Abstract
The authors hypothesized that published hypertension rates in Tanzania were influenced by the physiological response of individuals to blood pressure (BP) testing, known as the white‐coat effect (WCE). To test this, a representative sample of 79 participants from a baseline cohort of 2322 people aged 70 years and older were followed to assess BP using conventional BP measurement (CBPM) and ambulatory BP monitoring (ABPM). There was a significant difference between daytime ABPM and CBPM for both systolic BP (mean difference 29.7 mm Hg) and diastolic BP (mean difference 7.4 mm Hg). Rates of hypertension were significantly lower when measured by 24‐hour ABPM (55.7%) than by CBPM (78.4%). The WCE was observed in 54 participants (68.4%). The WCE was responsible for an increase in recorded BP. Accurate identification of individuals in need of antihypertensive medication is important if resources are to be used efficiently, especially in resource‐poor settings.
Globally, hypertension is a major cause of disability and early mortality.1, 2, 3 Previous reports of the prevalence of hypertension in sub‐Saharan Africa (SSA) have indicated it to be at least as common as in many high‐income regions.4, 5 A recent study by members of our team revealed the prevalence of hypertension to be 69.9% in persons aged 70 years and older in rural Tanzania.6 Furthermore, few of those who had hypertension had been previously diagnosed, and effective hypertension control was rare.
In most previously published studies of hypertension in SSA, conventional blood pressure measurement (CBPM) has been used to assess blood pressure (BP). However, the white‐coat effect (WCE), first observed by Mancia and colleagues in 1983,8 can lead to falsely elevated recorded BP values and mistaken diagnoses of hypertension, known as white‐coat hypertension (WCH).9 WCH is thought to be present in 15% to 30% of people diagnosed with hypertension based on CBPM.10
The use of ambulatory BP monitoring (ABPM) can overcome many of the problems of CBPM. ABPM readings are normally taken over a 24‐hour period, with the participant wearing a cuff and data recorded digitally. The use of ABPM is recommended by the National Institute for Health and Clinical Excellence in the United Kingdom for anyone with a CBPM ≥140/90 mm Hg.11 South African hypertension guidelines have been developed and recommend the use of ABPM in some situations.12 However, the impracticalities and cost of regular ABPM in resource‐poor settings is acknowledged.
Previous studies using ABPM to assess populations from SSA are few. Millen and colleagues13 used ABPM to assess the relationship between BP, salt intake, and insulin resistance in a cohort of 331 patients from South Africa. In 2014, Polonia and colleagues14 published a study comparing ABPM findings in untreated hypertensives living in Mozambique and Europe. Those in Mozambique were found to have higher BP, with a notably lower nighttime fall, than patients in Europe. However, reports on the nature and prevalence of the WCE and WCH from SSA are rare.
We aimed to investigate the influence of the WCE on CBPM‐recorded hypertension rates in a cohort of elderly people living in rural Tanzania. Our null hypothesis was that there was no significant difference between CBPM and ABPM recordings in this setting.
Methods
Ethical approval was obtained locally from Tumaini University's ethics committee and nationally from the Tanzanian National Institute of Medical Research.
All study participants provided their informed consent. For those who could not read and write, the purpose and implications of the study were verbally explained, and we obtained a thumbprint to indicate consent. In cases where participants were unable to consent, written assent was obtained from a close relative. Patients with hypertension, based on ABPM data, were given medication as appropriate and re‐educated about hypertension, stroke risk, and lifestyle changes. Medication and advice was given in accordance with Tanzanian guidelines by a local doctor or assistant medical officer.
Setting
Baseline data were collected between November 1, 2009, and July 31, 2010, and follow‐up data between March 6 and June 1, 2013. The Hai district of northern Tanzania is located on the southern slopes of Mount Kilimanjaro and includes a Demographic Surveillance Site (DSS) established in the early 1990s.15 On June 1, 2009, the census population of the DSS was recorded as 161,119, of whom 8869 were aged 70 years and older.
Baseline Study Population
Details of baseline data collection have already been published and brief details are presented below.6 We aimed to assess one quarter of the people aged 70 years and older in the DSS. Twelve villages from the 52 within the DSS were selected using a random number generator, giving a baseline cohort of 2232 people. Participants were assessed at a local health facility or at home if they were unable to travel.
