Abstract
The 24‐hour urine collection method is considered the gold standard for the estimation of ingested potassium and sodium. Because of the impracticalities of collecting all urine over a 24‐hour period, spot urine is often used for epidemiological investigations. This study aims to assess the agreement between spot urine and 24‐hour urine measurements to determine sodium and potassium intake. A total of 402 participants aged 25 to 64 years were randomly selected in South Benin. Spot urine was taken during the second urination of the day. Twenty‐four‐hour urine was also collected. Samples (2‐mL) were taken and then stored at ‐20°C. The analysis was carried out using potentiometric dosage. The agreement between spot urine and 24‐hour urine measurements was established using Bland‐Altman plots. A total of 354 results were analyzed. Daily sodium chloride and potassium chloride urinary excretion means were 10.2±4.9 g/24 h and 2.9±1.4 g/24 h, respectively. Estimated daily sodium chloride and potassium chloride means from the spot urine were 10.7±7.0 g/24 h and 3.9±2.1 g/24 h, respectively. Concordance coefficients were 0.61 at d=−0.5 g, (d±2SD=−11 g and 10.1 g) for sodium chloride and 0.61 at d=−1 g, (d±2SD=−3.8 g and 1.8 g) for potassium chloride. Spot urine method is acceptable for estimating 24‐hour urinary sodium and potassium excretion to assess sodium and potassium intake in a black population. However, the confidence interval for the mean difference, which is too large, makes the sodium chloride results inadmissible at a clinical level.
Excess sodium (Na) and low potassium (K) consumption increase the risk of hypertension and cardiovascular diseases.1, 2, 3 An estimation of dietary Na and K can be based on multiple methods: a study of sample portions, dietary surveys, and urine samples.4 Analysis of 24‐hour urine samples is considered the gold standard,4, 5, 6 as this method restitutes 85% to 90% of ingested Na and approximately 77% of K.7, 8 Advantages to this method include not being affected by subjectiveness in the description of diet and the desirability of test patients. It has two major constraints, however: the completeness of 24‐hour urine and the need to adhere to a strict timetable to avoid any issues with the collection process. Given these issues, the spot urine method (a one‐time sample) is used for epidemiological studies,9 although this method is limited by the variations in the presence of electrolytes, which occur on a daily basis.
Many studies have analyzed the correlation between multiple spot urine samples and 24‐hour urine samples for estimating daily K and Na excretions. Using mathematical formulae, Kawasaki and colleagues10 showed that there was a strong correlation between estimated 24‐hour Na and K values based on the Na and K levels of a spot urine sample taken during the second morning voiding urine specimen and actual Na and K levels from a 24‐hour sample (Na: r=0.72, P<.001; K: r=0.78, P<.001 [n=159]) in an adult Japanese population aged 20 to 79 years with no hypertension. Subsequently, Tanaka and colleagues11 developed formulae to estimate Na and K levels from a spot urine sample in a given population and to compare these numbers within given groups, but these formulae were not applicable to individuals. Recent studies carried out on American adults aged 21 years and older assessed estimated values of 24‐hour urinary Na excretion from spot urine samples taken at different times throughout the day. These studies showed better correlation (r=0.86, P<.001 [n=45]) between samples collected in the later afternoon/early evening before dinner with 24‐hour samples.12, 13 Another study carried out in patients with kidney failure in Korea showed a strong correlation between the mean value of three measured spot urinary Na and 24‐hour urinary Na values (r=0.47, P<.001 [n=305]).14 Kawamura and colleagues15 found a stronger correlation (r=0.85, P<.05 [n=24]) between measurements carried out on the second morning voiding urine and 24‐hour urine samples in hypertension patients in Japan. However, the correlation coefficient used by these authors to test the spot measurements against those of the 24‐hour samples did not test for concordance but for the link between these two measurements. The reference method for establishing concordance between two measurements is the one described by Bland and Altman.16, 17, 18
Two more recent studies using the Bland‐Altman plots have validated the use of spot urine samples in the estimation of Na and K excretion in Asian populations of both healthy individuals and those with hypertension.19, 20
There is no study on concordance between spot urine and 24‐hour urine samples in Africa, specifically in black populations, to assess Na and K intake. The hypothesis we are testing is that spot urine samples can be used in black tropical populations as an alternative method to estimate Na and K urine excretions.
