Abstract
The survival of patients with malignant hypertension (MHT) has considerably improved over the past decades. Data regarding the excess risk of mortality and the contribution of conventional cardiovascular risk factors are lacking. The authors retrospectively assessed cardiovascular risk factors and all‐cause mortality in 120 patients with a history of MHT and compared them with 120 normotensive and 120 hypertensive age‐, sex‐, and ethnicity‐matched controls. Total cholesterol, low‐density lipoprotein cholesterol, and body mass index were lower in MHT patients compared with hypertensive controls, whereas blood pressure, high‐density lipoprotein cholesterol, and smoking habit were similar. Median estimated glomerular filtration rate was lower in MHT patients compared with normotensive and hypertensive controls (both P<.01). The annual incidence of all‐cause mortality per 100 patient‐years was higher in MHT patients (2.6) compared with normotensive (0.2) and hypertensive (0.5) controls (both P<.01). Mortality of patients with a history of MHT remains high compared with normotensive and hypertensive controls. Patients with MHT had a more favorable cardiovascular risk profile compared with hypertensive controls but a higher prevalence of renal insufficiency.
Malignant hypertension (MHT) is a hypertensive emergency characterized by severe hypertension and acute microvascular complications including grade III or IV hypertensive retinopathy. If left untreated, the 5‐year survival rate is <5% mainly because of stroke, myocardial infarction, congestive heart failure, and end‐stage renal disease.1, 2, 3 With the availability of antihypertensive drugs and improved patient care, mortality has been markedly reduced to approximately 10% after 5 years.2, 4 This is still considerable, however, given the relatively young study populations, with an average age varying between 40 and 50 years at presentation.2, 5
Previous cohort studies, including our own, have shown that renal dysfunction is an important predictor of mortality in patients with MHT,2, 4 while other studies suggest a role of traditional cardiovascular risk factors such as excess smoking, decreased levels of high‐density lipoprotein (HDL), and poor blood pressure (BP) control.6, 7, 8, 9 However, most of these studies lack a control population thereby limiting the internal validity. Nonetheless, insight into the excess risk of cardiovascular disease and mortality in patients with a history of MHT is required to identify which preventive measures may further improve outcome of this extreme phenotype of hypertension‐related organ damage.
Therefore, the principle aim of this study was to quantify the excess mortality risk in patients with a history of MHT. The second aim was to investigate whether traditional cardiovascular risk factors contribute to the increased risk. To this end, we compared cardiovascular risk factors and all‐cause mortality of patients with a history of MHT with age‐, sex‐, and ethnicity‐matched normotensive (NT) and hypertensive (HT) controls.
Methods
Participants
We used a case‐control design to compare patients with a history of MHT with NT and HT controls. The selection of patients with a history of MHT has been previously described.4 Briefly, we searched the database of a large teaching hospital in Amsterdam, The Netherlands. The diagnosis at discharge is recorded in this database according to the International Classification of Diseases (ICD) codes. All charts of patients admitted between August 1992 and January 2010 with MHT or related diagnoses were reviewed for clinical criteria of MHT, including (1) diastolic BP ≥120 mm Hg and (2) presence of grade III or IV hypertensive retinopathy.10 Excluded were patients younger than 18 years, pregnant women, and patients who were already undergoing dialysis before admission. Patients referred from elsewhere were excluded to prevent referral bias.
Patients with a history of MHT were individually matched for age, sex, and ethnicity with NT and HT control patients from the Surinamese in The Netherlands: Study on Ethnicity and Health (SUNSET), a large population‐based study among noninstitutional adults.11 The SUNSET study was carried out between 2001 and 2003 to assess the cardiovascular risk profile of 35‐ to 60‐year‐old people of European, African, and South‐Asian origin in Amsterdam, in the catchment area of our hospital. In total, 1383 persons of South‐Asian, African, and European origin participated in an interview and physical examination and were followed through the national medical registration database until December 23, 2007. Self‐reported ethnicity was used for classification in ethnic groups. Black patients were from sub‐Saharan African descent, mainly from Ghana and Nigeria. Asian patients were mainly from the Sub‐Indian continent, whereas white patients were of West‐European ancestry. Patients with a history of MHT who were either younger or older than NT and HT controls from SUNSET were matched with controls closest to their own age.
