Abstract
The aim of this study was to review the effect of continuous positive airway pressure (CPAP) on blood pressure (BP) in patients with obstructive sleep apnea (OSA) and hypertension. Biomedical databases were searched for randomized controlled trials (RCTs) comparing CPAP with control among these patients. Seven RCTs reporting 24‐hour ambulatory BP were identified for meta‐analysis. CPAP was associated with significant reductions in 24‐hour ambulatory systolic blood pressure (SBP) (−2.32 mm Hg; 95% confidence interval [CI], −3.65 to −1.00) and diastolic blood pressure (DBP) (−1.98 mm Hg; 95% CI, −2.82 to −1.14). CPAP led to more significant improvement in nocturnal SBP than that in diurnal SBP. Subgroup analysis showed that patients with resistant hypertension or receiving antihypertensive drugs benefited most from CPAP. Meta‐regression indicated that CPAP compliance, age, and baseline SBP were positively correlated with decrease in 24‐hour DBP, but not reduction in 24‐hour SBP.
Obstructive sleep apnea (OSA) is a highly prevalent but often underdiagnosed clinical condition among hypertensive patients. The disease is characterized by recurrent obstruction of the upper airway, leading to repetitive episodes of apnea and intermittent hypoxia during sleep.1 Contemporary epidemiologic research has estimated that 4% to 6% of the general middle‐aged population is affected by OSA, and this number is believed to keep growing in the near future.2 Research has shown cross‐talks between OSA and hypertension, and this relationship is independent of concurrent demographic variables such as age and body mass index (BMI).3 Early data show that hypertension was found in more than 50% of OSA patients, while around one third of hypertensive patients have concurrent OSA.4 OSA has now been recognized as an important cause or risk factor predisposing to hypertension.5
Continuous positive airway pressure (CPAP) is currently an effective therapeutic strategy for patients with OSA.6 For patients with hypertension secondary to or complicated with OSA, blood pressure (BP) control can be theoretically improved if the apnea were effectively treated by CPAP given the causal links between OSA and hypertension. Practically, clinical and basic studies have shown that CPAP can regulate the BP control by a series of mechanisms including attenuation of sympathetic nervous overactivity.6, 7
Previous meta‐analyses of randomized controlled trials (RCTs) have shown that CPAP reduces BP in patients with OSA.8, 9, 10, 11, 12 However, the magnitude of BP reduction remains to be investigated because of a considerable proportion of normotensive patients enrolled in these studies. Moreover, in a review of published RCTs on this topic, including the ones most recently reported, the effect of CPAP on BP control among OSA remained a subject of debate.13, 14, 15, 16, 17, 18, 19, 20 Therefore, we performed the present systematic review and meta‐analysis of relevant RCTs aiming to comprehensively evaluate the role of therapeutic CPAP in BP control for OSA patients with hypertension.
Methods
Study Search Strategy
We searched the database of Medline, Embase, the Cochrane Central Register of Controlled Trials, and relevant Web sites (www.acc.org, www.tctmd.com, www.theheart.org, www.clinicaltrialresults.org) for target studies before July 2014 by using the following keywords: “sleep apnea syndrome” or “obstructive sleep apnea” or “OSA,” “hypertension” or “hypertensive,” and “continuous positive airway pressure” or “CPAP.”
Study Selection
Clinical trials based on the following predefined inclusion criteria were retrieved: (1) those enrolling adult participants with OSA and hypertension; (2) those comparing therapeutic CPAP treatment and control (no CPAP, sham‐CPAP, oral placebo tablet, or mere standard conservative care); (3) those reporting adequate data of 24‐hour ambulatory blood pressure monitoring; (4) those following up at least 1 month; and (5) those using a prospective randomized method. If multiple publications of the same trial were identified, only the most recent publication was included. Two investigators (Hu XY, Fan JQ) independently performed study selection and data extraction. Disagreements were resolved through discussion with the participation of a third investigator (Yin YH).
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Outcome Measures Meta‐Analyses' (PRISMA) guidelines.
Data Collection and Assessment of Quality
We extracted prespecified data elements from each trial, including study design, inclusion/exclusion criteria, intervention methods, patient characteristics, concomitant antihypertensive medication, CPAP compliance, and outcomes of 24‐hour ambulatory BP. The Jadad scale was used to evaluate the quality of the RCTs. A study with scores three or higher on the Jadad scale was considered high‐quality.