The aim of this study was to use the Bland‐Altman method to establish the concordance between measurements of spot urine and 24‐hour urine samples for the estimation of Na and K urine excretion in the Benin population (West Africa).
Patients and Methods
Study Population and Sampling
This was a cross‐sectional descriptive and analytical study conducted between November 2012 and September 2013. It was carried out on an adult, apparently healthy, population aged 25 to 64 years who lived in Benin in the town of Bohicon and the district of Tanvè for at least 6 months. The patients gave their informed consent. The study protocol was approved by the ethics committee of the Faculty of Health Sciences, University of Abomey‐Calavi (Benin). Patients who did not give their consent, those who had been visited twice with no results, and those who had a medical condition making the collection of urine samples or answering the questionnaire impossible (speech and understanding impediments, mental illness, pregnancy, and menstruation) were excluded.
Of the 402 participants, 200 lived in the urban areas (Bohicon) and 202 in the rural areas (Tanvè). The study was conducted using a cluster sampling technique with probability proportional to size.21, 22, 23, 24 The population sampled comprised residents of all city neighborhoods or villages. This information was provided by the National Institute of Statistics and Economic Analysis.25 Thirty clusters were selected in each of the two zones.
Urine Sample Collection
The particpants were invited to the healthcare center closest to their place of residence at 7:30 am. General and health‐related information (place of residence, sex, social status, education level, age, weight, height, blood pressure, hypertension status) was gathered. Two types of urine samples were taken: spot urine and 24‐hour urine. The spot urine sample was taken during the second urination of the day, generally before noon and was also the beginning of the 24‐hour urine collection. The start and end collection were recorded for each individual. To secure the procedure and to optimize urine collection, participants were monitored by nursing staff throughout the 24‐hour period and a doctor's note was given to participants who were employed.
A 5‐L plastic container was given to each participant. A second container could be added if necessary. The containers were kept closed during collecting process. Participants reported whether they had missed any urine, particularly during bowel movement (from a few drops to a significant amount). Once the collection process was completed, 2‐mL samples were made after homogenization of the urine and were then frozen at −20°C. The samples were transported to the laboratory of Biochemistry and Molecular Genetics at Limoges University Hospital in France, using the cold chain.
Analysis of Urine Samples
Urinary Na and K were determined by using the ion‐selective electrode method and urinary creatinine was measured by using the Jaffé kinetic method. Creatinine excretion was used to assess adequacy of the 24‐hour urine collection and to correct the Na and K measurements from the spot sample for the calculation of estimated 24‐hour Na and K measurements. All analyses were carried out automatically by a Cobas C8000 analyzer (Roche, Basel, Switzerland) for Na and K and module C701 for creatinine. Criteria for adequacy creatinine were as follow: 24‐hour creatinine >10 (women) and >15 (men) mg/kg body weight with a diuresis ≥500 mL.26
Glycosuria tests were performed for all patients using fast‐test strips (Siemens Multistix‐GP Reagent 8SG, Erlangen, Germany). We also looked inquired about the use of diuretics, as the use of a diuretic or a positive glycosuria can change daily excretions of Na and K as well as modify diuresis.27
Statistical Analysis
Data were entered in Epi data 3.1 (EpiData Association, Odense, Denmark) and were analyzed using StatView 5.0 software (SAS Institute, Cary, NC). Quantitative variables were expressed as mean±standard deviation (SD). Qualitative variables were expressed in frequency. Estimated 24‐hour urine Na and K from the spot samples were calculated using the following formulae:12, 13
| (1) |
| (2) |
Na SpotU and K SpotU=Na and K measured from spot urine sample
Creatinine SpotU=Urinary creatinine measured from spot
24HU creatinine=Urinary creatinine measured from 24‐hour urine collection
The correlation coefficient was determined to assess the link between the spot and 24‐hour measurements. Concordance between spot urine and 24‐hour urine methods was assessed using Bland‐Altman method and by determining the concordance coefficient (intraclass correlation coefficient). The software used was Medcalc 14.8 (Acacialan 22 B‐8400 Ostend, Belgium, 2014). On the X axis of the Bland‐Altman plots was the mean of the estimated_24HU measurements and the 24‐hour urine collection results. On the Y axis, the difference between the two measurements was obtained by subtracting the estimated results from the 24‐hour urine collection results. The limits of agreements were defined as: (1) d=mean difference between estimated results and measured results, and (2) SD=when multiplied by 1.96 gives a confidence interval within 95% of the limits of validity. The significance threshold used was 5% for all statistical analyses.