Data Collection and Definitions
Vital status was assessed by inquiry of the municipal administration registries. For patients with a history of MHT, the cause of death was retrieved from the medical file or from general physicians. In addition, we recorded follow‐up data on cardiovascular events of these patients. Data derived within 3 months after admission of patients with a history of MHT were censored, because death or cardiovascular events occurring during this period could be attributable to the acute episode of MHT. For NT and HT controls, data on the cause of death or the number of cardiovascular events were not available.
All conventional cardiovascular risk factors including age, sex, ethnicity, systolic and diastolic BP, body mass index (BMI), smoking, lipid profile, statin use, fasting glucose, presence of diabetes mellitus, plasma creatinine, and proteinuria were assessed at the entry of the SUNSET study for NT and HT controls. For patients with a history of MHT, age, sex, ethnicity, smoking status, and presence of left ventricular hypertrophy were assessed at initial admission. Left ventricular hypertrophy was considered present when detected by cardiac ultrasonography or by electrocardiography according to the Sokolow‐Lyon criteria. Systolic and diastolic BP, lipid profile, statin use, fasting glucose, prevalence of diabetes, BMI, plasma creatinine, and proteinuria were documented during a follow‐up visit at the outpatient department using a standardized risk assessment identical to the SUNSET study. Values assessed more than 2 years after admission were excluded. The median time between admission and cardiovascular risk assessment was 5 months, with an interquartile range (IQR) of 2 to 10 months after presentation.
Renal function was estimated according to the Modification of Diet in Renal Disease formula.12 Macroalbuminuria was defined as urinary protein excretion >300 mg/d on 24‐hour urine or >200 mg/L on a morning spot sample. All laboratory tests in patients with MTH and the NT and HT controls were performed in the hospital's central laboratory according to local protocols.
Statistical Analysis
Continuous data were expressed as mean and standard deviation or median and IQR for variables with a skewed distribution. Categoric data were expressed as number and percentages. Differences between groups for continuous variables were assessed by one‐way analysis of variance with post‐hoc least significant difference correction for parametric or Dunnet's post‐hoc correction for nonparametric distributions. Chi‐square tests were used for categoric variables. Annual incidence rates were calculated for mortality to account for differences in follow‐up duration. The annual incidence rates were expressed as the number of events per 100 person‐years of follow‐up. To assess the mortality over time, Kaplan‐Meier plots were generated to express 5‐year survival. The log‐rank test was used to assess differences in all‐cause mortality between groups. SPSS software was used for all analyses (version 19.0, Inc, Chicago, IL). A P value <.05 was considered significant.
Results
Characteristics of Patients With MHT at Presentation
A total of 120 patients admitted with MHT were included, with a mean age of 44 years (range, 19–79 years), 83 (69%) were men, and 60 (50%) were of West‐European ancestry. Mean BP at admission was 230±23/145±17 mm Hg. Neurologic symptoms consistent with hypertensive encephalopathy were present in 11 (9%) patients, and 66 (55%) patients had grade IV hypertensive retinopathy. Left ventricular hypertrophy was present in 95 (79%) patients. Hypertension was diagnosed prior to admission in 65 (54%) patients, and 39 (33%) patients were treated with antihypertensive medication. Median plasma creatinine at admission was 2.0 mg/dL with an IQR of 1.2 mg/dL to 4.5 mg/dL. A primary renal disease could be identified in 9 (8%) patients, and renovascular disease was diagnosed in 7 (6%) patients.
