Abstract
Beta‐trace protein (BTP) has emerged as a novel biomarker of cardiovascular risk. In this study, the authors aimed to assess the relationship between BTP levels and presence of atrial fibrillation in patients who had controlled hypertension (HTN) and normal renal function. A total of 80 controlled HTN patients with paroxysmal atrial fibrillation (PAF) and 80 age‐ and sex‐matched controls with controlled HTN were enrolled. Serum BTP levels were measured by enzyme‐linked immunosorbent assay. BTP levels were found to be significantly higher in patients with PAF (P<.001). Other parameters including mean systolic and diastolic blood pressure values, serum creatinine levels, and glomerular filtration rate were similar between the two groups. Along with left atrial diameter (odds ratio, 1.504; P<.001), BTP levels (odds ratio, 1.015; P<.001) were independently associated with the presence of PAF. BTP levels were increased in controlled HTN patients with PAF compared with controls, and this association was observed within normal renal functions as reflected by normal glomerular filtration rate.
Atrial fibrillation (AF) is the most common sustained arrhythmia and, therefore, constitutes a global health problem.1 It is known that AF most commonly occurs in the setting of underlying cardiovascular disease associated with hypertension (HTN), coronary artery disease (CAD), valvular heart disease, cardiomyopathies, and heart failure.1 Of these, HTN has been reported to be the most common comorbid chronic condition among Medicare beneficiaries with AF in all age groups (≥65 years and <65 years)2 and gained a place in risk stratification scores for ischemic cerebrovascular event in AF patients.1 Impaired renal function has been shown to be another independent risk factor for AF development.3, 4, 5
Beta‐trace protein (BTP), also known as prostaglandin D2 synthase, is a protein with low molecular mass belonging to the lipocalin protein family.6 BTP has been described as a more sensitive marker than serum creatinine in detecting impaired renal function, with performance comparable to that of cystatin C.7, 8 In addition, BTP has emerged as a novel biomarker of cardiovascular risk. Different studies have explored the role of BTP in HTN.9, 10 Increase in BTP levels has been proposed to reflect injuries in the renal tubules and arterioles induced by HTN.10
Up to now, BTP has not been investigated as a surrogate of renal or cardiovascular risk in patients with AF. Therefore, in this study, we aimed to assess the relationship between BTP levels and presence of AF in patients who had controlled HTN and normal renal function.
Methods
Study Population
In this observational study, we enrolled 80 controlled HTN patients with paroxysmal AF and 80 age‐ and sex‐matched control patients with controlled HTN.
Patients with controlled HTN who underwent regular visits in the outpatient clinics were monitored with 24‐hour ambulatory electrocardiography (Holter). Controlled HTN was defined as blood pressure values <140/90 mm Hg on at least two visits. Management of HTN was guided per recent guidelines.11 Patient group was defined as individuals who had self‐terminating AF episodes on Holter and no evidence of associated cardiopulmonary or other systemic disease except controlled HTN. The control group consisted of controlled HTN patients with no history of concomitant disease and no documented AF episodes on Holter.
A detailed medical history was taken from all patients and a thorough physical examination was performed to exclude any cardiac or noncardiac systemic disease. All patients underwent transthoracic echocardiography to assess chamber dimensions and valvular and systolic functions. Patients who were 65 years and older; who had a history of diabetes mellitus; heart failure; CAD; congenital, structural, or valvular heart disease; pulmonary disease; neurological disease; malignancy; autoimmune disease; recent infection; or operation were excluded from the study. None of the patients had impaired renal function, which was defined as a glomerular filtration rate (GFR) <60 mL/min/1.73 m2 using the Modification of Diet in Renal Disease (MDRD) formula. Informed consent was obtained from all participants. The study was in compliance with the principles outlined in the Declaration of Helsinki and approved by the institutional ethics committee.
Measurement of BTP Levels
Peripheral venous serum samples were obtained from patients during outpatient clinic visits for routine laboratory tests. Samples separated for determination of BTP level were centrifuged at 1000 × g for 10 minutes. BTP levels were measured using the Human Prostaglandin D Synthase, Lipocalin‐type ELISA kit (BioVendor, Cat. No. RD191113100R, Brno, Czech Republic).
