Abstract
Exaggerated blood pressure (BP) response to exercise predicts future hypertension. However, there is considerable lack of understanding regarding the mechanism of how this abnormal response is generated, and how it relates to the future establishment of cardiovascular disease. The authors studied 82 healthy male volunteers without cardiovascular risk factors. The participants were categorized into two age‐matched groups depending on their exercise systolic BP (ExSBP) rise after 3 minutes of exercise using a submaximal step test: exaggerated ExSBP group (hyper‐responders [peak SBP ≥180 mm Hg]) and low ExSBP responder group (hypo‐responders [peak SBP <180 mm Hg]). Forearm venous occlusion plethysmography and intra‐arterial infusions of acetylcholine (ACh), NG‐monomethyl‐L‐arginine (L‐NMMA), sodium nitroprusside (SNP), and norepinephrine (NE) were used to assess vascular reactivity. Proximal aortic compliance was assessed with ultrasound, and neurohormonal blood sampling was performed at rest and during peak exercise. The hyper‐responder group exhibited a significantly lower increase in forearm blood flow (FBF) with ACh compared with the hypo‐responder group (ΔFBF 215% [14] vs 332.3% [28], mean [standard error of the mean]; P<.001), as well as decreased proximal aortic compliance. The vasoconstrictive response to L‐NMMA was significantly impaired in the hyper‐responder group in comparison to the hypo‐responder group (ΔFBF −40.2% [1.6] vs −50.2% [2.6]; P<.05). In contrast, the vascular response to SNP and NE were comparable in both groups. Peak exercise plasma angiotensin II levels were significantly higher in the hyper‐responder group (31 [1] vs 23 [2] pg/mL, P=.01). An exaggerated BP response to exercise is related to endothelial dysfunction, decreased proximal aortic compliance, and increased exercise‐related neurohormonal activation, the constellation of which may explain future cardiovascular disease.
Atherosclerotic vascular disease is the major cause of death in Western societies. There is evidence suggesting that endothelial dysfunction occurs early and is the precursor to atheroma formation. Indeed, children and young adults with a family history of cardiovascular disease who may have a genetic predisposition to these diseases have been reported to have impaired endothelial function.1, 2 Importantly, endothelial dysfunction in the coronary circulation predicts future cardiac adverse events.3 Most, if not all, cardiovascular risk factors are associated with endothelial dysfunction, and risk factor modification leads to improvement in vascular function.
Since the process of atherosclerosis begins early in life,4 and endothelial dysfunction contributes to atherogenesis and precedes the development of morphological vascular changes, it is important to detect early functional structural changes.5 Indeed, for the cardiovascular disease burden to be reduced, primary prevention measures may be best targeted at individuals without overt vascular diseases, but with demonstrable endothelial dysfunction. There is, however, no simple method of assessing vascular endothelial function. We have previously shown that in hypertensive patients an exaggerated blood pressure (BP) response to a simple three‐minute exercise step test predicted the presence and degree of endothelial dysfunction, but these patients already had cardiovascular risk factors, thus limiting the applicability of the test to those with established atherosclerotic disease.6
On the other hand, it has been suggested that an exaggerated BP response to exercise occurring in patients with normal resting BP is predictive of risk for new‐onset hypertension.7 However, the mechanism(s) associated with exaggerated BP and by extension, contributing to future hypertension, remain poorly understood. We therefore set out to explore whether exaggerated exercise BP responses may be a surrogate marker of overall vascular health in healthy young men without cardiovascular risk factors. We also sought to explore the mechanism(s) behind the genesis of exaggerated BP responses during exercise and their relationship to future cardiovascular disease.
Methods
Participants and Design
From a local newspaper advertisement we screened 110 healthy men over a period of 2 months. All volunteers underwent baseline screening including health questionnaires, full medical examination, 24‐hour ambulatory BP monitoring (ABPM), fasting lipid profile, and glucose. Exclusion criteria included history of hypertension, coronary artery disease, diabetes, hyperlipidemia (fasting total cholesterol >4.5 mmol/L), renal impairment, cerebrovascular and peripheral vascular diseases, and tobacco smoking. We also excluded individuals with any first‐degree relative with any of the above diseases as well as individuals who engaged in sustained (more than twice weekly) physical exercise and athletes.
Suitable participants were then selected based on their exercise systolic BP (ExSBP) responses during a simple light weighted exercise step test.6, 8 The aim was to identify two groups of participants with contrasting ExSBPs. We defined hypo‐responders (low exercise BP) as those with peak ExSBP of <180 mm Hg and hyper‐responders (high exercise BP) as those with peak ExSBP of ≥180 mm Hg8 at peak exercise.
Written informed consent was obtained from each study participant, and the study had the approval of the Tayside committee on medical research ethics.
Procedures
Ambulatory BP Measurement (Screening Period)
Twenty‐four–hour ABPM took place at screening and before enrollment into the study to ensure normal BP. Normal daytime systolic and diastolic ambulatory BP was considered <135/85 mm Hg.