Quantitative Data Analysis
The absolute efficacy of CPAP was quantified by calculating the mean difference of outcomes (systolic and diastolic BP change). For parallel trials, it was calculated as the mean difference (CPAP minus control treatment) of the change (follow‐up minus baseline). For crossover trials, it was calculated as the mean difference between the end of the CPAP treatment and control periods. The computational method of Follmann and colleagues21 assumed a correlation coefficient of 0.5 between baseline and endpoint BP. Effect sizes and the corresponding 95% confidence intervals (CIs) were analyzed by pooling available data using the Comprehensive Meta‐Analysis V2 software (Biostat, Englewood, NJ). An α value of 0.05 was considered statistically significant. Heterogeneity was examined by I 2 index and the tau‐square test. I 2 statistic with values over 50% or between‐studies heterogeneity P<.10 indicated high heterogeneity, where random‐effects model would be performed instead of fixed‐effects model to compute the data.
Results
Literature Search and Quality Assessment
Figure 1 shows the detailed flow chart of the selection process of the studies. By using our predefined searching strategy, seven RCTs were consequently selected for data extraction and meta‐analysis.13, 14, 16, 17, 18, 19, 20 Table 1 summarizes the outcomes of quality assessment and Jadad scores of included studies (κ=0.91±0.06).
Figure 1.
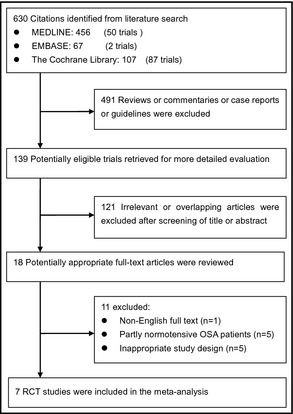
Flowchart of selected and identified studies. OSA indicates obstructive sleep apnea; RCT indicates randomized controlled trial.
Table 1.
Main Characteristic of the Trials Included in the Meta‐Analysis
| No. | Trial | Journal, Year of Publication | Study Duration, mo | Age Range, y | AHI, Events per h | Trial Type | Intervention/Control | Jadad Scale | Site |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Lloberes et al20 | J Hypertens, 2014 | 3 | >18 | ≥15 | Parallel RCT | CPAP+drugs/drugs | 3 | Barcelona, Spain |
| 2 | Martinez‐Garcia et al19 | JAMA, 2013 | 3 | 18–75 | ≥15 | Parallel RCT | CPAP+drugs/drugs | 3 | Valencia, Spain |
| 3 | Pedrosa et al18 | CHEST, 2013 | 6 | 30–65 | ≥15 | Parallel RCT | CPAP+drugs/drugs | 3 | Sao Paulo, Brazil |
| 4 | Lozano et al17 | J Hypertens, 2010 | 3 | 18–80 | ≥15 | Parallel RCT | CPAP+drugs/drugs | 2 | Barcelona, Spain |
| 5 | Duran‐Cantolla et al16 | BMJ, 2010 | 3 | 18–75 | ≥15 | Parallel RCT | CPAP/sham | 5 | Vitoria, Spain |
| 6 | Campos‐Rodriguez et al14 | CHEST, 2006 | 1 | 30–70 | ≥10 | Parallel RCT | CPAP/sham | 4 | Sevilla, Spain |
| 7 | Robinson et al13 | Eur Respir J, 2006 | 1 | >18 | >5 | Crossover RCT | CPAP/sham | 4 | Oxford, UK |
Abbreviations: AHI, apnea hypopnea index; CPAP, continuous positive airway pressure; RCT, randomized controlled trial.
Characteristics of the Studies and Patients Included
The main characteristics of included trials are presented in Table 2. Seven studies of 794 patients were analyzed. Three studies recruited patients with OSA and hypertension,13, 14, 16 and the other four trials selectively enrolled patients with OSA and resistant hypertension.17, 18, 19, 20 Untreated hypertensive patients at baseline were enrolled in one study.16 Of the seven RCTs, six studies were parallel14, 16, 17, 18, 19, 20 and one study was a crossover design.13 Four RCTs examined CPAP vs no CPAP17, 18, 19, 20 and three compared CPAP with sham‐CPAP.13, 14, 16 The treatment duration ranged from 1 to 6 months. All studies reported the changes in 24‐hour ambulatory BP, and the outcomes of diurnal and nocturnal ambulatory BP were available in five studies.16, 17, 18, 19, 20
Table 2.