Statistical Analysis
Normally distributed parameters were presented as mean±standard deviation and skewed parameters were expressed as median (interquartile range: minimum–maximum). Descriptive data were presented as frequencies and percentages and compared using chi‐square test. Comparisons between baseline characteristics were performed by independent Student t, Mann‐Whitney rank‐sum, Fisher exact, or chi‐square tests where appropriate. To determine associates of PAF presence, binomial logistic regression analysis was performed. Spearman's correlation analysis was done to investigate the correlation of parameters with serum levels of BTP. Receiver operating characteristics analysis was performed to determine the cut‐off levels of BTP for predicting PAF presence. Statistical analyses were performed using SPSS statistical software (version 20.0; SPSS Inc, Chicago, IL). A two‐tailed P<.05 was considered statistically significant.
Results
Our study population consisted of 80 hypertensive patients with PAF (52.81±7.40 years, 57.5% male) and 80 age‐ and sex‐matched hypertensive control patients with no documented AF episodes (53.41±6.05 years, 50% male). Baseline characteristics of the study population are shown in Table 1. Neither mean systolic (128.94 mm Hg vs 129.25 mm Hg, P=.728) or diastolic blood pressure (82.50 mm Hg vs 82.56 mm Hg, P=.935) values differed between the control and patient groups, respectively. The number of antihypertensive drugs also did not differ between groups (P=.685).
Table 1.
Baseline Characteristics of the Study Population (n=160)
| Control Group (n=80) | Paroxysmal Atrial Fibrillation Group (n=80) | P Value | |
|---|---|---|---|
| Demographic parameters | |||
| Male sex, No. (%) | 40 (50.00) | 46 (57.5) | .428 |
| Age, y | 53.41±6.05 | 52.81±7.40 | .575 |
| Clinical parameters | |||
| BMI, kg/m2 | 24.42±5.64 | 24.93±2.79 | .468 |
| Current smoking, No. (%) | 16 (20.00) | 24 (30.00) | .201 |
| Systolic blood pressure, mm Hga | 128.94±5.72 | 129.25±5.63 | .728 |
| Diastolic blood pressure, mm Hga | 82.50±4.64 | 82.56±5.03 | .935 |
| Antihypertensive drugs, No. (%) | |||
| 1 | 35 (43.75) | 32 (40.00) | .685 |
| 2 | 41 (51.25) | 43 (53.75) | |
| 3 | 4 (5.00) | 5 (6.25) | |
| Antihypertensive drugs, No. (%) | |||
| RAAS blockers | 33 (41.20) | 36 (45.00) | .750 |
| Calcium channel blockers | 34 (42.50) | 33 (41.20) | 1.000 |
| Beta‐blockers | 28 (35.00) | 31 (38.80) | .743 |
| Diuretics | 34 (42.50) | 33 (41.20) | 1.000 |
| Laboratory parameters | |||
| WBC count, ×109/L | 7.15±0.93 | 7.11±1.14 | .790 |
| Fasting blood glucose, mg/dL | 95.51±9.36 | 95.73±11. 83 | .900 |
| Triglycerides, mg/dL | 115.23±41.17 | 116.85±38.77 | .797 |
| LDL cholesterol, mg/dL | 116.68±29.88 | 125.05±27.92 | .215 |
| Serum creatinine, mg/dL | 0.80±0.13 | 0.80±0.14 | .882 |
| GFR, mL/min/1.73 m2 | 82.80 (60.40–129.50) | 80.36 (60.80–141.00) | .821 |
| BTP, ng/mL | 301.70 (154.28–568.00) | 364.51 (204.00–733.00) | <.001 |
| Echocardiographic parameters | |||
| LV end‐diastolic diameter, mm | 47.19±3.77 | 46.44±3.58 | .199 |
| LVEF, % | 66.36±3.13 | 66.36±3.42 | 1.000 |
| LA diameter, mm | 33.60±2.67 | 35.91±2.74 | <.001 |
Abbreviations: BMI, body mass index; BTP, beta‐trace protein; GFR, glomerular filtration rate; LA, left atrial; LDL, low‐density lipoprotein; LV, left ventricular; LVEF, left ventricular ejection fraction; RAAS, renin‐angiotensin‐aldosterone system; WBC, white blood cell. aSystolic and diastolic blood pressure values are expressed as the average of the values obtained at two outpatient clinic visits.
Left atrial (LA) diameter was significantly higher in patients with PAF when compared with controls (35.91±2.74 mm vs 33.60±2.67 mm, P<.001). Levels of BTP were also found to be significantly higher in patients with PAF (364.51 [204.00–733.00] vs 301.70 [154.28–568.00] ng/mL; P<.001). Other parameters including body mass index, smoking history, white blood cell count, serum creatinine levels, GFR, levels of low‐density lipoprotein cholesterol, triglyceride or fasting blood glucose, left ventricular ejection fraction, and left ventricular end‐diastolic diameter were similar between the two groups (Table 1).