ABPM was recorded using SpaceLabs model 90,207 recorders (Redmond, WA). Measurements of BP were taken every 15 minutes during the daytime (8 am to 10 pm) and every 30 minutes at night (10 pm to 8 am). Study participants were asked to continue their habitual daily life activities to reflect “active” BP during ABPM.
All eligible patients were invited to attend our institution for the study visit and after an overnight fast. All procedures related to the study took place on the same day in the following chronological order.
Resting and Exercise BP Measurement (Study Visit)
The patients rested in the seated position for at least 30 minutes, and resting and exercise BP assessments were performed. Resting BP was measured (mean of three measurements) before exercise in the seated position using a validated semiautomatic oscillometric monitor (OMRON 705CP, Matsusaka, Japan). ExSBP was assessed during exercise step test.6, 9 Briefly, this was a submaximal exercise step test using a step height of 17.5 cm and a stepping rate of 92 per minute set using a metronome. ExSBP was measured after 3 minutes of exercise, while the patient was still stepping, using a validated automated exercise BP monitor (Tango, SunTech Medical Instruments, Morrisville, NC).9 Resting seated BP and heart rate were measured after 3 minutes of exercise cessation to obtain recovery values.
Echocardiography and Proximal Aortic Compliance Assessment (Study Visit)
Patients attended our laboratory early in the morning following a 12‐hour overnight fast. All studies took place in a temperature‐controlled (21°C) room after the assessment of exercise BP. Echocardiographic examination took place first, and all studies were conducted by a single operator (NT). All echocardiographic parameters were measured in triplicate and averaged over three cardiac cycles. The following indices of left ventricular (LV) dimensions were recorded/derived from M‐mode measurements obtained at the level of the papillary muscles in the short‐axis view: LV internal diameter in diastole, LV internal diameter in systole, fractional shortening, and LV mass index. The left atrial size was measured in the parasternal long‐axis view (anteroposterior). To assess diastolic function, transmitral Doppler indices (peak velocities of the early E and late A waves and E/A ratio) and E deceleration time (E DT) and color M‐mode flow propagation velocity (CMMFPV) were measured as previously described.10 To assess aortic wall mechanical properties (compliance) we employed the ultrasonic method described by Stefanadis11 and Dernellis.12 The following aortic wall mechanically derived parameters were assessed: (1) Aortic distensibility was calculated from the formula: ΔA: (A×PP)=π×[(AoS/2)2−(AoD/2)2]: [π×(AoD/2)2×PP], with A denoting the cross‐sectional luminal area and PP the pulse pressure.13 A was estimated as the product of π by r2; where r was the AoS/2 or AoD/2. (2) Aortic stiffness index (β) was calculated13 as β=ln (SBP/DBP)/(AoS–AoD)/AoD (pure number), where SBP is systolic BP and DBP is diastolic BP. A single investigator (NT) undertook all measurements.
Resting and Exercise Blood Sampling (Study Visit)
To obtain resting and peak exercise blood samples, an intravenous indwelling cannula was inserted into a large antecubital fossa vein attached to a three‐way stock port and connected to a short Y connector tube to allow easy withdrawal of blood. Blood sampling for plasma electrolytes, aldosterone and plasma renin activity (PRA), norepinephrine, and angiotensin II (AngII) was performed after resting BP assessment and in an upright position. Repeated sampling for the same blood parameters took place immediately after cessation of the exercise, and also in an upright position (henceforth peak). Blood samples were placed in chilled lithium‐heparin tubes separated and centrifuged immediately at 3000 rpm at 4°C, and the plasma was separated and stored at −70°C until assayed as a batch. Catecholamines were extracted through adsorption on alumina after the addition of dihydroxybenzylamine used as an internal standard. After elution from the alumina through the addition of 0.2 mol/L HClO4, the catecholamines were then separated and measured by high‐performance liquid chromatography.14
Angiotensin I and II were measured by specific radioimmunoassays after SepPak extraction of plasma as previously described.15 Finally, the methods as well as reproducibility of the rest of the laboratory assays have been previously described.6
Vascular Studies (Study Visit)
All vascular studies took place after the exercise BP assessment and were conducted by the same operator (NT) following an overnight fast and after echocardiography. Alcohol and caffeine‐containing beverages were avoided for at least 24 hours before the day of the study. Following a supine rest of 30 minutes, the nondominant brachial artery was cannulated with a 27‐gauge steel needle mounted onto a 16‐gauge polyethylene epidural catheter under local anesthesia with 1% lidocaine. Forearm blood flow (FBF) was measured simultaneously in both arms by strain‐gauge venous occlusion plethysmography as previously described.16 BP and heart rate were noninvasively recorded in the noninfused (control) arm before each infusion (OMRON, HEM‐705CP; Omron Healthcare, Inc, Lake Forest, IL).