Results are expressed in grams of sodium chloride (NaCl) and potassium chloride (KCl), as this is the form in which Na and K are excreted in urine. The Na and K results can be deduced by conversion (1 g NaCl=0.4 g Na, 1 g KCl≈0.5 g K).28, 29
Results
Description of the Population
Of the 402 spot and 24‐hour samples collected, 48 analysis results were rejected because of abnormal creatinine levels26 or because of the use of diuretics, positive glycosuria, or issues with the analysis at the laboratory (Figure 1). Therefore, 354 laboratory analysis results were kept for the concordance study. The general characteristics of the patients are summarized in Table 1. The distribution of men and women between the rural and urban areas was not significantly different.
Figure 1.
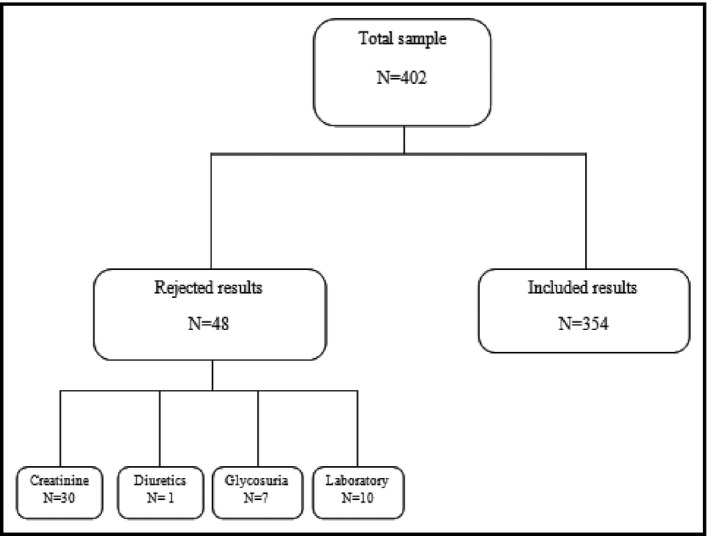
Flow diagram of the study.
Table 1.
General and Health Characteristics of Study Patients
| Variables | Number | Percentage | Mean±SD | Min–Max |
|---|---|---|---|---|
| Zone | ||||
| Urban | 184 | 51.9 | ||
| Rural | 170 | 48.1 | ||
| Sex | ||||
| Male | 172 | 48.6 | ||
| Female | 182 | 51.4 | ||
| Social status | ||||
| Office workers | 96 | 27.3 | ||
| Workers | 74 | 21 | ||
| Farmers and resellers | 181 | 51.5 | ||
| Education level | ||||
| ≤Primary | 234 | 66.1 | ||
| >Primary | 120 | 33.9 | ||
| Age, y | 354 | 43.0±11.3 | 25–64 | |
| BMI, kg/m2 | 354 | 24.33±4.9 | 15.8–44.6 | |
| Systolic BP, mm Hg | 354 | 128.0±22.0 | 88.0–241.0 | |
| Diastolic BP, mm Hg | 354 | 79.0±16.0 | 47.0–162.0 | |
| Hypertensive (known) | 67 | 18.9 | ||
Abbreviations: BP, blood pressure; SD, standard deviation. Body mass index (BMI) is expressed as weight (kg)/height2 (m2).