Univariate regression analysis revealed that LA diameter (odds ratio [OR], 1.356; 95% confidence interval [CI], 1.194–1.540; P<.001) and levels of BTP (OR, 1.014; 95% CI, 1.008–1.021; P=.001) were significantly associated with the presence of PAF in patients with controlled HTN (Table 2). Multivariate regression analysis showed that LA diameter (OR, 1.504; 95% CI, 1.279–1.769; P<.001) and BTP levels (OR, 1.015; 95% CI, 1.009–1.022; P<.001) were independently associated with presence of PAF (Table 2).
Table 2.
Binomial Regression Analysis for Determining Associates of AF Presence
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| BTP, ng/mL | 1.014 (1.008–1.021) | <.001 | 1.015 (1.009–1.022) | <.001 |
| LA diameter, mm | 1.356 (1.194–1.540) | <.001 | 1.504 (1.279–1.769) | <.001 |
| LV end‐diastolic diameter, mm | 0.946 (0.868–1.030) | .198 | 1.044 (0.932–1.169) | .458 |
Abbreviations: AF, atrial fibrillation; BTP, beta‐trace protein; CI, confidence interval; LA, left atrial; LV, left ventricular; OR, odds ratio.
Receiver operating characteristic analysis showed that serum BTP level with a cut‐off of 330.14 ng/mL predicted presence of PAF in controlled HTN patients with a sensitivity and specificity of 75% and 74%, respectively (area under the curve, 0.824; 95% CI, 0.757–0.891; P<.001) (Figure).
Figure 1.
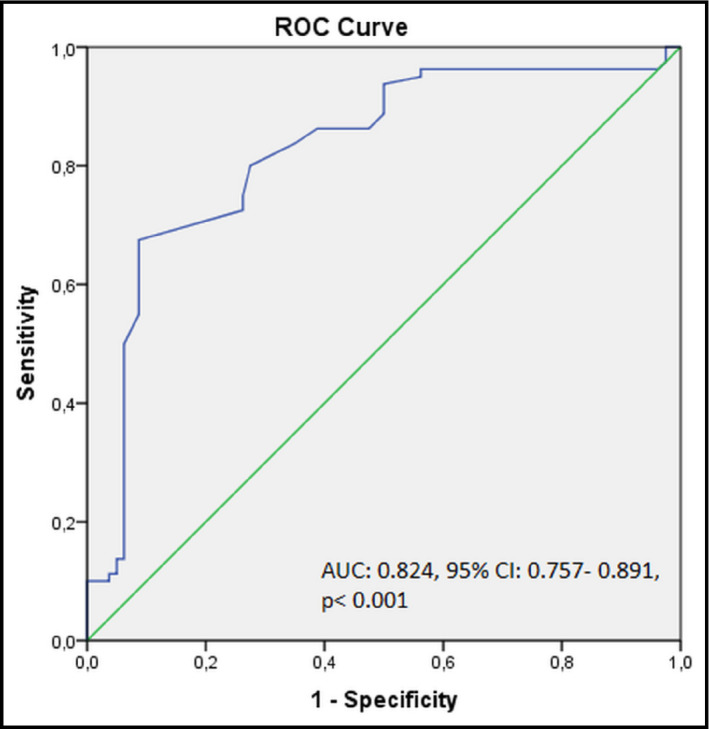
Receiver operating characteristic (ROC) analysis results for sensitivity and specificity of serum beta‐trace protein levels in prediction of paroxysmal atrial fibrillation presence. AUC indicates area under the curve; CI, confidence interval.
Spearman's correlation analysis showed that there was a significant correlation between serum BTP levels and GFR (r=0.239, P=.002), but not with any other baseline parameters.
Discussion
In the present study, we demonstrated the association of serum BTP levels with PAF in controlled HTN patients. To the best of our knowledge, this is the first study demonstrating the relationship between BTP levels and presence of PAF. Findings of our study indicate that higher serum BTP levels are associated with PAF presence in controlled HTN patients with normal renal function.
BTP has been proposed to have a role in the pathogenesis of cardiovascular disease. One study reported that BTP is expressed in both endocardium and myocardium of normal patients and the stenotic site in patients with stable angina and is secreted into the coronary circulation.12 Previous studies have reported prognostic value for BTP in CAD12, 13, 14, 15, 16 and acute heart failure.17 Hirawa and colleagues10 compared BTP levels in normotensive and hypertensive patients, where they found higher levels of BTP in HTN patients. In the present study, we demonstrate for the first time that BTP levels are elevated in patients with PAF when compared with age‐ and sex‐matched control patients.