Hemodynamic Measurements and Drug Infusions
FBF was measured during the last 2 minutes after each infusion period, and was expressed as mL/min per 100−1mL forearm volume according to the Whitney method.17 Resting baseline FBFs were obtained at least 30 minutes after needle placement to ensure that the blood flow in the cannulated arm had stabilized. After resting baseline FBF measurements, each study patient received intra‐arterial infusions of incremental doses of acetylcholine [Ach] (Miochol, CIBA Vision, Atlanta, GA), sodium nitroprusside [SNP] (David Bull Laboratories, Sydney, Australia), and NG‐monomethyl‐L‐arginine N (Clinalfa Merck, Bubendorf, Switzerland). The muscarinic agonist ACh was used to assess endothelium‐dependent vasodilatation (stimulated nitric oxide [NO] release), while SNP, an exogenous source of NO, was used to assess endothelium‐independent vasodilatation. Cumulative dose‐response curves were constructed after infusions of ACh 25 nmol/min, 50 nmol/min, and 100 nmol/min and SNP 4.2 nmol/min, 12.6 nmol/min, and 37.8 nmol/min; each incremental dose was for 5 minutes. The endothelial‐dependent vasoconstriction was assessed using the competitive NO synthase antagonist NG‐monomethyl–L‐arginine (L‐NMMA) infused at 1 μmol/min, 2 μmol/min, and 4 μmol/min, again, each for 5 minutes. Norepinephrine (60 nmol/L, 120 nmol/L, and 240 nmol/L), each infused for 5 minutes, was used as a control vasoconstrictor (endothelium‐independent vasoconstrictor). An identical protocol of drug infusion order was kept for all study visits and patients were unaware of the substance infused. FBF (mL per 100 mL of forearm volume) was expressed as percentage change in FBF from baseline immediately preceding each drug infusion. Finally, a random sequence to infuse the vasoactive substances to investigate the L‐arginine‐NO pathway would not have been possible; L‐NMMA produces prolonged vasoconstriction, hence it should ideally be infused last in the sequence.18 Norepinephrine always preceded L‐NMMA infusion in our study, but there is no evidence to suggest that this choice of infusion order might have affected the sensitivity to subsequent infusion, albeit the return to baseline was ensured.19
Statistical Analysis
Five recordings of FBF at each infusion step were measured for both infused and control arms. Because BP and baseline forearm flow did not vary significantly during visits, the FBF ratio between infused and control arms in response to drugs was expressed as a percentage of the ratio measured during the placebo period [ΔFBF% (mean) standard error)]. Clinical characteristics between groups were compared by unpaired t test, while FBF measurements for individual treatments were compared using two‐way analysis of variance. Wilcoxon rank‐sum test was used when variables were not normally distributed. Factors potentially affecting responses to ACh in the two groups were identified by linear regression analysis. In a multivariate model, the following were used as predictors of maximum ΔFBF% response to ACh (100 nmol/min): age, daytime and 24‐hour ambulatory SBP and DBP, plasma potassium, serum fasting total and low‐density lipoprotein cholesterol, E/A ratio, CMMFPV, and plasma resting and exercise neurohormones. A two‐tailed P<.05 was considered significant.
Spearman's correlation coefficients were calculated to assess univariate associations between both ExSBP to exercise testing and duration of exercise with diverse cofactors. Furthermore, we implemented logistic multivariable regression models after adjustment for significant cofactors identified from univariate analyses, setting as a dependent variable the ExSBP to exercise testing.
Reproducibility of the Vascular Studies and Exercise BP
Sensitivity and reproducibility of the methods (forearm venous occlusion plethysmography and echocardiography) performed in our laboratory have been previously reported.6, 10 The coefficient of variation for measurements of aortic wall–related mechanical properties (distensibility index) was 3%. Since all patients underwent two consecutive exercise step tests over short periods of time (14±4 days), the intraclass correlation coefficient for ExSBP was 0.6.
Results
Baseline Anthropometric and Hemodynamic Characteristics
Six patients were excluded after the ABPM‐led screening period because of elevated BP. Three patients also failed to attend after their screening visit. A total of 44 of 110 screened patients (age range, 21–40 years) exhibited exaggerated SBP response to exercise (hyper‐responders) during screening. Thus, the final study population consisted of 41 hyper‐responders and 41 hypo‐responders. Despite the different ExSBP values, the two groups had similar clinic and daytime ambulatory BP values and were well‐matched for anthropometric characteristics (Tables 1 and 2). There were no significant differences between groups with regards to fasting cholesterol levels and electrolytes (Table 3). In addition, the baseline (resting) neurohormonal status was not different between the two groups (Table 3).
Table 1.