The overall NaCl (Na) excretion mean based on the 24‐hour urine collection was as follows: 10.2±4.9 (4.0±1.9) g/24 h vs 10.7±7 (4.2±2.8) g/24 h for the estimated_24HU excretion mean (spot). The overall KCl (K) excretion mean for the 24‐hour urine collection was 2.9±1.4 (1.4±0.7) g/24 h vs 3.9±2.1 (1.9±1.0) g/24 h for the estimated_24HU excretion mean (spot) (Table 2).
Table 2.
Estimated and Measured Levels of NaCl and KCl
| Variables | Measured | Estimated | |
|---|---|---|---|
| Mean±SD | Mean±SD | Mean±SD | |
| Diuresis, L | 2.8±1.2 | ||
| Creatinine spot urine, mg/L | 1164.3±865.5 | ||
| 24HU Creatinine, mg/L | 629.1±403.5 | ||
| 24HU_NaCl, g | 10.2±4.9 | 10.7±7 | |
| 24HU_Na, g | 4.0±1.9 | 4.2±2.8 | |
| 24HU_KCl, g | 2.9±1.4 | 3.9±2.1 | |
| 24HU_K, g | 1.4±0.7 | 1.85±1.0 |
Abbreviations: 24HU, 24‐hour urine; K, potassium; Na, sodium; SD, standard deviation. Creatinine levels in mg/L and sodium chloride (NaCl) and potassium chloride (KCl) levels in g/L from the 24‐hour samples were multiplied by the diuresis for the levels per 24 hours. Conversion was performed to take the NaCl and KCl measurements initially in mEq to g/L (1 mEq×1/17 for NaCl and 1 mEq×1/13 for KCl, respectively).
Correlation Between Estimated and Measured Na and K Excretion
There was a significant positive correlation between estimated values (NaCl and KCl) and measured values: r=0.65 (P<.001) for NaCl and r=0.76 (P<.001) for KCl, respectively (Figure 2). In the subgroup of participants with “hypertensive known” status (n=67), correlation results were: r=0.48 (P<.001) for NaCl and r=0.76 (P<.001) for KCl.
Figure 2.
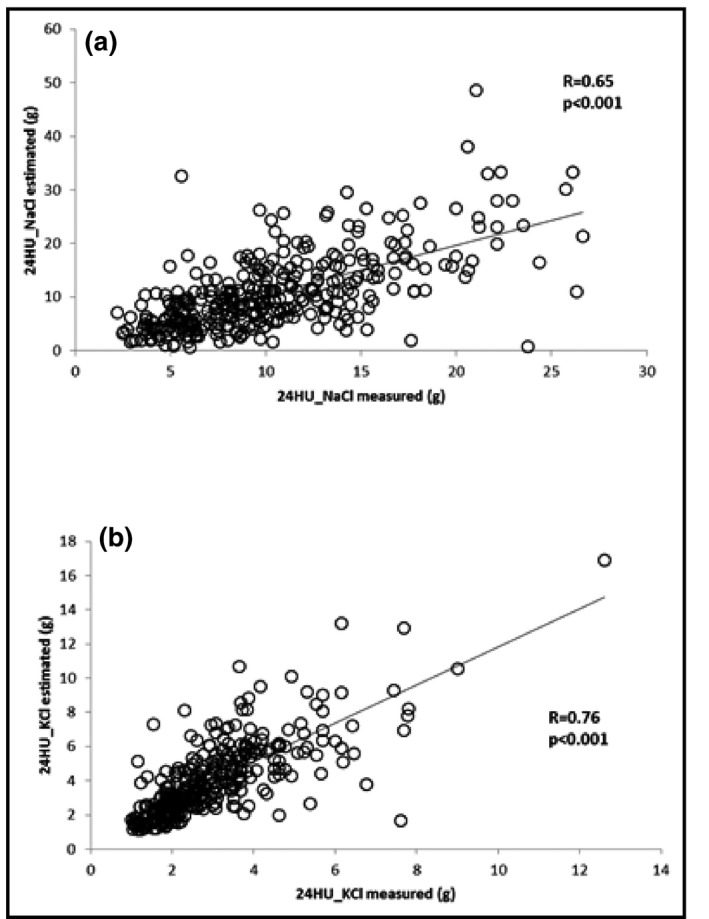
Correlation between measured 24‐hour urine sodium chloride (24HU_NaCl) and estimated 24HU_NaCl (a) and measured 24‐hour urine potassium chloride (24HU_KCl) and estimated 24HU_KCl (b).