Baseline Clinical Characteristic of the Study Populations
| Trial | Stduy Duration, mo | Sample Size, No. | CPAP Patients, No. | Antihypertensive Drugs at Baseline | Antihypertensive drugs (Mean±SD), No. | CPAP Pressure (Mean±SD), cm/H2O | CPAP Compliance (Mean±SD), h/Night | Mean Age, y | Male, % | BMI (Mean±SD), kg/m2 | AHI (Mean±SD), No./night | ESS (Mean±SD), No. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lloberes et al20 | 3 | 58 | 29 | Yes | NR | 9.6±1.5 | 5.7±1.5 | 58.7±9.5 | 72.4 | 32.0±4.9 | 50.0±20.3 | 7.3±4 |
| Martinez‐Garcia et al19 | 3 | 194 | 98 | Yes | 3.7±0.9 | 8.5±2.1 | 5±1.9 | 56.0±9.5 | 68.6 | 34.1±5.4 | 40.4 ±18.9 | 9.1±3.7 |
| Pedrosa et al18 | 6 | 35 | 19 | Yes | 4 (4–5)a | 11.5±0.5 | 6.02±0.33 | 56±1 | 77.0 | 32 (28–39)b | 29 (24–48)c | 10±1 |
| Duran‐Cantolla et al16 | 3 | 340 | 169 | No | 0 | 8.8±1.6 | 4.5±1.7 | 53.2±10.2 | 79.0 | 31.9±5.7 | 44.5±24.6 | 10.3±4.2 |
| Lozano et al17 | 3 | 64 | 29 | Yes | 3.41±0.57 | 9.62±1.54 | 5.6±1.52 | 59.2±9.9 | 75.9 | 30.8±5 | 52.67±21.5 | 6.14±3.30 |
| Campos‐Rodriguez et al14 | 1 | 68 | 34 | Yes | 2.0±0.9 | 9.5±1.9 | 5.0±1.4 | 55.3±9.6 | 55.8 | 35.7±5.6 | 58.3±24.6 | 15.0±3.9 |
| Robinson et al13 | 1 | 35 | 18 | Yes | NR | 10.4±1.5 | 5.2±2.1 | 54±8 | 88.6 | 33.2±5.3 | 28.1 (18.0–38.0) | 5.3 (3.0–7.0)d |
Abbreviations: CPAP, continuous positive airway pressure; NR, not reported; SD, standard deviation. aRepresenting the mean number of antihypertensive drugs and 95% confidence interval. bRepresenting the mean baseline body mass index (BMI) and 95% confidence interval. cRepresenting the mean baseline apnea hypopnea index (AHI) and 95% confidence interval. dRepresenting the mean baseline Epworth Sleepiness Scale (ESS) and 95% confidence interval.
The diagnoses of OSA in the included trials were all made by polysomnography monitoring. The majority of enrolled patients were documented as moderate to severe OSA (apnea hypopnea index ≥15). The mean age of participants in individual trials varied from 53.2 to 59.2 years. All included trials predominantly recruited males patients (mean percentage 74%). The mean BMIs were all beyond 30 kg/m2. The mean Epworth Sleepiness Scale ranged from 5 to 15 (total score 24), while the mean CPAP adherence ranged from 4.5 hours to 6.0 hours per night. There were no significant differences in the patients' main baseline characteristics between CPAP and control group.
Pooled Analysis
Pooled outcome was displayed as forest plot. As illustrated in the forest plot in Figure 2, the net change in 24‐hour ambulatory systolic blood pressure (SBP) and diastolic blood pressure (DBP) was significantly decreased in patients receiving CPAP therapy compared with those in the control group (change in SBP, −2.32 mm Hg, 95% confidence interval [CI], −3.65 to −1.00, P=.001; change in DBP, −1.98 mm Hg, 95% CI, −2.82 to −1.14, P<.001, respectively), without significant heterogeneity (I 2=0% for SBP, I 2=21% for DBP, respectively).
Figure 2.
