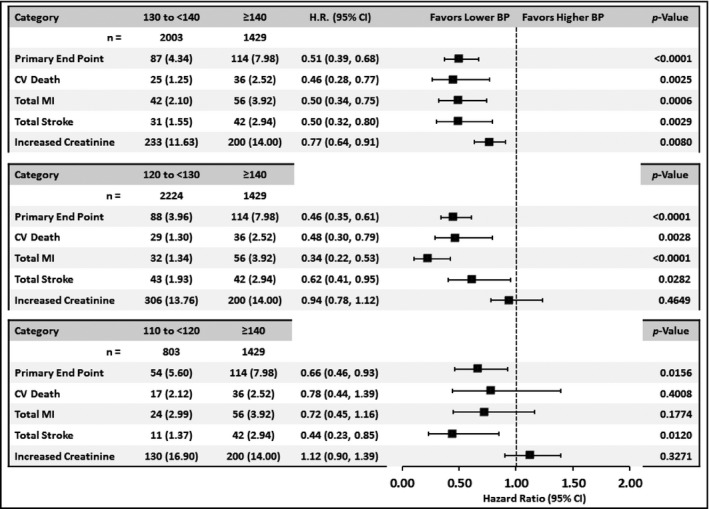
Comparison in diabetic patients of event rates from major cardiovascular, stroke, and renal endpoints between patients in the achieved systolic blood pressure (SBP) group >140 mm Hg and each of the other SBP categories. HR indicates hazard ratio; CI, confidence interval; BP, blood pressure; CV, cardiovascular; MI, myocardial infarction.
Figure 3.
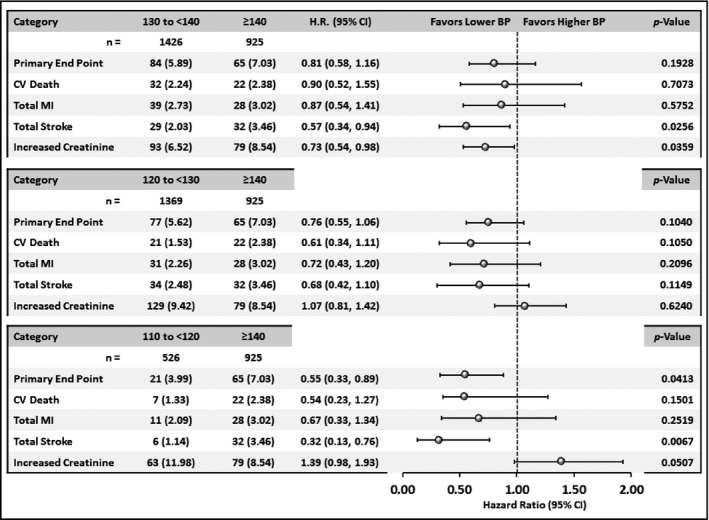
Comparison in nondiabetic patients of event rates from major cardiovascular, stroke, and renal endpoints between patients in the achieved systolic blood pressure (SBP) group >140 mm Hg and each of the other SBP categories. HR indicates hazard ratio; CI, confidence interval; BP, blood pressure; CV, cardiovascular; MI, myocardial infarction.
For the diabetic patients, those with an achieved SBP in the range <140 mm Hg or <130 mm Hg had significantly lower event rates than the ≥140 mm Hg group for CV outcomes. The subgroup below 120 mm Hg (compared with ≥140mm Hg) also showed benefits for the primary endpoint and for stroke but not myocardial infarction or CV death.
In contrast, for the nondiabetic cohort there was a slowly progressive decline in event rates for all endpoints in the three strata below 140 mm Hg, but only in the SBP group <120 mm Hg was the difference vs >140 mm Hg significant for the primary study endpoint.