Concordance Between Estimated and Measured Na and K Excretion
The assessments of the concordance between estimated and measured NaCl and KCl are presented in Figure 3 and Figure 4 for NaCl and KCl, respectively. The concordance coefficients were identical both for NaCl and KCl (r=0.61). The mean differences were: d=−0.5 g, (d±2SD=−11 and 10.1 g) for NaCl and d=−1 g (d±2SD=−3.8 and 1.8 g) for KCl. In the subgroup of participants with “hypertensive known” status, agreements limits of Bland‐Altman plots were: d=−0.6 g; d±2SD=−17.0 and 15.7 g for NaCl and d=−1.0 g; d±2SD=−3.9 and 1.7 g for KCl.
Figure 3.
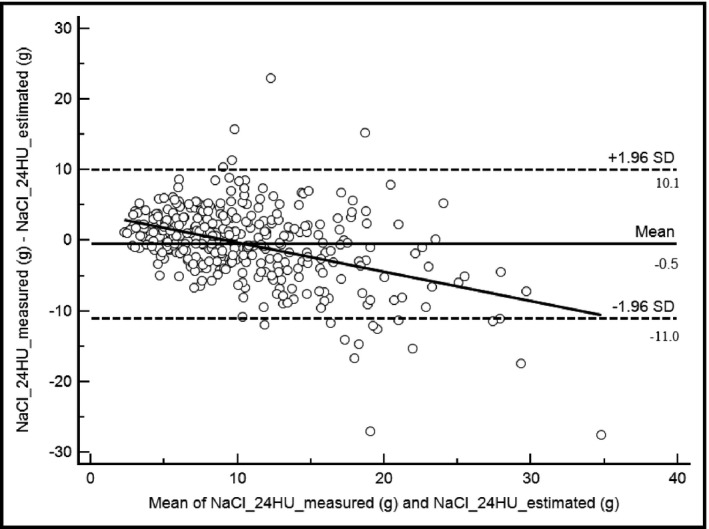
Bland‐Altman plots and trend curve for sodium chloride (NaCl) measurements. Mean, −0.5; 95% confidence interval [CI], −1.0 to 0.1. Lower limit, −11.0; 95% CI, −11.9 to −10.0. Upper limit, 10.1; 95% CI, 9.1–11.0. Intraclass coefficient, 0.61; 95% CI, 0.55–0.66; R=0.42. 24HU indicates 24‐hour urine.
Figure 4.
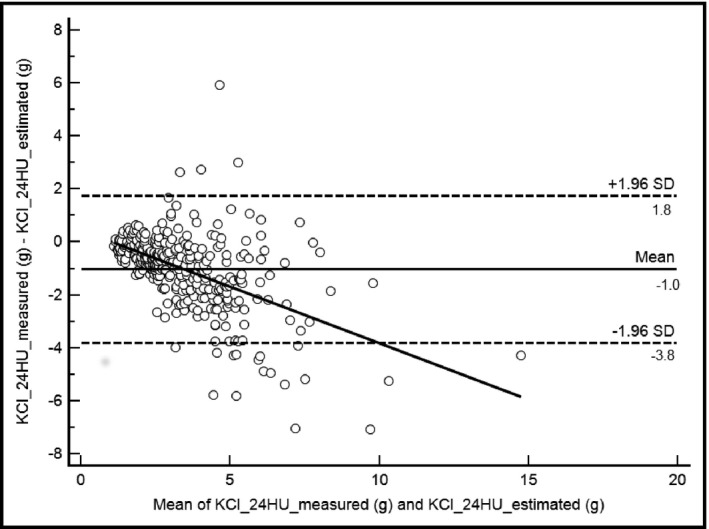
Bland‐Altman plots and trend curve for potassium chloride (KCl) measurements. Mean, −1.0; 95% confidence interval [CI], −1.1 to −0.8. Lower limit, −3.8; 95% CI, −4.05 to −3.54. Upper limit, 1.8; 95% CI, 1.5–2.0. Intraclass coefficient, 0.61; 95% CI, 0.56–0.66; R, 0.51. 24HU indicates 24‐hour urine; SD, standard deviation.