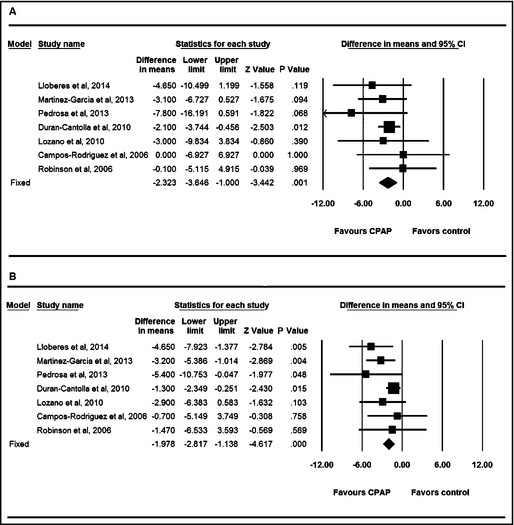
Forest plot for 24‐hour ambulatory systolic blood pressure (A) and diastolic blood pressure (B). CI indicates confidence interval; CPAP, continuous positive airway pressure.
Five studies with 691 patients were pooled for the subset analysis of diurnal and nocturnal BP change. Nocturnal BP was significantly decreased by CPAP treatment compared with control (pooled SBP change −2.74 mm Hg, 95% CI, −4.26 to −1.23, P<.001; pooled DBP change −1.75 mm Hg, 95% CI, −2.79 to −0.71, P=.001, respectively) (Table 3) without significant heterogeneity across trials (I 2=0%, I 2=0%, respectively).
Table 3.
Results of Subgroup Analysis of 24‐h Ambulatory Blood Pressure Change
| Pooled Effects | Trials, No. | Mode | Difference in Means | Standard Error | (Lower limit, Upper Limit) | P Value |
|---|---|---|---|---|---|---|
| Diurnal SBP change | 5 | Random | −3.58 | 2.28 | (−8.04, 0.89) | .117 |
| Diurnal DBP change | 5 | Random | −2.85 | 1.39 | (−5.58, −0.12) | .041 |
| Nocturnal SBP change | 5 | Fixed | −2.74 | 0.77 | (−4.26, −1.23) | <.001 |
| Nocturnal DBP change | 5 | Fixed | −1.75 | 0.53 | (−2.79, −0.71) | .001 |
| 24‐h SBP baseline drugs=0 | 1 | Fixed | −2.10 | 0.84 | (−3.74, −0.46) | .012 |
| 24‐h SBP baseline drugs>0 | 6 | Fixed | −2.73 | 1.14 | (−9.83, 3.83) | .016 |
| 24‐h SBP hypertension | 3 | Fixed | −1.81 | 0.78 | (−3.34, −0.29) | .020 |
| 24‐h SBP resistant hypertension | 4 | Fixed | −3.88 | 1.36 | (−6.55, −1.22) | .004 |
| 24‐h DBP baseline drugs=0 | 1 | Fixed | −1.30 | 0.54 | (−2.35, −0.25) | .015 |
| 24‐h DBP baseline drugs>0 | 6 | Fixed | −3.19 | 0.72 | (−4.59, −1.79) | <.001 |
| 24‐h DBP hypertension | 3 | Fixed | −1.28 | 0.51 | (−2.28, −0.27) | .013 |
| 24‐h DBP resistant hypertension | 4 | Fixed | −3.65 | 0.79 | (−5.19, −2.10) | <.001 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Although the pooled mean difference of diurnal DBP was found significantly to be −2.85 mm Hg (95% CI, −5.58 to −0.12, P=.041), the pooled diurnal SBP change was −3.58 mm Hg (95% CI, −8.04 to 0.89, P=.117) without statistical significance. A significant heterogeneity for diurnal SBP and DBP was found to be 89% and 88%, respectively.
Additionally, subgroup analysis for 24‐hour BP was performed to identify the magnitude of the effect of CPAP in different subgroups. The pooled results from trials of resistant hypertension17, 18, 19, 20 showed that OSA patients with resistant hypertension had a significantly greater reduction in 24‐hour SBP change (−3.88 mm Hg; 95% CI, −6.55 to −1.22, P=.004), as well as 24‐hour DBP change (−3.65 mm Hg; 95% CI, −5.19 to −2.10, P<.001) after CPAP intervention, as compared with controls.