Follow‐up Study Population
We pragmatically chose to follow‐up a random sample of those living in two of the villages. All survivors were stratified according to hypertensive grade (normotensive, grade I, II, or III) from baseline measurement in 2010 and then those within each stratum were randomized. Stratified random sampling was preferred to random sampling to ensure an even spread of BPs across the sample. The participants for ABPM were then seen strictly in the order on the list. If we were unable to find a participant after three attempts, or if they had moved villages or refused, we moved on to the next person on the list. As for baseline assessment, participants were assessed at a local health facility or at home if they were unable to travel.
Assessments
Conventional BP Measurement
Seated BP was recorded in the right arm using an appropriately sized cuff, with the arm supported at the level of the heart. In accordance with the World Health Organization (WHO) STEPwise Approach to Surveillance (STEPS) protocol,16 three measurements were taken 1 minute apart after 5 minutes of resting quietly, with an average taken of the last two readings. If there was a marked difference (20 mm Hg systolic or 10 mm Hg diastolic) between the second and third readings, further readings were taken. A calibrated A&D UA‐767 (A&D Instruments Ltd, Abingdon, UK) BP monitor was used to record BP.17
At baseline, CBPM was taken by UK‐based research doctors (M.D. and F.D.) or a trained local census enumerator. At follow‐up, measurements were taken by the enumerators.
Ambulatory BP Monitoring
ABPM was conducted using electronic Diasys Integra II devices (DIASYS integra; Novacor SA, Rueil‐Malmaison, France), which is recommended for use by the European Society of Hypertension (ESH).18 Devices were set up in line with Novacor guidelines and the procedure run as far as possible according to recommendations from the ESH on ambulatory measurements, with modifications as outlined below.10, 19 Measurements were based on the auscultatory mode, with oscillometric mode as back up, and recorded every hour during the day and night. One‐hour intervals were used, instead of 20‐minute intervals, as we were uncertain as to the acceptability of the devices. We considered any values outside the margins of 50 mm Hg to 250 mm Hg for systolic BP and 30 mm Hg to 150 mm Hg for diastolic BP as invalid. Based on our experience of sleeping patterns in Hai district, daytime was defined as 5 am to 10 pm and nighttime as 10 pm to 5 am. Monitor fitting, checking, removal, and data logging were conducted by census enumerators, supervised by UK‐based medical students (A.I. and J.T.).
Other Assessments
Demographic data were collected as part of the baseline assessment and details confirmed during follow‐up.
Hypertension Definitions
Conventional BP Measurement
Hypertension was defined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg. Further classification of severity was undertaken according to ESH guidelines (Table 3).20 Isolated systolic hypertension was defined as systolic BP ≥140 mm Hg and diastolic BP <90 mm Hg.
Table 3.
Association Between Presence of the WCE and Presence of Hypertension by Conventional BP Measurement
| Hypertension Present (≥140/90 mm Hg) | Grade II Hypertension Present (≥160/100–179/109 mm Hg) | Grade III Hypertension Present (≥180/110 mm Hg) | Isolated Systolic Hypertension | |
|---|---|---|---|---|
| Systolic BP | 171.8 (26.79) | 169.6 (17.03) | 205.0 (16.46) | 162.4 (19.27) |
| Diastolic BP | 89.7 (12.06) | 92.3 (10.14) | 98.1 (13.60) | 81.6 (6.22) |
| WCE present (n=54) | 48 (88.9%) | 14 (61.1%) | 19 (35.2%) | 26 (48.1%) |
| WCE absent (n=25) | 14 (56.0%) | 4 (16.0%) | 1 (4.0%) | 10 (40.0%) |
| Significance | χ2 (1)=10.945, P=.001 | χ2 (1)=13.966, P<.001 | χ2 (1)=8.790, P=.003 | χ2 (1)=0.457, P=.499 |
Abbreviations: BP, blood pressure; WCE, white‐coat effect. Values are presented as mean (standard deviation) or number (percentage).