Baseline Demographic and Morphological Characteristics of the Study Population
| Parameter | Hyper‐Responders | Hypo‐Responders | P Value |
|---|---|---|---|
| No. | 41 | 41 | |
| Age, y | 34 (7) | 33 (8) | .08 |
| Height, cm | 179 (5.2) | 177 (5.5) | .3 |
| Weight, kg | 81 (9.5) | 77 (8) | .2 |
| BMI, kg/m2 | 26 (0.8) | 25 (0.9) | .2 |
| Forearm circumference/length, cm | 28.5 (2.4)/31 (1.5) | 28.3 (1.7)2/30 (1.6) | .7/.5 |
| Absolute baseline FBF, mL−1100−1 forearm volume | 3.2 (0.8) | 3.5 (1.9) | .3 |
Abbreviations: BMI, body mass index; FBF, forearm blood flow. Data are expressed as mean (standard deviation) except for FBF, which is expressed as mean (standard error).
Table 2.
Hemodynamic Parameters of the Study Population
| Parameter | Hyper‐Responders | Hypo‐Responders | P Value |
|---|---|---|---|
| Daytime ABP, mm Hg | 125 (6)/73 (8) | 126 (7)/75 (5) | .6 |
| Resting SBP, mm Hg | 127 (9) | 125 (11) | .7 |
| Resting DBP, mm Hg | 76 (9) | 77 (8) | .7 |
| ExSBP, mm Hg | 190 (18) | 141 (26) | <.001 |
| Rest/exercise HR, beats per min | 70 (10)/108 (12) | 69 (9)/104 (11) | .6/.3 |
| Recovery SBP, mm Hg | 135 (13) | 121 (11) | .02 |
| Recovery HR, beats per min | 75 (9) | 73 (17) | .8 |
Abbreviations: ABP, ambulatory blood pressure; DBP, diastolic blood pressure; ExSBP, exercise systolic blood pressure; HR, heart rate; SBP, systolic blood pressure. Data are expressed as mean (standard deviation).
Table 3.
Biochemical and Neurohormonal Parameters of the Study Population
| Parameter | Hyper‐Responder | Hypo‐Responder | P Value |
|---|---|---|---|
| Serum potassium, mmol/L | 4.3 (0.2) | 4.2 (0.2) | .6 |
| Plasma glucose, mmol/L | 4.5 (0.2) | 4.8 (0.5) | .06 |
| Serum creatinine, μmol/L | 96.6 (7) | 97 (10) | .8 |
| Total cholesterol, mmol/L | 4.2 (0.8) | 4 (1) | .3 |
| Plasma LDL, mmol/L | 3 (0.8) | 3.2 (0.9) | .3 |
| Serum triglycerides, mmol/L | 1.5 (0.2) | 1.3 (0.2) | .1 |
| Plasma urate, ng/mL/h | 0.31 (0.04) | 0.39 (0.3) | .4 |
| Plasma aldosterone, pg/mL | 35 (8) | 38 (9) | .1 |
| Plasma renin activity, ng/mL/h | 0.4 (0.02) | 0.4 (0.06) | .4 |
| Plasma norepinephrine, pg/mL | 711 (211) | 650 (156) | .3 |
| Plasma epinephrine, pg/mL | 49 (21) | 53 (22) | .3 |
| Plasma angiotensin II, pg/mL | 13 (2) | 15 (3) | .1 |
| Peak plasma aldosterone, pg/mL | 47 (8) | 49 (11) | .1 |
| Peak plasma renin activity, ng/mL/h | 0.9 (0.1) | 1 (0.1) | .3 |
| Peak plasma norepinephrine, pg/mL | 890 (120) | 911 (140) | .3 |
| Peak plasma epinephrine, pg/mL | 64 (11) | 72 (22) | .3 |
| Peak plasma angiotensin II, pg/mL | 31 (1) | 23 (1.9) | .01 |
Abbreviation: LDL, low‐density lipoprotein. Data are expressed as mean (standard deviation).
Effects of Endothelium‐Dependent Vasoactive Substances on FBF (Ach and L‐NMMA)
ACh produced a marked dose‐dependent increase in FBF in both groups (Figure 1). However, the hyper‐responder group had significantly less endothelium‐dependent vasodilatation (ΔFBF% 119 [14], 173 [19], and 215 [14] of the baseline value) in response to ACh (25, 50, 100 nmol/min, respectively) compared with the hypo‐ responders (226 [30], 255 [31], 332.3 [28] mean [standard error of the mean]; P<.001 for the whole dose range). Similarly, the vascular vasoconstrictive response to L‐NMMA was also significantly blunted in the hyper‐responders compared with hypo‐responders (max ΔFBF% −40.2 [1.6] vs −50.2 [2.6]; P<.05 for the whole dose range) (Figure 2).
Figure 1.