Comparison of Cardiovascular Risk Profiles at Baseline
Patients with a history of MHT were well‐matched for age, sex, and ethnicity with HT and NT controls (Table 1). Systolic and diastolic BP levels during follow‐up were higher in patients with a history of MHT compared with NT controls (both P<.01) but were not different from HT controls (P=1.0 for systolic and P=.30 for diastolic BP). BMI of MHT patients was similar compared with NT controls and lower compared with HT controls (P<.01). Smoking habits did not differ between the groups. Patients with a history of MHT had lower total cholesterol and low‐density lipoprotein (LDL) cholesterol levels compared with HT and NT controls (P<.01), while statins were more frequently prescribed to patients with a history of MHT (9%) compared with HT (3%) and NT (2%) controls (P=.02). After excluding MHT patients who used statins, mean plasma total cholesterol and LDL cholesterol remained significantly lower in patients with a history of MHT compared with HT controls (P<.01 for both total cholesterol and LDL cholesterol). HDL cholesterol was similar in all groups. Triglycerides were comparable in patients with a history of MHT and HT, but lower in NT controls (P<.01). There were no differences in fasting glucose levels among groups (P=.19); however, diabetes mellitus was more prevalent in HT controls compared with both NT controls and MHT patients (P<.01). Median estimated glomerular filtration rate (eGFR) of patients with a history of MHT (33; IQR, 14–68 mL/min/1.73 m2) was lower compared with NT (82; IQR, 71–112 mL/min/1.73 m2) and HT (83; IQR, 71–109 mL/min/1.73 m2) controls (P<.01). Macroalbuminuria was more often present in patients with a history of MHT compared with NT and HT controls (P<.01). Sixteen of 120 patients (13%) with MHT needed kidney replacement therapy at the follow‐up visit compared with none in the groups with NT and HT.
Table 1.
Baseline Characteristics
| NT | HT | MHT | P Value | |
|---|---|---|---|---|
| Patients, No. | 120 | 120 | 120 | – |
| Follow‐up (IQR), mo | 66 (62–70) | 67 (62–70) | 62 (24–103) | .54 |
| Age, y | 44 (8) | 44 (6) | 44 (12) | – |
| Men, No. (%) | 83 (69) | 83 (69) | 83 (69) | – |
| Black, No. (%) | 57 (48) | 57 (48) | 57 (48) | – |
| White, No. (%) | 60 (50) | 60 (50) | 60 (50) | – |
| Asian, No. (%) | 3 (3) | 3 (3) | 3 (3) | – |
| Systolic BP, mm Hg | 116 (12) | 144 (15) a | 144 (23) a | <.01 |
| Diastolic BP, mm Hg | 75 (7) | 93 (8) a | 91 (15) a | <.01 |
| Total cholesterol, mg/dL | 204 (40) | 214 (43) | 196 (44) b | <.01 |
| LDL cholesterol, mg/dL | 130 (37) | 133 (40) | 114 (38) a,b | <.01 |
| HDL cholesterol, mg/dL | 56 (14) | 55 (16) | 54 (18) | .50 |
| Triglycerides, mg/dL | 90 (51) | 131 (99) a | 132 (96) a | <.01 |
| Statin prescribed, No. (%) | 2 (2) | 4 (3) | 10 (8) | <.05 |
| Antihypertensive drugs, No. (%) | 0 | 24 (20) | 81 (68) b | <.01 |
| Fasting plasma glucose, mg/dL | 97 (23) | 103 (22) | 97 (23) | .19 |
| Diabetes mellitus, No. (%) | 5 (4) | 12 (10) | 5 (4) | <.01 |
| Body mass index, kg/m2 | 25.9 (4.8) | 28.2 (5.4) a | 26.1 (5.1) b | <.01 |
| Current Smoker, No. (%) | 67 (56) | 60 (50) | 52 (43) | .19 |
| Plasma creatinine, (IQR), mg/dL | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 2.0 (1.2–3.4) a,b | <.01 |
| eGFR, (IQR), mL/min/1.73 m2 | 82 (71–112) | 83 (71–109) | 33 (14–68) a,b | <.01 |
| Macroalbuminuria, No. (%) | 0 | 4 (3) | 66 (55) | <.01 |
Abbreviations: eGFR, estimated glomular filtration rate; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein; SD, standard deviation. Cardiovascular risk factors at first follow‐up visit in patients with history of malignant hypertension (MHT) as compared with baseline values of age‐, sex‐, and ethnicity‐matched normotensive (NT) and hypertensive (HT) controls from the same area of residence. Values are expressed as means and SD, median and IQR, or numbers and percentage. a P<.05 vs NT. b P<.05 vs HT.