Comparisons Among the Achieved BP Strata
Figures 4 and 5 provide further data on the relationships between achieved BPs and clinical event rates. Considering the primary endpoint, it is apparent that there are differences between diabetic and nondiabetic patients in these relationships. Figure 4 demonstrates that whereas event rates for the primary endpoint appear to fall progressively throughout the BP strata in nondiabetic patients, this effect appears to start reversing below systolic values of 120 mm Hg in the diabetic patients. Indeed, considering CV mortality, one of the components of the primary endpoint, it is apparent that its event rates, which were significantly reduced in the <140 mm Hg and <130 mm Hg strata, were no longer significantly different when SBP was <120 mm Hg. In addition, Figure 5 indicates that in the diabetic patients the event rate for myocardial infarction was significantly higher at an SBP of <120 mm Hg than at <130 mm Hg, possibly providing evidence for the so‐called J‐curve phenomenon. Only stroke among the primary endpoint components did not increase below 120 mm Hg.
Figure 4.
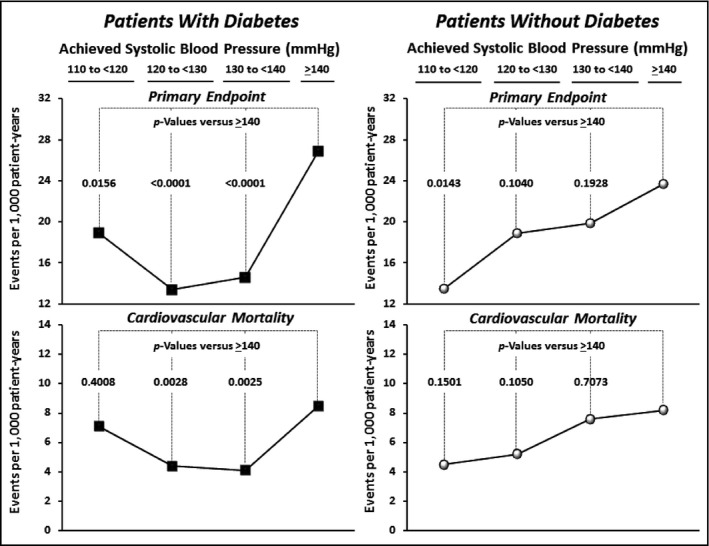
Event rates (per 1000 patient years) within each of the achieved systolic blood pressure (SBP) categories for the primary study endpoint (cardiovascular [CV] death or nonfatal myocardial infarction or stroke) and for CV death alone shown separately for patients with and without diabetes. None of the differences between adjacent categories was significant except for >140 mm Hg vs <140 mm Hg in the diabetes patients.
Figure 5.
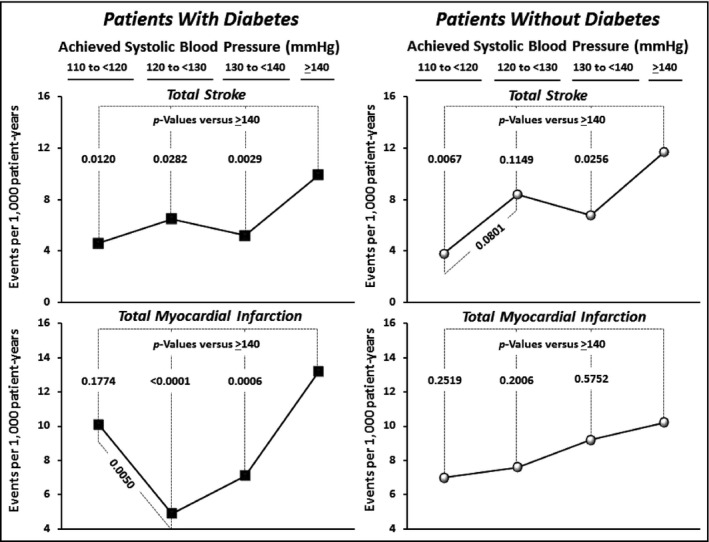
Event rates (per 1000 patient years) within each of the achieved systolic blood pressure (SBP) categories for total (fatal + nonfatal) stroke and myocardial infarction shown separately for patients with and without diabetes.