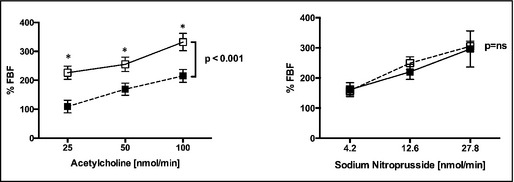
Percentage changes in flow ratio (infused/noninfused) from baseline preceding each drug infusion for three dose levels of acetylcholine (ACh) and sodium nitroprusside (SNP). (○) indicates hypo‐responders and (•) indicates hyper‐responders. *P<.05. **P<.001.
ExSBP and age were significant independent predictors (P<.001 and P<.01, respectively) of the maximum ΔFBF% to ACh (index of vascular reactivity) in a stepwise multivariate analysis. Although recovery ExSBP responses were significantly different between the two groups (Table 3) and did not reach statistical significance as predictor of the maximum ΔFBF% to ACh either on univariate of multivariate model of analysis.
Effects of Endothelium‐Independent Vasoactive Substances on FBF (SNP and NE)
The responses to sodium nitroprusside and norepinephrine (NO‐independent responses) were not different between groups, indicating that the forearm resistance arteries in these two groups were functionally, rather than structurally, different (P=.56 and P=.55, respectively) (Figure 2).
Figure 2.

Percentage changes in flow ratio (infused/noninfused) from baseline preceding each drug infusion for three dose levels of NG‐monomethyl‐L‐arginine (L‐NMMA) and norepinephrine (NDR). (○) indicates hypo‐responders and (•) indicates hyper‐responders. *P<.05. **P<.001.
Effects of Exercise on Neurohormonal Status and Aortic Wall Mechanical Properties
Resting AngII, aldosterone, and renin levels were similar in both groups (Table 3). However, the hyper‐responder group had significantly higher levels of peak exercise AngII in comparison to hypo‐responders (Table 3). Similarly, there was a trend for higher peak aldosterone plasma levels (Table 3). There were no differences in peak norepinephrine plasma levels (Table 3).
Although there were no significant differences in LV wall thickness and ejection fraction, the indirect indices of diastolic function in the ExSBP group increased myocardial stiffness as evidenced by a trend toward higher CMMFPV (Table 4).
Table 4.
Echocardiographic and Aortic Compliance Parameters of the Study Population
| Parameter | Hyper‐Responders | Hypo‐Responders | P Value |
|---|---|---|---|
| LVIDs, mm | 4.5 (0.3) | 4.8 (0.4) | .6 |
| LVIDs, mm | 3.3 (0.3) | 3.5 (0.4) | .6 |
| IVSd, cm | 1 (0.1) | 1.06 (0.1) | .3 |
| PWd, cm | 0.8 (0.1) | 0.9 (0.1) | .2 |
| LA, cm | 2.9 (0.3) | 2.8 (0.6) | .5 |
| Emax, cm/s | 72 (14) | 75 (17) | .1 |
| Amax, cm/s | 48 (9) | 50 (7) | .2 |
| E/A ratio | 1.6 (0.3) | 1.7 (0.5) | .1 |
| E DT, cm/s | 200 (33) | 190 (15) | .1 |
| CMMFVP, cm/s | 41 (9) | 46 (6) | .06 |
| Aortic distensibility, mm Hg−110−3 | 3.1 (0.5) | 2.7 (0.6) | .01 |
| Aortic index, β | 8 (1.1) | 7.7 (1.3) | .02 |
Abbreviations: CMMFV, color M‐mode flow propagation velocity; E DT, E deceleration time; IVSd, interventricular septal at end‐diastole; LA, left atrium; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; PWd, posterior wall thickness at end‐diastole. Data are expressed as mean (standard deviation). Bold values indicate significance.
Lastly, hyper‐responders had lower aortic distensibility, which was paralleled by similarly increased aortic stiffness index, suggesting reduced arterial elasticity and proximal aortic compliance in this group (Table 4).
Discussion
Our findings add three important pieces of information to the existing knowledge of the etiology of exaggerated BP response during exercise. We found that an exaggerated SBP response to submaximal exercise was closely related to underlying systemic endothelial dysfunction, reduced proximal aortic compliance, and high exercise‐induced AngII levels in otherwise healthy sedentary volunteers.