BTP is now regarded as a more sensitive marker than serum creatinine in detecting impaired renal function, with performance comparable to that of cystatin C.7, 8 Elevated BTP levels in patients with PAF may be explained by the fact that chronic kidney disease is associated with increased AF risk, as shown in a prospective population‐based study.4 In our study, all patients had normal renal function according to GFR based on the MDRD formula. Previous studies have shown that low GFR is associated with poor outcome in AF patients4, 18, 19 and recurrence after AF ablation.20, 21, 22 However, findings in our study demonstrate an association between AF presence and serum BTP levels in patients who had GFR values within normal limits.
Although our study demonstrates an association, it does not elucidate exact pathophysiological mechanisms; however, it is possible to speculate on possible links. First, although HTN is an established risk factor for decreased kidney function, some evidence supports a reverse association.9 Therefore, the association demonstrated in our study may be linked with the ability of BTP to reflect impaired renal function even in patients with normal GFR in HTN patients. Impaired renal function results in an increased cardiovascular risk and is commonly associated with the development of AF3 and its complications.23 Atrial pressure elevation is known to predispose to AF development. Acute increase in atrial pressure leads to electrophysiologic changes, while chronic atrial stretch induces structural remodeling.24 Knowing that in the heart BTP has been localized to myocardial cells and atrial endocardial cells,12 elevated serum BTP levels may reflect the increase in atrial pressure or atrial stretch leading to AF. Furthermore, Taga and colleagues25 previously demonstrated that laminar shear stress stimulated the production of BTP by upregulating the expression of lipocalin‐type prostaglandin G synthase in endothelial cells. Whether endothelial BTP secretion plays a role in AF pathogenesis or increased serum levels are only innocent bystanders should be further investigated.
Study Limitations
Our study has some limitations. First, this is a single‐center study with a small number of patients; however, the study population was well‐defined and we excluded patients who had conditions that could have an effect on BTP levels. Second, this is a hypothesis‐generating clinical study, therefore a pathophysiological link between BTP and presence of PAF in HTN patients cannot be proposed with these data. Our results do not reveal a causal relationship but demonstrate an association.
Conclusions
We demonstrated that serum levels of BTP were increased in controlled HTN patients with PAF compared with control patients, and this association was observed within normal renal function reflected by normal GFR. Our study suggests that elevated serum BTP levels in patients with controlled HTN may require a closer follow‐up for early recognition of AF and prevention of related unfavorable outcomes (eg, thromboembolism and heart failure), such as performing Holter monitoring in each office visit. This group of patients may also require more strict control of blood pressure. The importance of compliance with lifestyle interventions should be emphasized and renin‐angiotensin‐aldosterone system blockers should be the first‐choice drug for antihypertensive treatment. In case the exact pathophysiologic link is demonstrated in further studies, BTP may also constitute a therapeutic target in the future.
Disclosure
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Acknowledgment
None.
J Clin Hypertens (Greenwich). 2016;18:439–443. DOI: 10.1111/jch.12703. © 2015 Wiley Periodicals, Inc.
References
- 1. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Welcome to the Chronic Conditions Data Warehouse Web site. www.ccwdata.org. Accessed September 11, 2015.