Renal Endpoint
Unlike the CV endpoints, the effects of BP levels on renal function were parallel in the diabetic and nondiabetic cohorts. In both cohorts, as indicated in Figures 2 and 3, the endpoint of a ≥50% increase in serum creatinine was significantly lower in the 10 mm Hg systolic range below 140 mm Hg than ≥140 mm Hg, but further reductions in SBP to the range below 130 mm Hg, and again to the range below 120 mm Hg, were associated with increases in this outcome. Figure 6 demonstrates that at any level of SBP, adverse renal findings were considerably higher in the diabetic patients than in the nondiabetic patients (P<.05 or stronger for each BP category). For all patients combined, this endpoint was more than 50% higher in the diabetic than the nondiabetic cohort (HR, 1.56; 1.40–1.76; P<.0001).
Figure 6.
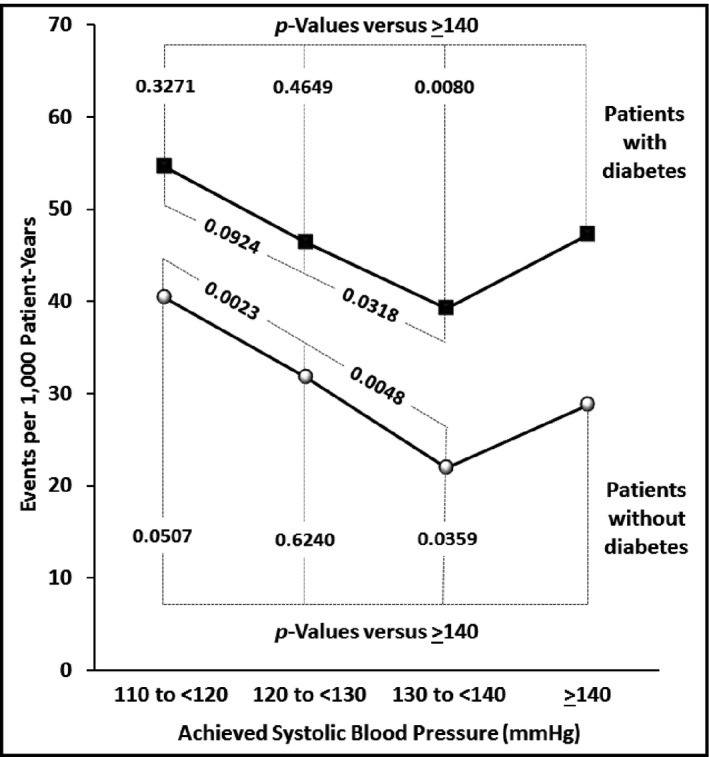
Event rates (per 1000 patient years) within each of the achieved systolic blood pressure (SBP) categories for the renal endpoint (increased from baseline in serum creatinine of >50%) shown separately for patients with and without diabetes.
Safety
Treatment in the ACCOMPLISH trial was generally well tolerated. Detailed information on adverse treatment effects has been provided previously.15
Discussion
During treatment in this trial, the distribution of patients across the achieved SBP categories was very similar for the diabetic and nondiabetic patients. However, there was a clear difference between these patient groups in the relationships between systolic BPs and CV outcomes.
Events in Patients With Diabetes
In the diabetic cohort, the composite primary endpoint (CV death, nonfatal myocardial infarction, or nonfatal stroke), as well as the individual components of this endpoint, were all significantly lower in the <140 mm Hg SBP category than in the uncontrolled (≥140 mm Hg) category. Event rates at <130 mm Hg were similar to those at <140 mm Hg, but there was a loss of CV benefits at BPs <120 mm Hg. In fact, myocardial infarction rates were higher at <120 mm Hg than at <130 mm Hg, and both myocardial infarction and CV mortality rates were no longer significantly reduced when compared with the uncontrolled category. Even so, total event rates for the composite primary endpoint were still significantly lower in the <120 mm Hg group than in the uncontrolled group.