In addition, in the seven RCTs, the patients who received antihypertensive medication at baseline had significantly more reduction in 24‐hour ambulatory SBP (−2.73 mm Hg; 95% CI, −4.96 to −0.51, P=.016) and 24‐hour ambulatory DBP (−3.19 mm Hg; 95% CI, −4.59 to −1.79, P<.001) than those in the control group.
By using further sensitivity analyses for the above pooled comparisons, there was no significant alteration of the statistical significance of these primary results by using a one‐study removing method.
Meta‐Regression
Meta‐regression demonstrated that effective CPAP pressure (P<.001), CPAP compliance (P<.001), patient age (P<.05), OSA severity (P<.001), treatment duration (P<.001), and baseline SBP (P<.001) were positively correlated with improvement in diurnal BP by CPAP treatment. The analysis also showed that CPAP compliance (P=.016), age (P=.024), and baseline SBP (P=.036) were positively associated with reduction in 24‐hour ambulatory DBP by CPAP (detailed in Table 4).
Table 4.
Results of Meta‐Regression of the 24‐h Ambulatory and Diurnal BP Change
| Explanatory Variable | Trials, No. | Patients, No. | 24‐h Ambulatory SBP (P Value) | 24‐h Ambulatory DBP (P Value) | Diurnal SBP (P Value) | Diurnal DBP (P Value) |
|---|---|---|---|---|---|---|
| Effective CPAP pressure, cm/H2O | 7 | 794 | .730 | .214 | <.001 | <.001 |
| CPAP compliance, Hours per night | 7 | 794 | .328 | .016 | <.001 | <.001 |
| Mean age, y | 7 | 794 | .328 | .024 | .004 | .003 |
| Male (decimal) | 7 | 794 | .681 | .324 | .460 | .792 |
| Baseline BMI, kg/m2 | 7 | 794 | .673 | .707 | .082 | .547 |
| Baseline AHI, No. | 7 | 794 | .941 | .649 | <.001 | <.001 |
| Baseline ESS, No. | 7 | 794 | .840 | .122 | .621 | .880 |
| Study duration, mo | 7 | 794 | .097 | .206 | <.001 | <.001 |
| Antihypertensive drugs, No. | 5 | 701 | .405 | .053 | <.001 | <.001 |
| Baseline SBP, mm Hg | 7 | 794 | .201 | .036 | <.001 | <.001 |
| Crossover vs parallel trial | 7 | 794 | .368 | .842 | NA | NA |
Abbreviations: AHI, apnea hypopnea index; BMI, body mass index; BP, blood pressure; CPAP, continuous positive airway pressure; DBP, diastolic blood pressure; ESS, Epworth Sleepiness Scale; NA, not available; SBP, systolic blood pressure.
Assessment of Publication Bias
To assess for the risk of publication bias, funnel plots of standard error and difference in means were constructed, with no significant asymmetry detectable, as shown in Figure 3.
Figure 3.
Funnel plot for 24‐hour ambulatory systolic blood pressure (A), 24‐hour ambulatory diastolic blood pressure (B), nocturnal systolic blood pressure (C), nocturnal diastolic blood pressure (D), diurnal systolic blood pressure (E), and diurnal diastolic blood pressure (F).
Discussion
Mechanism Related to BP‐Lowering Effect With CPAP
As a result of pharyngeal collapse, OSA induces intermittent hypoxemia and CO2 retention, with oxygen saturation dropping to as much as <60% in severe cases This leads to disordered autonomic and hemodynamic responses during sleep, particularly featured by chemoreflex‐mediated sympathetic activity and peripheral blood vessel constriction effect, consequently resulting in a high level of blood pressure through the night.22, 23 In addition, induced by recurrent hypoxemia, vasoactive substance such as endothelin and angiotensin, or other pathological effects such as oxidative stress, systemic inflammation and insulin resistance may all contribute to the upregulation of blood pressure.24, 25, 26, 27 To treat the obstructive apneas, CPAP can hold the collapsed airway open, improve the patients' ventilation situation by which CPAP can attenuate subsequent overactivity of sympathetic nervous axis and relevant disorders, and thus decrease BP during sleep.28
Previous Studies and Findings of the Present Meta‐Analysis
Which patient group benefits the most by BP improvement from CPAP therapy remains a subject of interest. Existing evidence suggested that there were viable effects of CPAP on BP in patients with OSA. Previous meta‐analyses10, 11, 12 enrolling a considerable number of normotensive patients showed a near 2‐mm Hg BP reduction after CPAP treatment. By including OSA patients complicated with hypertension, the pooled results of the present meta‐analysis demonstrated a weighted mean reduction in 24‐hour ambulatory SBP of 2.32 mm Hg and DBP of 1.98 mm Hg, suggesting a greater magnitude in BP reduction among hypertensive and OSA patients.