Ambulatory BP Measurement
Participants were given information regarding the purpose of ABPM and what to expect from the monitor during the 24‐hour period of use. It was also emphasized that they were free to remove the monitor at any point for any reason (eg, discomfort, fatigue). In accordance with the most recent update of the ESH guidelines on ambulatory measurements, hypertension, when measured using ABPM, was defined as systolic BP ≥130 mm Hg or diastolic BP ≥80 mm Hg averaged across the full 24‐hour period.10 Where data relating to daytime measurement only were presented, a cutoff of ≥135/85 mm Hg was used.19 For nighttime measurement, a cutoff of ≥120/70 mm Hg was used. Masked hypertension was defined as hypertension on ABPM over 24 hours in the absence of CBPM hypertension. A clinically significant WCE was defined as a difference between CBPM and daytime ABPM of >20 mm Hg systolic BP and/or 10 mm Hg diastolic BP.21, 22
A drop in either systolic or diastolic BP of ≤10% was used to define those without a significant nighttime dip in BP (nondippers).23
Statistical Analysis
Data relating to BP were broadly normally distributed, although age data were not. Confidence intervals (CIs) for proportions and odds ratios (OR) were calculated based on the assumptions of the binomial distribution. CIs for continuous variables were calculated from the normal distribution. When comparing BP measurements, paired and independent sample (equal variance assumed) t tests were used as appropriate. For data relating to age, Mann‐Whitney U tests were used to compare groups. For categorical data (eg, sex, hypertension present/absent), chi‐square tests were used. Two‐tailed tests were used throughout. For CBPM in 2013, there was one missing value for systolic and diastolic values; the mean was imputed. For t tests comparing BP data, some multiple comparisons were made and for this reason the significance level was set at 1% for these tests. For all other tests, the significance level was set at 5%.
Results
Of persons approached for inclusion (n=83), one participant was excluded for taking daily hypertensive medication. Only one other participant was excluded, this was for having systolic BP >250 mm Hg by CBPM; they were immediately referred to specialist medical services. One person refused to undergo ABPM and one person removed the monitor before recording was complete. Therefore, ABPM data were collected in 79 participants. All participants were in sinus rhythm clinically, with no evidence of atrial fibrillation on radial pulse palpation, or 12‐lead electrocardiography from baseline assessment in 2010.24 Based on 2010 CBPM recordings, 19 individuals (24.1%) were normotensive, 22 (27.8%) had grade I hypertension, 17 (21.5%) had grade II hypertension, and 21 (26.6%) had grade III hypertension.
Not all recordings were valid and for logistical reasons it was occasionally necessary to remove the monitors before the full 24 hours of recording was completed. Although it was not always clear why a recording was not made or was invalid, sleeping position, arm position, or engagement in activity during the recording were likely reasons. Nevertheless, the median number of recordings made per participant was 19 (interquartile range, 17–20). Furthermore, there was no significant correlation between the number of recordings undertaken and either systolic BP (r=0.174, P=.130) or diastolic BP (r=0.094, P=.414) or the magnitude of the systolic WCE (r=0.047, P=.684) or diastolic WCE (r=0.010, P=.929).
Comparison of Baseline and Follow‐Up Cohorts
The baseline characteristics in 2010 of those followed‐up and those not followed‐up (n=2144) are compared in Table 1. There were no significant differences in age, sex, or baseline BP readings between those followed‐up and those not followed‐up. At baseline, there was also no evidence of clustering of baseline hypertension cases by village, with no significant difference in systolic BP (t=1.437, P=.155) and diastolic BP (t=0.266, P=.795) measurements between the two villages.
Table 1.