Therefore, the ideal SBP treatment target for CV protection in hypertensive patients with diabetes appears to be <140 mm Hg or possibly <130 mm Hg. This finding is in agreement with the results of the ACCORD trial in diabetic hypertensive patients in which, with the exception of stroke, CV event rates were not different between the <140 mm Hg and <120 mm Hg targets.12 The findings in our diabetic cohort raise the possibility of a U‐curve effect—as suggested by the increase in coronary events below 120 mm Hg—and appear to be consistent with data in diabetic patients in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET)7 and a long‐term observation in primary care patients with type 2 diabetes in Sweden.18
Patients Without Diabetes
In contrast to the diabetic patients, clinical event rates in the nondiabetic cohort, with the exception of stroke, were not lower in the <140 mm Hg category than in the uncontrolled range. Indeed, only in the <120 mm Hg SBP range was the primary endpoint significantly lower than at ≥140 mm Hg. Based on this observation, it appears that the ideal SBP target in nondiabetic hypertensive patients could be as low as <120 mm Hg. Moreover, unlike the diabetic patients, the nondiabetic cohort did not appear to experience adverse effects at this low BP level despite the fact that more than 70% of them had histories of coronary disease that potentially could have made them susceptible to reduced coronary perfusion. This finding is in keeping with the recently published results of the SPRINT trial.14 That study, like ACCORD,13 compared CV outcomes in patients randomized to systolic treatment targets of <140 mm Hg or <120 mm Hg, although SPRINT studied only nondiabetic patients with a high‐risk profile. SPRINT reported a 25% lower event rate in its composite CV primary outcome and a 27% lower rate in total death in the patients allocated to the more intensively treated <120 mm Hg group. Taken together, ACCORD and SPRINT emphasize the benefits of <120 mm Hg in high‐risk nondiabetic patients but not in patients with diabetes. Our current findings, derived from the ACCOMPLISH trial, provide conclusions that are consistent with those studies.
Strokes and BP
For both diabetic and nondiabetic patients, the lowest stroke rates occurred below 120 mm Hg. For the diabetic patients, this creates a clinical dilemma since CV outcomes—particularly myocardial infarction and CV mortality—might actually be higher at <120 mm Hg. The ACCORD trial similarly identified these differing effects of low BP on CV and stroke outcomes.13 However, for high‐risk nondiabetic patients, decision making is more straightforward since all major outcomes (except renal function) are best prevented when BP is <120 mm Hg.
Our findings of the relationship between BP and stroke draw attention to a further observation. In Figure 5, it is apparent that there is an inflection point in this relationship: for both diabetic and nondiabetic patients, event rates for stroke are significantly lower at <140 mm Hg than >140 mm Hg, but at <130 mm Hg, there is a small increase in event rates before a clear further reduction is again observed at <120 mm Hg. We have noted that there may be similar inflection points for stroke rates in other studies.7, 19
Changes in Renal function
In this study, adverse effects on kidney function were defined as an increase in serum creatinine of ≥50%. These increases were probably not due to the trial's drug therapy because at the start of the trial almost all the patients were already taking ACE inhibitors or angiotensin receptor blockers, agents that are well recognized for increasing serum creatinine.20
The diabetic and nondiabetic patients demonstrated directionally similar changes in renal function at the achieved SBP categories. Specifically, the lowest event rates for increased creatinine were in the SBP range 130 mm Hg to 139 mm Hg for both cohorts. There were significantly higher event rates at 140 mm Hg or above as well as in the <130 mm Hg range and, most of all, in the <120 mm Hg range. These findings could influence the choice of target BPs in patients with chronic kidney disease in whom it may be critical to minimize further deterioration in renal function, and where achieving a range between 130 mm Hg and 139 mm Hg may thus be an appropriate clinical target. The adverse effects of 50% increases in serum creatinine on the progression of renal disease has been reported by others.21
Finally, it can be pointed out that despite the parallel effects on renal function of the achieved BPs in the diabetic and nondiabetic groups, event rates for the renal endpoint were more than 50% higher in the diabetic patients, confirming the heightened susceptibility of people with diabetes to chronic kidney disease.