Cardiovascular Events in Patients With a History of MHT
During a median follow‐up of 62 months, cerebrovascular accidents were the most frequently observed cardiovascular event in patients with a history of MHT (n=5). Other events that occurred in MHT patients were myocardial infarction (n=4), angina pectoris (n=2), and peripheral artery disease (n=2). In addition, 2 cardiovascular events (1 stroke and 1 case of angina pectoris) occurred within 3 months after admission.
Comparison of All‐Cause Mortality
Eighteen patients with a history of MHT died during follow‐up. One patient died within 3 months after admission of a malignancy and was excluded from survival analysis. Causes of death of the remaining 17 patients with history of MHT included cardiovascular events (n=6), malignancy (n=2), infectious disease (n=2), and renal failure (n=2) and was uncertain for 5 patients. Of the control patients, 1 NT and 3 HT patients died during follow‐up. Annual incidence rate of all‐cause mortality per 100 years of follow up was significantly higher in patients with a history of MHT (2.6) compared with both NT (0.2) and HT (0.5) controls (both P<.05 [Table 2). Log‐rank test of 5‐year all‐cause mortality showed a higher mortality rate in patients with history of MHT compared with both NT and HT controls (both P<.05 [Figure]).
Table 2.
Annual Incidence Rates of All‐Cause Mortality
| Total Observation, y | NT 677 | HT 665 | MHT 648 |
|---|---|---|---|
| Deaths | 1 | 3 | 17 |
| Annual incidence rate | 0.2 | 0.5 | 2.6 |
| RR (95% CI) compared with NT | 1 | 3.1 (0.3–29.4) | 17.8 (2.4–133.6) a |
| RR (95% CI) compared with HT | 0.3 (0.0–3.1) | 1 | 5.8 (1.7–19.8) b |
Abbreviations: CI, confidence interval; HT, hypertensive controls; MHT, patients with malignant hypertension; NT, normotensive controls; RR, relative risk. Annual incident rate of all‐cause mortality per 100 person‐years of follow‐up in patients with a history of malignant hypertension (MHT) as compared with age‐, sex‐, and ethnicity‐matched normotensive and hypertensive controls from the same residence area. a P<.05 compared with NT. b P<.05 compared with HT.
Figure 1.
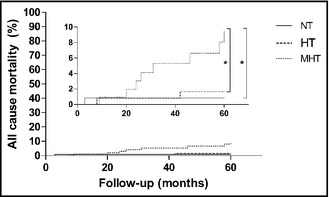
Five‐year all‐cause mortality for each group. NT indicates normotensive controls; HT, hypertensive controls; MHT, patients with a history of malignant hypertension. *P<.05 on log‐rank test for MHT compared with NT and HT. The inner panel shows an enlargement of the outer figure.
There was no difference in the average age of deceased patients with a history of MHT compared with NT and HT controls. Comparison of deceased and surviving patients with a history of MHT showed that patients who died during follow‐up tended to be older (51±19 years vs 43±11 years), were less often male (41% vs 75%, P=.01), and tended to smoke more often (71% vs 46%). There were no significant differences in BP (152±24/90±17 mm Hg vs 143±21/89±13 mm Hg), and other cardiovascular risk factors between deceased and surviving patients with MHT. eGFR (42 [IQR 16–105] mL/min/1.73 m2 vs 37 [IQR 25–58] mL/min/1.73 m2) was similar in deceased and surviving patients with a history of MHT, whereas macroalbuminuria tended to be present more often in deceased patients (65% vs 54%).