Comparison of Demographic Data and BP Readings in 2010 Between the Baseline Cohort Not Seen in 2013 and Patients Seen in 2013
| Baseline Cohort Not Followed‐Up (n=2144) | Baseline Cohort Followed‐Up in 2013 (n=79) | Significance of Difference | |
|---|---|---|---|
| Age in 2010, y | Median: 76 (IQR 72–81) | Median: 76 (IQR 72–80) | U=80390.5, z=−0.769, P=.442 |
| 70–74 | 838 (39.1%) | 36 (45.6%) | |
| 75–79 | 602 (28.1%) | 20 (25.3%) | |
| 80–84 | 328 (15.3%) | 14 (17.7%) | |
| 85 and older | 376 (17.5%) | 9 (11.4%) | |
| Male | 930 (43.3%) | 42 (53.2%) | χ2 (1)=2.966, P=.085 |
| Female | 1214 (56.6%) | 37 (46.8%) | |
| Mean systolic BP 2010 (standard deviation) | 160.8 (33.95) | 161.8 (30.81) | t=0.236 (df 2221), P=.813 |
| Mean diastolic BP 2010 (standard deviation) | 86.6 (16.28) | 87.3 (14.57) | t=0.375 (df 2221), P=.708 |
| Hypertension present 2010 (140/90 mm Hg) | 1494 (69.7%) | 59 (74.7%) | χ2 (1)=0.905, P=.341 |
Abbreviations: BP, blood pressure; df, degrees of freedom; IQR, interquartile range.
Use of Antihypertensive Medication
A total of 21 of 79 participants (26.6%) followed‐up were taking antihypertensive medication in 2010. At 3‐year follow‐up, no participants were taking antihypertensive medication regularly. No one had taken antihypertensives on the day ABPM was started, and participants were told to abstain from taking medication during monitoring. Only three patients reported taking antihypertensive medication in the preceding week, and a further nine patients reported taking antihypertensive medication in the last month. In those no longer taking medication, the predominant reason given for stopping medication was a lack of availability (n=14); cost (n=5) and adverse side effects (n=1) were also cited.
Comparison of Ambulatory and Conventional BP
Table 2 shows mean BP readings and prevalence of hypertension within the cohort at baseline. The difference in systolic BP measurements (t=0.135, P=.893) and diastolic BP measurements (t=0.628, P=.532) was not significant between baseline and follow‐up. There was a significant difference between daytime ABPM and CBPM recorded in 2013 for both systolic BP (mean difference, 29.7 mm Hg; 95% CI, 24.2–35.2; t=10.826; P<.001) and diastolic BP (mean difference, 7.4 mm Hg; 95% CI, 4.9–9.8; t=6.015; P<.001). Furthermore, despite the lower threshold used for the presence of hypertension with 24‐hour ABPM (130/80 mm Hg), rates of hypertension were also significantly lower when measured by ABPM (difference in proportions, 22.8%; 95% CI for difference, 8.6–37.0) than CBPM.
Table 2.
Comparison of Conventional and Ambulatory Blood Pressure Data at Follow‐Up in 2013
| Mean Systolic Blood Pressure (Standard Deviation) | Mean Diastolic Blood Pressure (Standard Deviation) | Hypertension Present, No. (%) | |
|---|---|---|---|
| Conventional blood pressure measurement | 161.9 (30.80) | 86.6 (12.98) | 62 (78.5) |
| 24‐hour ambulatory blood pressure monitoring | 128.5 (18.57) | 76.7 (9.70) | 44 (55.7) |
| Daytime ambulatory blood pressure monitoring | 132.2 (20.55) | 79.2 (10.73) | 37 (46.8) |
| Nighttime ambulatory blood pressure monitoring | 122.5 (19.72) | 73.2 (10.51) | 55 (69.6) |
Diurnal Variation
Nighttime hypertension was surprisingly common, found in 55 patients (69.6%). As shown in Table 2, mean pulse pressure was higher when assessed by CBPM (75.3 mm Hg) than by daytime ABPM (53.0 mm Hg). The mean diurnal variation was 9.6 mm Hg for systolic BP and 6.1 mm Hg for diastolic BP. Only 22 patients (27.8%) had a significant nighttime dip in both systolic and diastolic BP. Although mean ABPM recordings across the whole 24 hours were similar for dippers and nondippers (129.7/77.4 mm Hg and 128.3/77.0 mm Hg, respectively), dippers had higher mean daytime BP (138.7/82.4 mm Hg) and lower mean nighttime BP (113.2/68.1 mm Hg) than nondippers (129.7/78.0 mm Hg and 126.2/75.1 mm Hg, respectively). The nighttime decrease (but not the daytime increase) in systolic (t=2.730, P=.008) and diastolic (t=2.799, P=.006) BP was significant.