- 3. Xu D, Murakoshi N, Sairenchi T, et al. Anemia and reduced kidney function as risk factors for new onset of atrial fibrillation (from the Ibaraki prefectural health study). Am J Cardiol. 2015;115:328–333. [DOI] [PubMed] [Google Scholar]
- 4. Sciacqua A, Perticone M, Tripepi G, et al. Renal disease and left atrial remodeling predict atrial fibrillation in patients with cardiovascular risk factors. Int J Cardiol. 2014;175:90–95. [DOI] [PubMed] [Google Scholar]
- 5. Buiten MS, de Bie MK, Rotmans JI, et al. The dialysis procedure as a trigger for atrial fibrillation: new insights in the development of atrial fibrillation in dialysis patients. Heart. 2014;100:685–690. [DOI] [PubMed] [Google Scholar]
- 6. Risch L, Lisec I, Jutzi M, et al. Rapid, accurate and non‐invasive detection of cerebrospinal fluid leakage using combined determination of beta‐trace protein in secretion and serum. Clin Chim Acta. 2005;351:169–176. [DOI] [PubMed] [Google Scholar]
- 7. Priem F, Althaus H, Birnbaum M, et al. Beta‐trace protein in serum: a new marker of glomerular filtration rate in the creatinine‐blind range. Clin Chem. 1999;45:567–568. [PubMed] [Google Scholar]
- 8. Priem F, Althaus H, Jung K, Sinha P. Beta‐trace protein is not better than cystatin C as an indicator of reduced glomerular filtration rate. Clin Chem. 2001;47:2181. [PubMed] [Google Scholar]
- 9. Huang M, Matsushita K, Sang Y, et al. Association of kidney function and albuminuria with prevalent and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2015;65:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirawa N, Uehara Y, Yamakado M, et al. Lipocalin‐type prostaglandin D synthase in essential hypertension. Hypertension. 2002;39(2 Pt 2):449–454. [DOI] [PubMed] [Google Scholar]
- 11. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 12. Eguchi Y, Eguchi N, Oda H, et al. Expression of lipocalin‐type prostaglandin D synthase (beta‐trace) in human heart and its accumulation in the coronary circulation of angina patients. Proc Natl Acad Sci USA. 1997;94:14689–14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Urade Y, Eguchi N. Lipocalin‐type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins Other Lipid Mediat. 2002;68–69:375–382. [DOI] [PubMed] [Google Scholar]
- 14. Cipollone F, Fazia M, Iezzi A, et al. Balance between PGD synthase and PGE synthase is a major determinant of atherosclerotic plaque instability in humans. Arterioscler Thromb Vasc Biol. 2004;24:1259–1265. [DOI] [PubMed] [Google Scholar]
- 15. Manzano‐Fernandez S, Lopez‐Cuenca A, Januzzi JL, et al. Usefulness of beta‐trace protein and cystatin C for the prediction of mortality in non ST segment elevation acute coronary syndromes. Am J Cardiol. 2012;110:1240–1248. [DOI] [PubMed] [Google Scholar]
- 16. Inoue T, Eguchi Y, Matsumoto T, et al. Lipocalin‐type prostaglandin D synthase is a powerful biomarker for severity of stable coronary artery disease. Atherosclerosis. 2008;201:385–391. [DOI] [PubMed] [Google Scholar]
- 17. Manzano‐Fernandez S, Januzzi JL Jr, Boronat‐Garcia M, et al. β‐trace protein and cystatin C as predictors of long‐term outcomes in patients with acute heart failure. J Am Coll Cardiol. 2011;57:849–858. [DOI] [PubMed] [Google Scholar]
- 18. Wang D, Liu M, Hao Z, Tao W. Association between reduced kidney function and clinical outcomes after ischaemic stroke with atrial fibrillation. Eur J Neurol. 2014;21:160–166. [DOI] [PubMed] [Google Scholar]
- 19. You L, Wang P, Lv J, et al. The role of high‐sensitivity C‐reactive protein, interleukin‐6 and cystatin C in ischemic stroke complicating atrial fibrillation. J Huazhong Univ Sci Technolog Med Sci. 2010;30:648–651. [DOI] [PubMed] [Google Scholar]
- 20. Tokuda M, Yamane T, Matsuo S, et al. Relationship between renal function and the risk of recurrent atrial fibrillation following catheter ablation. Heart. 2011;97:137–142. [DOI] [PubMed] [Google Scholar]
- 21. Chao TF, Lin YJ, Chang SL, et al. Associations between renal function, atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Circ J. 2011;75:2326–2332. [DOI] [PubMed] [Google Scholar]
- 22. Neumann T, Wojcik M, Berkowitsch A, et al. Cryoballoon ablation of paroxysmal atrial fibrillation: 5‐year outcome after single procedure and predictors of success. Europace. 2013;15:1143–1149. [DOI] [PubMed] [Google Scholar]
- 23. Soliman EZ, Prineas RJ, Go AS, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J. 2010;159:1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agoston G, Szilagyi J, Bencsik G, et al. Impaired adaptation to left atrial pressure increase in patients with atrial fibrillation. J Interv Card Electrophysiol. 2015;44:113–118. [DOI] [PubMed] [Google Scholar]
- 25. Taba Y, Sasaguri T, Miyagi M, et al. Fluid shear stress induces lipocalin‐type prostaglandin D(2) synthase expression in vascular endothelial cells. Circ Res. 2000;86:967–973. [DOI] [PubMed] [Google Scholar]