Exaggerated Exercise BP and Endothelial Dysfunction
In the resting state, shear stress causes a continuous (basal) NO release, modulating the peripheral vascular tone in favor of a vasodilated state. Normally, the peripheral vascular resistance falls during exercise as a result of peripheral vasodilatation. This is partly attributable to enhanced NO release during exercise via vascular wall shear stress.20 It is likely that reduced NO bioactivity or endothelial dysfunction limits exercise peripheral vasodilatation that normally buffers against an exaggerated rise in ExSBP. Given the logistic difficulties in assessing endothelial function during acute exercise, we used the resting NO bioactivity status as a benchmark of vascular health. The hypothesis was that resting (baseline) systemic endothelial dysfunction would adversely affect exercise hemodynamics contributing to exaggerated BP response. In our study, we found that patients with ExSBP and without overt cardiovascular risk factors had endothelial dysfunction as evidenced by reduced NO bioactivity. Our findings extend the observations of other groups linking ExSBP to systemic endothelial dysfunction albeit with different methods and/or vascular beds assessed.21, 22 Although it is likely that additional factors contribute to the genesis of ExSBP, the fact that endothelial dysfunction may be a major contributor is not a surprise. Indeed, an exaggerated BP response to exercise has also been reported to be a powerful predictor of future hypertension in children and young adults, and it is also an independent marker of cardiovascular morbidity and mortality in middle‐aged men.23, 24, 25 Conceivably, in younger persons, exaggerated ExSBP results primarily from a failure to reduce total peripheral resistance during exercise, and may be related to early structural vascular changes that precede progression to hypertension. The fact that endothelial dysfunction is a systemic condition may explain why peripheral endothelial function (microvascular and macrovascular) correlates with endothelial function in the coronary arteries.26
Exaggerated Exercise BP, Arterial Stiffness, and Diastolic Dysfunction
Aerobic exercise dilates muscular arteries and reduces arterial pressure augmentation,27 an effect that will ultimately enhance ventricular‐vascular coupling and reduce the pressure load on the left ventricle. In our study, which extends the findings of Fazio and colleagues,28 we found that aortic distensibility was decreased and this was paralleled by reduced aortic compliance in healthy men with ExSBP. A similar close relationship of progressive changes in arterial stiffness and future hypertension in young normotensives was also reported by Dernellis and colleagues12 using the same ultrasound‐based method of aortic compliance assessment. It is possible that an unfavorable interplay between impaired arterial vasodilation secondary to reduced NO bioactivity and increased arterial stiffness leads to relative ventricular‐vascular coupling failure and increased vascular resistance, which is not counterbalanced by flexible vascular compliance. Following that concept, intermittent “exposure” to high BP during exercise coupled with reduced compliance could lead to increased myocardial stiffness, myocardial geometry, and possibly ventricular dysfunction.29 Indeed, the findings of our study of a trend towards increased CMMFV––an index of diastolic function and myocardial relaxation––may provide additional weight to this hypothesis. It is possible that patients with ExSBP move through the spectrum of cardiovascular disease severity depending on their age; at a younger age, only subtle cardiovascular changes can be detected, while later on more obvious target organ damage will develop.30 Intuitively, the latter is the group of patients who will require early recognition.
Exaggerated BP Resting and Peak Exercise Neurohormonal Activation
The sympathoadrenal and renin‐angiotensin systems play an important role in BP control and regulation of cardiovascular function during exercise. The renin‐angiotensin and sympathoadrenal systems do not operate independently but mutually interact with each other in accomplishing their cardiovascular regulatory function. An exaggerated BP response to exercise has been thought to be related to excess stimulation of the sympathetic nervous system and increased activation of the renin‐angiotensin‐aldosterone system.30, 31, 32 In particular, β‐adrenergic receptor sensitivity (βAS) seems to play an important role in eliciting both the vascular as well as myocardial response to exercise, as evidenced by an increased number of activated βAS lymphocytes mediated by a β2AR mechanism.33 In this study, we found that AngII levels at peak exercise were significantly higher at peak exercise in patients with exaggerated exercise BP response compared with hypo‐responders. This finding is intriguing, and extends the findings of Shim and colleagues.32 Although we cannot ascertain whether higher AngII plasma levels are caused by increased release (whether vascular or systemic) or reduced degradation, this certainly indicates a wider neurohormonal dysregulation in determining ExSBP response. All plasma neurohormones increased significantly at peak exercise in both groups; however, not to the extent reported in other studies.32, 34 The reason for this discrepancy may well lie in the magnitude of exercise (in terms of aerobic capacity) during submaximal step testing, which is equivalent to routine daily activities and might not be sufficient enough to increase systemic catecholamine concentration to the point of reaching statistical significance.
Study Limitations
As mentioned in the discussion, it is likely that exaggerated BP response to exercise has multifactorial components, and several other factors may contribute variably to its genesis. We focused on stimulated and basal NO release to assess endothelial function, large artery stiffness, and basal/peak exercise neurohormonal activation. It is possible, however, that other factors such endothelium‐derived hyperpolarizing factor,35 increased sympathetic outflow, asymmetric dimethyl arginine,36 and insulin levels37 may have contributed either collectively or in isolation to ExSBP and certainly this merits further investigation. Additionally, we considered only few of the possible mechanisms affecting both vascular and myocardial responsiveness to exercise. It is possible that more complex interactions might have also taken place, such as an interplay between genetic polymorphism and NO synthase activity38 or G protein–coupled receptor kinases in the regulation of vascular tone.33 We did not investigate the above primarily because of the relatively small patient sample size.