WCE and WCH
The WCE was observed in 54 participants (68.4%). It was not associated with education levels (χ2=2.093, P=.148), age (U=661.0, z=−0.148, P=.882), or sex (χ2=0.392, P=.531). However, those exhibiting the WCE were much more likely to have hypertension by CBPM than those without the WCE (Table 3. Isolated systolic hypertension was not associated with the presence of the WCE. The presence of the WCE was also associated with significantly higher CBPM pulse pressure (t=3.369, P=.001), systolic BP (t=4.741, P<.001), and diastolic BP (t=4.408, P<.001). The Figure shows histograms of the extent of the systolic and diastolic WCE when comparing CBPM and daytime ABPM.
Figure 1.
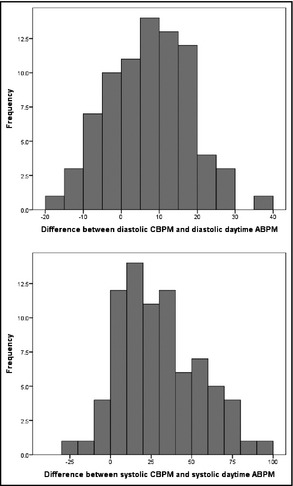
Systolic and diastolic white‐coat effect.
WCH was present in 22 participants (27.8%), as measured using average 24‐hour measurement; 40 participants (50.6%) were true hypertensives, four participants (5.1%) had masked hypertension, and the remaining 13 participants (16.5%) were normotensive.
Discussion
The WCE was responsible for an increase in recorded BP in more than two thirds of our cohort of elderly, rural‐dwelling Tanzanians. Furthermore, the WCE results in the incorrect classification of more than a quarter of individuals as hypertensive, when ABPM suggested that they were normotensive. Mean daytime systolic BP was almost 30 mm Hg lower when measured using ABPM compared with daytime measurement using CBPM.
Our findings in relation to the WCE support those of a study from Yemen, which compared CBPM readings between two separate home visits in a nationally representative sample of 10,242 people aged 15 to 69 years.25 Hypertension rates were 35% lower at the second visit compared with the first. Furthermore, those who would have been treated for hypertension based on their first visit recordings, but not their second, were found to have a lower cardiovascular disease risk score. The study findings highlight the need to target scarce resources more effectively.26
The lack of association of the WCE with age, sex, or education level is interesting. We had considered that either generally higher education levels in men or a greater familiarity with medical procedures in women (eg, during pregnancy) may have resulted in a sex disparity in the prevalence of the WCE. The WCE was significantly more common in true hypertensives, although the reason for this is unclear. It may be that BP in hypertensive patients is more prone to fluctuation. Indeed, some studies have suggested that greater propensity for increased BP in response to stress may mean that those with WCH are still at increased risk from adverse outcomes, although this effect may be small compared with that observed in sustained hypertensives.27, 28, 29, 30, 31
Although masked hypertension was not common, relatively high nocturnal BP resulted in nighttime hypertension in more than two thirds of participants. Reported nocturnal hypertension rates are based on a much lower cutoff (120/70 mm Hg), and it is not clear how important this finding is in understanding the nature of hypertension in this cohort, although our findings support those of Polonia and colleagues14 in Mozambique. However, data from other cohorts suggest that it is one of the most important determinants of cardiovascular risk.7, 32, 33 Nondippers were relatively common in our cohort and a prospective study investigating the relationship between this phenomenon and increased stroke, heart disease, and mortality risk is merited.
Limitations
The main limitation of our study is the relatively small cohort in whom ABPM was measured. Nevertheless, our data are unique for SSA. Further work looking at larger cohorts in this and other populations is planned. We also took the decision to monitor BP every hour only. Although monitoring every 20 minutes is recommended, we were unsure as to the acceptability of ABPM in this setting and in this elderly population. In practice, only one participant removed the monitor before they were revisited by a member of the study team and no adverse events were reported. Future work will investigate the acceptability of monitoring every 20 minutes.