Study Limitations
As noted earlier, an analysis of CV, stroke, and renal outcomes on the basis of achieved BPs was a prespecified part of the ACCOMPLISH study plan. However, many of the details of this analysis were inevitably established after the trial was completed so it should again be noted that some parts of what is reported here should be regarded as exploratory or post hoc. In addition, it should be acknowledged that ACCOMPLISH was not originally designed to explore the outcomes effects of different BP goals and thus did not randomize patients to the BP categories considered in this paper. Beyond the formal drug titration requirements of the study protocol it can be assumed that factors such as discretionary clinical decisions by investigators, the antihypertensive effectiveness of the study drugs, and the compliance of patients with their treatment regimens would have contributed to the achieved BP levels.
For the purposes of the present study, we used the standard triple major CV composite outcome (CV death or nonfatal myocardial infarction or nonfatal stroke) rather than the broader CV composite used in the original ACCOMPLISH report.15 The reason for this decision was to make our findings more parallel to other recent clinical trials that have utilized this three‐part major CV outcomes composite. Similarly, it should be noted that the decision to use 10 mm Hg systolic ranges to compare the effects of different achieved BP targets with outcomes was made post hoc. It can also be pointed out that to obtain adequate power for our analysis, we pooled patients randomized to the ACCOMPLISH trial's two therapeutic regimens. It is possible that results might have been different had the effects of a single treatment strategy been explored.
The number of patients, especially in the nondiabetic cohort, was relatively small, particularly when divided into the 10 mm Hg categories. Even so, we believe that the trends we were able to show adequately support our conclusions regarding these high‐risk nondiabetic patients. Likewise, the <120 mm Hg diabetes group, in which some important findings were noted, was the smallest of the diabetes categories, although we believe that it was still sufficiently strong to enable the exploratory analyses reported here. In this report, we have generally avoided direct comparisons between the diabetic and nondiabetic cohorts. As shown in Table 1, there were substantial baseline differences between these patients in their clinical histories and findings, such that comparisons of outcomes would be of questionable value.
Another issue is that this study was performed in patients with an average age in the high 60s and thus does not necessarily provide information or guidance relevant to the management of patients in younger age groups. Similarly, the patients we studied were originally recruited for the ACCOMPLISH trial and selected for their high‐risk histories and therefore may not provide information that can be extrapolated to individuals with hypertension but without other major risk factors.
Conclusions
For patients with hypertension and diabetes, the optimal SBP target is <140 mm Hg or <130 mm Hg. At levels below 120 mm Hg, coronary event rates may start to increase—a finding that is consistent with other trials7, 18—although overall CV protection is still significantly better than in the uncontrolled (>140 mm Hg) category. In contrast, for patients without diabetes, the optimal BP target for preventing CV events appears to be <120 mm Hg. However, for both diabetic and nondiabetic patients, stroke is best prevented below 120 mm Hg, a finding that merits discussion with high‐risk patients when deciding on how aggressively to treat their hypertension. On the other hand, renal function is best preserved at SBPs in the range of 130 mm Hg to 139 mm Hg. Again, this finding could provide guidance in selecting BP targets in patients whose renal function is a major clinical concern.