Additionally, the role of endothelin in determining vascular responses both at rest and during exercise has not been examined. We used a standardized submaximal exercise method (step test) and SBP to assess BP responses to exercise. Submaximal exercise is not thought to be effort‐related and is relatively independent of physical fitness, therefore appealing to a wider population.39 Indeed, the workload produced during the three‐minute step test (five metabolic equivalents [Mets]) mimics the activities of daily life and may be more physiologically relevant.8 The fact that in a small sample of the normal population vascular reactivity was significantly correlated to age, ExSBP and peak exercise AngII is intriguing. However, in contrast to previous larger studies, we did not find any relationship with other cardiovascular risk factors such as cholesterol. The size of the sample examined may have been insufficient to detect this finding. Finally, volunteer selection was biased towards young men. We have included relatively young adults to obviate the need for age correction in NO vasodilation given the natural decline of endothelial dysfunction with aging. The exclusion of young women was also motivated by the favorable effect of estrogens on NO bioactivity in premenopausal women.
We assessed resting indices of LV relaxation (diastolic function). Undoubtedly, more useful information could have been provided if we had carried out an assessment of diastolic function during stress40; however, given the supine nature of the test this was not possible.
Conclusions
We have shown that the constellation of altered peripheral functional capacity (endothelial function), increased central arterial stiffness, and excess of peak exercise AngII are associated with the genesis of the unique phenomenon of ExSBP. The individual weight of participation of each of the above factors to ExSBP cannot be determined. However, our study introduces a simple exercise step test as a potential detector of very early cardiovascular target organ creating the necessary groundwork for its future introduction into clinical routine practice of cardiovascular risk assessment.
Sources of Funding
Interdepartmental from the University of Dundee.
Conflicts of interest/disclosures
None.
Acknowledgments
We are indebted to the staff of the Hypertension Research Centre for the support provided for the completion of this study.
J Clin Hypertens (Greenwich). 2015;17:837–844. DOI: 10.1111/jch.12629. © 2015 Wiley Periodicals, Inc.
References
- 1. Taddei S, Virdis A, Mattei P, et al. Defective L‐arginine‐nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94:1298–1303. [DOI] [PubMed] [Google Scholar]
- 2. Celermajer DS, Sorensen KE, Gooch VM, et al. Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 3. Suwaidi JA, Hamasaki S, Higano ST, et al. Long‐term follow‐up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 4. Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–990. [DOI] [PubMed] [Google Scholar]
- 5. Santulli G. Coronary heart disease risk factors and mortality. JAMA. 2012;307:1137; author reply 8. [DOI] [PubMed] [Google Scholar]
- 6. Tzemos N, Lim PO, MacDonald TM. Exercise blood pressure and endothelial dysfunction in hypertension. Int J Clin Pract. 2009;63:202–206. [DOI] [PubMed] [Google Scholar]
- 7. Nakashima M, Miura K, Kido T, et al. Exercise blood pressure in young adults as a predictor of future blood pressure: a 12‐year follow‐up of medical school graduates. J Hum Hypertens. 2004;18:815–821. [DOI] [PubMed] [Google Scholar]
- 8. Lim PO. Dundee Step Test: a simple method of measuring blood pressure to exercise. J Hum Hypertens. 1999;13:521–526. [DOI] [PubMed] [Google Scholar]
- 9. Lim PO, Shiels P, MacDonald TM. The Dundee Step Test: a novel exercise test suitable for the outpatient management of hypertension [letter]. J Hypertens. 1998;16:1701. [PubMed] [Google Scholar]
- 10. Tzemos N, Lim PO, Wong S, et al. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008;51:1525–1530. [DOI] [PubMed] [Google Scholar]
- 11. Stefanadis C, Stratos C, Boudoulas H, et al. Distensibility of the ascending aorta: comparison of invasive and non‐invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J. 1990;11:990–996. [DOI] [PubMed] [Google Scholar]
- 12. Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45:426–431. [DOI] [PubMed] [Google Scholar]
- 13. Pannier BM, Avolio AP, Hoeks A, et al. Methods and devices for measuring arterial compliance in humans. Am J Hypertens. 2002;15:743–753. [DOI] [PubMed] [Google Scholar]
- 14. Yee KM, Pringle SD, Struthers AD. Circadian variation in the effects of aldosterone blockade on heart rate variability and QT dispersion in congestive heart failure. J Am Coll Cardiol. 2001;37:1800–1807. [DOI] [PubMed] [Google Scholar]
- 15. Farquharson CA, Struthers AD. Gradual reactivation over time of vascular tissue angiotensin I to angiotensin II conversion during chronic lisinopril therapy in chronic heart failure. J Am Coll Cardiol. 2002;39:767–775. [DOI] [PubMed] [Google Scholar]