In Hai district, patterns of sleep and wake tend to vary from those seen in more industrialized regions. Most people tend to go to bed earlier and rise much earlier. For this reason we defined the sleep period as 10 pm to 5 am. The use of sleep diaries was not practical since many of our participants did not have access to a reliable watch or clock. Furthermore, given that monitoring was only conducted hourly, we were reluctant to discard any data collected at sleep‐wake transition times. We recognize that this may have biased our data, particularly in relation to possible lowering of mean daytime measurements. It is also possible that many participants may have slept poorly during ABPM as a result of wearing the monitor. This may have been partly responsible for the relatively small fall in BP.
Although not all possible recordings were valid or recording was cut short for logistical reasons, the majority of data were collected. However, the lack of correlation between the number of recordings made and both BP levels and the extent of the WCE suggests that this did not significantly bias our results.
Conclusions
There was a significant difference between mean CBPM and ABPM readings and a substantial WCE in our cohort. We recognize that screening large populations using ABPM is not feasible. Self‐monitoring or allowing a greater length of time between repeat measurements (including making two separate visits) should be considered as alternative methods of obtaining more reliable data on BP levels in this setting. In this resource‐poor setting, accurate identification and cost‐effective treatment for persons most at need is more important than ever.
Disclosures
There were no conflicts of interest for any of the authors. Data collection in 2010 was supported in part by a grant from the Peel Medical Research Trust for Dr Matthew Dewhurst. Ashleigh Ivy and Jonathan Tam conducted data collection in 2013 in part fulfillment of an MRes degree at Newcastle University. The sponsors of this study had no role in designing the study; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Author contributions
Design/conception: Richard Walker, Matthew Dewhurst, and William Gray. Literature search: Richard Walker, Matthew Dewhurst, William Gray, and Ashleigh Ivy. Data collection: Felicity Dewhurst, Matthew Dewhurst, Jane Rogathi, Ashleigh Ivy, and Jonathan Tam. Data analysis: William K. Gray and Matthew Dewhurst. Interpretation of results: Richard Walker, Matthew Dewhurst, Paul Chaote, William K. Gray, Ashleigh Ivy, and Jonathan Tam. Writing of paper and review: Richard Walker, William K. Gray, Ashleigh Ivy, Jonathan Tam, Matthew Dewhurst, Felicity Dewhurst, Paul Chaote, and Jane Rogathi.
Acknowledgments
We wish to acknowledge the help of all health care workers, officials, carers, and family members who assisted in identification of cases, examination and assessment, and in data collection.
J Clin Hypertens (Greenwich). 2015;17:389–394. DOI: 10.1111/jch.12501. © 2015 Wiley Periodicals, Inc.
References
- 1. Murray CJ, Vos T, Lozano R, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 2. O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case–control study. Lancet. 2010;376:112–123. [DOI] [PubMed] [Google Scholar]
- 3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Addo J, Smeeth L, Leon DA. Hypertension in sub‐saharan Africa: a systematic review. Hypertension. 2007;50:1012–1018. [DOI] [PubMed] [Google Scholar]
- 5. Ibrahim MM, Damasceno A. Hypertension in developing countries. Lancet. 2012;380:611–619. [DOI] [PubMed] [Google Scholar]
- 6. Dewhurst MJ, Dewhurst F, Gray WK, et al. The high prevalence of hypertension in rural‐dwelling Tanzanian older adults and the disparity between detection, treatment and control: a rule of sixths? J Hum Hypertens. 2013;27:374–380. [DOI] [PubMed] [Google Scholar]
- 7. Franklin SS, Thijs L, Hansen TW, et al. White‐coat hypertension: new insights from recent studies. Hypertension. 2013;62:982–987. [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, Bertinieri G, Grassi G, et al. Effects of blood‐pressure measurement by the doctor on patient's blood pressure and heart rate. Lancet. 1983;2:695–698. [DOI] [PubMed] [Google Scholar]
- 9. Pickering TG, James GD, Boddie C, et al. How common is white coat hypertension? JAMA. 1988;259:225–228. [PubMed] [Google Scholar]
- 10. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 11. National Institute for Health and Clinical Excellence (NICE) . Hypertension: Clinical Management of Primary Hypertension in Adults (CG127). London, UK: NICE; 2011. [Google Scholar]
- 12. Seedat YK, Rayner BL. South African hypertension guideline 2011. S Afr Med J. 2012;102(1 Pt 2):57–83. [PubMed] [Google Scholar]
- 13. Millen AM, Norton GR, Majane OH, et al. Insulin resistance and the relationship between urinary Na(+)/K(+) and ambulatory blood pressure in a community of African ancestry. Am J Hypertens. 2013;26:708–716. [DOI] [PubMed] [Google Scholar]
- 14. Polonia J, Madede T, Silva JA, et al. Ambulatory blood pressure monitoring profile in urban African black and European white untreated hypertensive patients matched for age and sex. Blood Press Monit. 2014;19:192–198. [DOI] [PubMed] [Google Scholar]
- 15. Adult Morbidity and Mortality Project (AMMP) . Policy implications of adult morbidity and mortality; final report. 2004. http://research.ncl.ac.uk/ammp/finrep/. Accessed July 25, 2014.