Discussion
We show that despite considerable improvement in survival over the past decades, patients with a history of MHT remain at increased risk of dying compared with age‐, sex‐, and ethnicity‐matched NT and HT controls. Cardiovascular risk factors seem to be of little influence on the excess mortality, as total and LDL cholesterol, obesity, and prevalence of diabetes mellitus were higher in HT controls compared with patients with MHT, while smoking habit was comparable. BP levels were also similar in MHT patients and HT controls, suggesting that adherence to antihypertensive medication in patients with a history of MHT may have improved after admission to the hospital. However, renal function was significantly impaired in patients with MHT compared with NT and HT controls, suggesting that renal dysfunction may be an important contributor to the higher mortality rate observed in patients with a history of MHT.
There is ample evidence that renal dysfunction increases the risk of cardiovascular and all‐cause mortality, with both decreased eGFR and proteinuria contributing individually to this increased risk.13, 14, 15, 16 In fact, all‐cause mortality is similar in patients with chronic kidney disease (CKD) when compared with diabetic patients without CKD.17 In the present study, we observed that patients with a history of MHT who died during follow‐up more often had macroalbuminuria compared with surviving patients with a history of MHT. In addition, 16 (13%) patients with a history of MHT were on kidney replacement therapy.
Hypertension is associated with clustering of cardiometabolic risk factors, including obesity, diabetes, and dyslipidemia.18 HT control patients indeed had an increased cardiometabolic risk as demonstrated by the higher BMI, LDL, and triglyceride levels and higher prevalence of diabetes mellitus compared with NT controls. However, there was no evidence of clustering of cardiometabolic risk factors in patients with a history of MHT except for higher triglyceride levels. The apparent lack of cardiovascular risk factor clustering in patients with a history of MHT contradicts previous reports which show that these patients had higher plasma triglycerides, lower plasma HDL cholesterol, and more often smoked compared with either nonmalignant HT or NT controls.6, 7, 8, 9 Differences in the proportion of smoking patients could be explained by temporal changes in smoking behavior as studies on associations between MHT and cardiovascular risk date back more than 30 years. In addition, previous studies did not use matched control patients to account for differences in socioeconomic status or cultural background, potentially influencing cardiometabolic risk factors and smoking behavior. In the present study, control groups were derived from the same residence area and were individually matched for age, sex, and ethnicity with MHT patients to limit differences in socioeconomic status and cultural background. With regard to the aforementioned difference in HDL cholesterol, timing of the blood collections may have been relevant as HDL levels were previously assessed in the acute phase of MHT. Because HDL cholesterol is an acute phase reactant, the lower HDL cholesterol levels in that study may have been influenced by the inflammatory response associated with MHT. To avoid influence of these acute effects, cardiovascular risk profile was completed with a fasting venous blood sample after patients were discharged from the hospital and BP‐lowering treatment was instituted.
Despite the lack of an unfavorable cardiovascular risk profile compared with HT and NT controls, 13 (11%) patients with a history of MHT experienced cardiovascular events during follow‐up. The implication of this discrepancy between estimated cardiovascular risk and the observed number of cardiovascular events is that risk predictors based on traditional risk factors such as the Framingham score underestimate the risk in patients with a history MHT. Our data show that patients with a history of MHT should be considered high‐risk patients and suggest, in line with European Society of Hypertension recommendations, that prediction models for cardiovascular risk should be avoided in these patients.
Study Strengths and Limitations
Our study is the first to compare the survival and cardiovascular risk factors of a large group of consecutive patients with a history of MHT with that of NT and HT controls. Limitations include firstly its retrospective nature. As a result of coding errors, some patients with a history of MHT could have been missed. To overcome this, we performed a sensitivity analysis showing that no patients with a history of MHT who visited the emergency department between 1992 and 2008 were missed.4 Secondly, the age range of SUNSET participants was limited to 35 to 60 years, whereas the patients with a history of MHT were aged 19 to 79 years. Nonetheless, most patients with a history of MHT who fell outside this age range were younger than 35, implying a lower mortality risk, and the mean age of deceased patients was similar among all groups. Thirdly, the recruitment window of NT and HT controls from SUNSET was considerably smaller, leading to a much smaller variation in follow‐up time compared with that in the patients with a history of MHT. To account for this, the annual incidence rate of all‐cause mortality was calculated. Because the median follow‐up time was similar, we estimate that the influence on our results is limited. Finally, average follow‐up BP was similar in patients admitted from 1992 to 2000 compared with those admitted from 2001 to 2010 (data not shown), indicating that introduction of new antihypertensive medication during the recruitment period did not change BP control rate.