Furthermore, subgroup analysis including trials of resistant hypertension17, 18, 19, 20 showed that OSA patients with resistant hypertension had a more substantial reduction in 24‐hour SBP and 24‐hour DBP change after CPAP intervention as compared with overall patients. This result was also echoed by early studies.29 It was extrapolated that CPAP may be a first‐choice adjunctive treatment strategy for OSA patients with refractory hypertension.
Effective CPAP therapy and antihypertensive pharmacotherapy are both considered to be of importance in the improvement of BP control.30 In the systematic review of included trials, six studies13, 14, 17, 18, 19, 20 in which the patients received antihypertensive medication appeared to achieve a greater BP reduction than those without antihypertensive drugs enrolled in one study.16 This synergic efficacy was also shown by our subgroup meta‐analysis, indicating that the combination therapy of CPAP and antihypertensive drug therapy should be recommended as a standard treatment for OSA and hypertension.
The present meta‐analysis revealed that CPAP had significant BP‐lowering effects mainly on nocturnal SBP; however, its impact on diurnal SBP seemed modest. Mechanism research has showed that CPAP continuously supports the collapsed airway and prevents hypoxia events, thus balancing autonomic nervous activity and regulating BP during the night.7, 31 Immediate nocturnal BP improvement can be achieved by the direct effect of CPAP in minutes,29 whereas the regulation of daytime BP appears to be more multifactorial, involving patients' physical activity, emotional stress, dietary habits, and smoke and alcohol consumption.30
To some extent, the effect of nocturnal BP regulation by CPAP treatment may also have an indirect impact on the increased daytime BP. Our meta‐regression analysis further demonstrated that effective CPAP pressure, better CPAP compliance, elder age, more sever OSA, longer treatment duration, and higher baseline SBP may be positive predictors of greater reduction of daytime BP by CPAP treatment. These findings were partly mirrored by previous results.16, 19 Our meta‐regression also found that CPAP compliance, age, and baseline SBP were positively correlated with the efficacy of CPAP treatment on control of 24‐hour DBP, and no factors were associated with the improvement of 24‐hour SBP.
Study Strengths and Limitations
There are several strengths in the current meta‐analysis. By adopting predefined study selection criteria, all included trials were prospective randomized studies whose methodological quality was considered to be relatively high. Meta‐analysis of randomized trials can provide confident evidence if the between‐study bias is appropriately controlled. Moreover, the main baseline characteristics such as patient demographic profile were comparable between the treatment and control groups, and the time range of the included trials performed was within the recent decade. These features suggest a low risk of selection bias across trials and show an adequate power of our analysis.
Several limitations should be addressed when interpreting our results. First, most of the studies had a relative small sample size, and the risk of potential bias could not be completely excluded in view of intrinsic difference in study design. Second, the intervention applied in the control group was not univariate and included sham‐CPAP or no CPAP treatment; however, data from several studies17, 18, 19 showed that the adherence of sham‐CPAP was relatively low during follow‐up. Thus, a sham‐CPAP intervention may be unlikely to alter our primary results as relative to no CPAP. Because of a limited follow‐up period and a lack of individual data, hard endpoints such as incidence of cardiovascular events, mortality, or other adverse events were not analyzed in the current study.
Conclusions
For OSA and hypertensive patients, CPAP treatment results in a significant reduction in 24‐hour ambulatory BP, which is characterized by nocturnal BP. Those with resistant hypertension or taking antihypertensive pharmacotherapy are shown to have more substantial BP reduction. CPAP adherence, age, and baseline SBP are positively correlated with the decrease of 24‐hour DBP. In addition to aforementioned factors, effective CPAP pressure, OSA severity, and treatment duration may also predict improvement in diurnal BP control. None are associated with the reduction in 24‐hour SBP.
Disclosures
This work was partly supported by Sunshine Cardiovascular Research Foundation of Chinese Medical Doctor Association.
Conflicts of Interest
None.