Disclosures
MAW: consulting: Boehringer Ingelheim, Eli Lilly and Company, Forest; speaking: Arbor Pharmaceuticals. GLB: Clinical trials: Bayer; consultant: Takeda Pharmaceuticals, AbbVie, CVRx, Janssen, Eli Lilly and Company/Boehringer‐Ingelheim, Medtronic, Astra‐Zeneca, Novartis, GSK, Bayer, Daichi‐Sankyo. BP: consultant: Bayer, Merck, Astra Zeneca, Boehringer Ingelheim, Forrest Laboratories, Relypsa, scPharmaceuticals, PharMain, Tricida, DaVinci Biosciences, Stealth Peptides, KBP BioSciences, AuraSense; stock options: Relypsa, scPharmaceuticals, PharMain, KBP BioSciences, AuraSense, DaVinci Biosciences, Galectin Therapeutics, Tricida; patent pending: site‐specific delivery of eplerenone to the myocardium. DSMBs: Novartis Pharmaceuticals, Inc, J&J, Oxygen Biotherapeutics; events committee: Juventis. DHZ: employee of Novartis Pharmaceuticals, Inc. TH: employee of Novartis Pharmaceuticals, Inc. JNB: consulting: National Heart, Lung, and Blood Institute, Eli Lilly and Company, Amgen, Arbor Pharmaceuticals, Janssen, Medtronic, Novartis, ReCor; speaker: Arbor Pharmaceuticals, Janssen; grant support: National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases. MRW: scientific advisory board: Relypsa, Akebia, Janssen, Astra, Boehringer‐Ingelheim, Boston Scientific, Ablative Solutions; clinical trial steering committee: Sandoz, Amgen. EJV: consulting: Novartis, Amgen, Merck; grant support: National Heart, Lung, and Blood Institute, Novartis, Amgen, Pfizer, Alynylam, Medtronic Foundation. KJ: none.
J Clin Hypertens (Greenwich). 2016;18:299–307. doi: 10.1111/jch.12816. © 2016 Wiley Periodicals, Inc.
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. National high blood pressure education program coordinating committee: seventh report of the Joint National Committee on prevention, detection, evaluation and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, et al. Task Force Members . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 3. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 5. Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. [DOI] [PubMed] [Google Scholar]
- 6. Cooper‐DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sleight P, Redon J, Verdecchia P, et al. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens. 2009;27:1360–1369. [DOI] [PubMed] [Google Scholar]
- 8. Weber MA, Bakris GL, Hester A, et al. Systolic blood pressure and cardiovascular outcomes during treatment of hypertension. Am J Med. 2013;126:501–508. [DOI] [PubMed] [Google Scholar]
- 9. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 10. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 11. Wright JTJr, Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499–503. [DOI] [PubMed] [Google Scholar]
- 12. Bangalore S, Gong Y, Cooper‐DeHoff RM, et al. 2014 Eighth Joint National Committee panel recommendation for blood pressure targets revisited: results from the INVEST study. J Am Coll Cardiol. 2014;64:784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103–2116. DOI: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators . Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 16. Weber MA, Bakris GL, Dahlöf B, et al. Baseline characteristics in the Avoiding Cardiovascular events through Combination therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial: a hypertensive population at high cardiovascular risk. Blood Press. 2007;16:13–19. [DOI] [PubMed] [Google Scholar]
- 17. Jamerson KA, Bakris GL, Wun CC, et al; Rationale and design of the avoiding cardiovascular events through combination therapy in patients living with systolic hypertension (ACCOMPLISH) trial: the first randomized controlled trial to compare the clinical outcome effects of first‐line combination therapies in hypertension. Am J Hypertens. 2004;17:793–801. [DOI] [PubMed] [Google Scholar]
- 18. Sundström J, Sheikhi R, Ostgren CJ, et al. Blood pressure levels and risk of cardiovascular events and mortality in type‐2 diabetes: cohort study of 34,009 primary care patients. J Hypertens. 2013;31:1603–1610. [DOI] [PubMed] [Google Scholar]
- 19. Redon J, Mancia G, Sleight P, et al; ONTARGET Investigators . Safety and efficacy of low blood pressures among patients with diabetes: subgroup analyses from the ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial). J Am Coll Cardiol. 2012;59:74–83. [DOI] [PubMed] [Google Scholar]
- 20. Ruggenenti P, Remuzzi G. Dealing with renin‐angiotensin inhibitors, don't mind serum creatinine. Am J Nephrol. 2012;36:427–429. [DOI] [PubMed] [Google Scholar]
- 21. Hirsch S1, Hirsch J, Bhatt U, Rovin BH. Tolerating increases in the serum creatinine following aggressive treatment of chronic kidney disease, hypertension and proteinuria: pre‐renal success. Am J Nephrol. 2012;36:430–437. [DOI] [PubMed] [Google Scholar]