- 16. World Health Organization . The WHO STEPS instrument: the WHO STEPwise approach to chronic disease risk factor surveillance (STEPS). Geneva: World Health Organization. http://www.who.int/chp/steps/STEPS_Instrument_v2.1.pdf. Accessed February 25, 2011. [Google Scholar]
- 17. Rogoza AN, Pavlova TS, Sergeeva MV. Validation of A&D UA‐767 device for the self‐measurement of blood pressure. Blood Press Monit. 2000;5:227–231. [DOI] [PubMed] [Google Scholar]
- 18. O'Brien E, Waeber B, Parati G, et al. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Brien E, Asmar R, Beilin L, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701. [DOI] [PubMed] [Google Scholar]
- 20. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 21. Myers MG, Haynes RB, Rabkin SW. Canadian Hypertension Society guidelines for ambulatory blood pressure monitoring. Am J Hypertens. 1999;12(11 Pt 1):1149–1157. [DOI] [PubMed] [Google Scholar]
- 22. O'Brien E. Ambulatory blood pressure monitoring in the management of hypertension. Heart. 2003;89:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Brien E, Parati G, Stergiou G. Ambulatory blood pressure measurement: what is the international consensus? Hypertension. 2013;62:988–994. [DOI] [PubMed] [Google Scholar]
- 24. Dewhurst MJ, Adams PC, Gray WK, et al. Strikingly low prevalence of atrial fibrillation in elderly Tanzanians. J Am Geriatr Soc. 2012;60:1135–1140. [DOI] [PubMed] [Google Scholar]
- 25. Modesti PA, Rapi S, Bamoshmoosh M, et al. Impact of one or two visits strategy on hypertension burden estimation in HYDY, a population‐based cross‐sectional study: implications for healthcare resource allocation decision making. BMJ Open. 2012;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Modesti PA, Agostoni P, Agyemang C, et al. Cardiovascular risk assessment in low‐resource settings: a consensus document of the European Society of Hypertension Working Group on Hypertension and Cardiovascular Risk in Low Resource Settings. J Hypertens. 2014;32:951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Franklin SS, Thijs L, Hansen TW, et al. Significance of white‐coat hypertension in older persons with isolated systolic hypertension: a meta‐analysis using the International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes population. Hypertension. 2012;59:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verdecchia P, Reboldi GP, Angeli F, et al. Short‐ and long‐term incidence of stroke in white‐coat hypertension. Hypertension. 2005;45:203–208. [DOI] [PubMed] [Google Scholar]
- 29. Pierdomenico SD, Cuccurullo F. Prognostic value of white‐coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens. 2011;24:52–58. [DOI] [PubMed] [Google Scholar]
- 30. Kollias A, Ntineri A, Stergiou GS. Is white‐coat hypertension a harbinger of increased risk? Hypertens Res. 2014;37:791–795. [DOI] [PubMed] [Google Scholar]
- 31. Stergiou GS, Asayama K, Thijs L, et al. Prognosis of white‐coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675–682. [DOI] [PubMed] [Google Scholar]
- 32. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. [DOI] [PubMed] [Google Scholar]
- 33. Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. [DOI] [PubMed] [Google Scholar]