- 16. Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double‐blind, crossover study. Circulation. 2001;104:511–514. [DOI] [PubMed] [Google Scholar]
- 17. Whitney RJ. The measurement of volume changes in human limbs. J Physiol (London). 1953;121:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vallance P, Collier J, Moncada S. Effects of endothelium‐derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997–1000. [DOI] [PubMed] [Google Scholar]
- 19. Calver A, Collier J, Vallance P. Forearm blood flow responses to a nitric oxide synthase inhibitor in patients with treated essential hypertension [see comments]. Cardiovasc Res. 1994;28:1720–1725. [DOI] [PubMed] [Google Scholar]
- 20. Gilligan DM, Panza JA, Kilcoyne CM, et al. Contribution of endothelium‐derived nitric oxide to exercise‐induced vasodilation. Circulation. 1994;90:2853–2858. [DOI] [PubMed] [Google Scholar]
- 21. Chang HJ, Chung J, Choi SY, et al. Endothelial dysfunction in patients with exaggerated blood pressure response during treadmill test. Clin Cardiol. 2004;27:421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stewart KJ, Sung J, Silber HA, et al. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens. 2004;17:314–320. [DOI] [PubMed] [Google Scholar]
- 23. Dlin RA, Hanne N, Silverberg DS, Bar‐Or O. Follow‐up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J. 1983;106:316–320. [DOI] [PubMed] [Google Scholar]
- 24. Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium‐dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. [DOI] [PubMed] [Google Scholar]
- 25. Mundal R, Kjeldsen SE, Sandvik L, et al. Exercise blood pressure predicts cardiovascular mortality in middle‐aged men. Hypertension. 1994;24:56–62. [DOI] [PubMed] [Google Scholar]
- 26. Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. [DOI] [PubMed] [Google Scholar]
- 27. Munir S, Jiang B, Guilcher A, et al. Exercise reduces arterial pressure augmentation through vasodilation of muscular arteries in humans. Am J Physiol Heart Circ Physiol. 2008;294:H1645–H1650. [DOI] [PubMed] [Google Scholar]
- 28. Fazio S, Palmieri EA, Izzo R, et al. An exaggerated systolic blood pressure response to exercise is associated with cardiovascular remodeling in subjects with prehypertension. Ital Heart J. 2005;6:886–892. [PubMed] [Google Scholar]
- 29. Herkenhoff FL, Lima EG, Goncalves RA, et al. Doppler echocardiographic indexes and 24‐h ambulatory blood pressure data in sedentary middle‐aged men presenting exaggerated blood pressure response during dynamical exercise test. Clin Exp Hypertens. 1997;19:1101–1116. [DOI] [PubMed] [Google Scholar]
- 30. Miyai N, Arita M, Morioka I, et al. Ambulatory blood pressure, sympathetic activity, and left ventricular structure and function in middle‐aged normotensive men with exaggerated blood pressure response to exercise. Med Sci Monit. 2005;11:CR478–CR484. [PubMed] [Google Scholar]
- 31. Favier R, Pequignot JM, Desplanches D, et al. Catecholamines and metabolic responses to submaximal exercise in untrained men and women. Eur J Appl Physiol. 1983;50:393–403. [DOI] [PubMed] [Google Scholar]
- 32. Shim CY, Ha JW, Park S, et al. Exaggerated blood pressure response to exercise is associated with augmented rise of angiotensin II during exercise. J Am Coll Cardiol. 2008;52:287–292. [DOI] [PubMed] [Google Scholar]
- 33. Santulli G, Trimarco B, Iaccarino G. G‐protein‐coupled receptor kinase 2 and hypertension: molecular insights and pathophysiological mechanisms. High Blood Press Cardiovasc Prev. 2013;20:5–12. [DOI] [PubMed] [Google Scholar]
- 34. Staessen J, Fagard R, Hespel P, et al. Plasma renin system during exercise in normal men. J Appl Physiol. 1987;63:188–194. [DOI] [PubMed] [Google Scholar]
- 35. Ozkor MA, Hayek SS, Rahman AM, et al. Contribution of endothelium‐derived hyperpolarizing factor to exercise‐induced vasodilation in health and hypercholesterolemia. Vasc Med. 2015;20:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kayrak M, Bacaksiz A, Vatankulu MA, et al. G‐protein‐coupled receptor kinase 2 and hypertension: molecular insights and pathophysiological mechanisms. Circ J. 2010;74:1135.20453387 [Google Scholar]
- 37. Papavasileiou MV, Thomopoulos C, Antoniou I, et al. Impaired glucose metabolism and the exaggerated blood pressure response to exercise treadmill testing in normotensive patients. J Clin Hypertens (Greenwich). 2009;11:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santulli G, Cipolletta E, Sorriento D, et al. CaMK4 gene deletion induces hypertension. J Am Heart Assoc. 2012;1:e001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palatini P. Exercise haemodynamics in the normotensive and the hypertensive subject. Clin Sci (Colch). 1994;87:275–287. [DOI] [PubMed] [Google Scholar]
- 40. Ha JW, Oh JK, Pellikka PA, et al. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr. 2005;18:63–68. [DOI] [PubMed] [Google Scholar]