J Clin Hypertens (Greenwich). 2015;17:215–222. DOI: 10.1111/jch.12472. © 2015 Wiley Periodicals, Inc.
Drs Hu, Fan, and Chen contributed equally to this paper.
References
- 1. Devulapally K, Pongonis RJ, Khayat R. OSA: the new cardiovascular disease: part II: overview of cardiovascular diseases associated with obstructive sleep apnea. Heart Fail Rev. 2009;14:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. [DOI] [PubMed] [Google Scholar]
- 3. Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis. 2009;51:434–451. [DOI] [PubMed] [Google Scholar]
- 4. Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea‐hypopnea and related clinical features in a population‐based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–689. [DOI] [PubMed] [Google Scholar]
- 5. Pataka A, Riha RL. Continuous positive airway pressure and cardiovascular events in patients with obstructive sleep apnea. Curr Cardiol Rep. 2013;15:385. [DOI] [PubMed] [Google Scholar]
- 6. Parati G, Lombardi C, Hedner J, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens. 2012;30:633–646. [DOI] [PubMed] [Google Scholar]
- 7. Abboud F, Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest. 2014;124:1454–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta‐analysis of placebo‐controlled randomized trials. Arch Intern Med. 2007;167:757–764. [DOI] [PubMed] [Google Scholar]
- 9. Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta‐analysis of randomized controlled trials. Lung. 2007;185:67–72. [DOI] [PubMed] [Google Scholar]
- 10. Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta‐analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fava C, Dorigoni S, Dalle VF, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta‐analysis. Chest. 2014;145:762–771. [DOI] [PubMed] [Google Scholar]
- 12. Schein AS, Kerkhoff AC, Coronel CC, et al. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta‐analysis with 1000 patients. J Hypertens. 2014;32:1762–1773. [DOI] [PubMed] [Google Scholar]
- 13. Robinson GV, Smith DM, Langford BA, et al. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27:1229–1235. [DOI] [PubMed] [Google Scholar]
- 14. Campos‐Rodriguez F, Grilo‐Reina A, Perez‐Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo‐controlled trial. Chest. 2006;129:1459–1467. [DOI] [PubMed] [Google Scholar]
- 15. Barbe F, Duran‐Cantolla J, Capote F, et al. Long‐term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–726. [DOI] [PubMed] [Google Scholar]
- 16. Duran‐Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. [DOI] [PubMed] [Google Scholar]
- 17. Lozano L, Tovar JL, Sampol G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161–2168. [DOI] [PubMed] [Google Scholar]
- 18. Pedrosa RP, Drager LF, de Paula LK, et al. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144:1487–1494. [DOI] [PubMed] [Google Scholar]
- 19. Martinez‐Garcia MA, Capote F, Campos‐Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–2415. [DOI] [PubMed] [Google Scholar]
- 20. Lloberes P, Sampol G, Espinel E, et al. A randomized controlled study of CPAP effect on plasma aldosterone concentration in patients with resistant hypertension and obstructive sleep apnea. J Hypertens. 2014;32:1650–1657. [DOI] [PubMed] [Google Scholar]
- 21. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–773. [DOI] [PubMed] [Google Scholar]
- 22. Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic‐nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. [DOI] [PubMed] [Google Scholar]
- 23. Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol (1985). 1989;67:2101–2106. [DOI] [PubMed] [Google Scholar]
- 24. Gjorup PH, Sadauskiene L, Wessels J, et al. Abnormally increased endothelin‐1 in plasma during the night in obstructive sleep apnea: relation to blood pressure and severity of disease. Am J Hypertens. 2007;20:44–52. [DOI] [PubMed] [Google Scholar]
- 25. Meier‐Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C‐reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. [DOI] [PubMed] [Google Scholar]
- 26. Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta‐analysis. J Clin Sleep Med. 2013;9:1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zamarron C, Valdes CL, Alvarez‐Sala R. Pathophysiologic mechanisms of cardiovascular disease in obstructive sleep apnea syndrome. Pulm Med. 2013;2013:521087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2013. Sep 24[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29. Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21:241–247. [DOI] [PubMed] [Google Scholar]
- 30. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 31. Baguet JP, Barone‐Rochette G, Tamisier R, et al. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol. 2012;9:679–688. [DOI] [PubMed] [Google Scholar]