Conclusions
Mortality is increased in patients with a history of MHT compared with matched normotensive and hypertensive controls. Patients with MHT had a favorable cardiovascular risk profile compared with HT controls but had severe renal dysfunction. Since uncontrolled hypertension is the only modifiable predictor of long‐term renal outcome and mortality in patients with MHT,4 tight BP control should be the primary goal in the management of patients with history of MHT.
Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Acknowledgments
This was presented during the European Society of Hypertension (ESH) conference in June 2013 in Milan.
J Clin Hypertens (Greenwich). 2014;16:122–126. DOI: 10.1111/jch12243. ©2013 Wiley Periodicals, Inc.
References
- 1. Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1939;196:332–339. [DOI] [PubMed] [Google Scholar]
- 2. Lane DA, Lip GY, Beevers DG. Improving survival of malignant hypertension patients over 40 years. Am J Hypertens. 2009;22:1199–1204. [DOI] [PubMed] [Google Scholar]
- 3. Lip GY, Beevers M, Beevers DG. Complications and survival of 315 patients with malignant‐phase hypertension. J Hypertens. 1995;13:915–924. [DOI] [PubMed] [Google Scholar]
- 4. Amraoui F, Bos S, Vogt L, van den Born BJ. Long‐term renal outcome in patients with malignant hypertension: a retrospective cohort study. BMC Nephrol. 2012;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Born BJ, Koopmans RP, Groeneveld JO, van Montfrans GA. Ethnic disparities in the incidence, presentation and complications of malignant hypertension. J Hypertens. 2006;24:2299–2304. [DOI] [PubMed] [Google Scholar]
- 6. Bloxham CA, Beevers DG, Walker JM. Malignant hypertension and cigarette smoking. BMJ. 1979;1:581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edmunds E, Landray MJ, Li‐Saw‐Hee FL, et al. Dyslipidaemia in patients with malignant‐phase hypertension. QJM. 2001;94:327–332. [DOI] [PubMed] [Google Scholar]
- 8. Isles C, Brown JJ, Cumming AM, et al. Excess smoking in malignant‐phase hypertension. BMJ. 1979;1:579–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tuomilehto J, Elo J, Nissinen A. Smoking among patients with malignant hypertension. Br Med J (Clin Res Ed). 1982;284:1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Arterial hypertension. World Health Organ Tech Rep Ser. 1978;628:57. [PubMed] [Google Scholar]
- 11. Bindraban NR, van Valkengoed IG, Mairuhu G, et al. Prevalence of diabetes mellitus and the performance of a risk score among Hindustani Surinamese, African Surinamese and ethnic Dutch: a cross‐sectional population‐based study. BMC Public Health. 2008;8:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 13. Chronic Kidney Disease Prognosis Consortium . Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet. 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 15. Tonelli M, Muntner P, Lloyd A, et al. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154:12–21. [DOI] [PubMed] [Google Scholar]
- 16. Kidney Disease Outcomes Quality Initiative (K/DOQI) . K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–S290. [PubMed] [Google Scholar]
- 17. Tonelli M, Muntner P, Lloyd A, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population‐level cohort study. Lancet. 2012;380:807–814. [DOI] [PubMed] [Google Scholar]
- 18. Weycker D, Nichols GA, O'Keeffe‐Rosetti M, et al. Risk‐factor clustering and cardiovascular disease risk in hypertensive patients. Am J Hypertens. 2007;20:599–607. [DOI] [PubMed] [Google Scholar]