Discussion
To our knowledge, this is the first study to assess concordance between spot and 24‐hour urine collection measurements in black African populations. Our results showed a significant positive correlation between measurements of spot urine and 24‐hour urine. Agreement between NaCl (estimated vs measured) showed good concordance (r=0.61); however, the limits remain too large (10.5 g) (Figure 3) and show that spot urine can widely overestimate or underestimate urinary NaCl levels. It cannot be used in a clinical setting.
The concordance assessment of KCl was good (r=0.61) and the agreement limits of the Bland‐Altman plots were acceptable from a clinical point of view, with a confidence interval of 2.8 g (Figure 4). In the subgroup of participants with “hypertensive known” status, the agreement was similar to those obtained for the general population for KCl (d=−1.0 g; d±2SD=−3.9 and 1.7 g), while the results were even clinically unacceptable for NaCl (d=−0.6 g; d±2SD=−17.0 and 15.7 g). A spot urine sample taken during the second urination of the day can therefore be used to determine urinary K excretion and then dietary K levels.
Furthermore, analysis of the trend graph derived from the Bland‐Altman plots shows that for KCl levels <5 g/24 h, estimated values and measured values are almost identical whereas for levels above 5 g, the spot urine overestimates K values. These results suggest that the spot urine technique is a very good method to determine KCl urinary excretion, particularly for levels <5 g within the study population.
The urinary excretion results in this study suggest very high Na intake and low K intake according to World Health Organization recommendations.28, 29
Validity of Our Results
Methodology
In this study, we assessed concordance using the Bland‐Altman method, which is the gold standard, whereas other studies simply assessed the correlation between spot urine and 24‐hour urine measurements to validate the use of spot urine samples to determine Na and K intakes. It should be noted that these two methods can be strongly correlated but systematically different. The advantage of the Bland‐Altman method is that by assessing the concordance between the measurements, it allows the user to decide whether the concordance is satisfactory (particularly to decide whether the difference between the two results is clinically acceptable).16, 17, 18
Urine Completeness and the Use of Creatinine
In this study, 30 results were rejected because of abnormal urine creatinine levels. Despite the safeguards put in place and the instructions given to participants, this could be due to error during the collection process. Furthermore, there are no established standards for creatinine levels in black populations despite it being known that creatinine excretion levels differ greatly depending on ethnicity and race and could be higher in black populations.30 Finally, there are large differences in the standards used for the validation of creatinine in urine excretion assessment studies, making comparisons difficult. We applied standards using only lower limits depending on sex.26 Another method for the validation of 24‐hour urine completeness uses oral administration of pills containing para‐aminobenzoic acid, a nontoxic biomarker that could have been used as an alternative to creatinine. However, this requires strict observance of the prescription,31, 32 and various allergic reactions have been reported.33
Moreover, various factors can affect urinary excretion of creatinine, including muscle mass, consumption of protein‐rich foods, intense physical activity, age, and weight.33, 34, 35 These factors were controlled in the study population. Patients' eating habits mainly consisted of cereals, tubers, vegetables, and fish, whereas meat and milk were less readily available,36 and they had been instructed to not change their diet. There was no intense physical exercise during the study period.
Study Limitations
This study has some limitations. First, we did not assess the diet composition of participants, mainly because dietary instruments (eg, food frequency questionnaire, food composition tables) have not been specifically developed for Benin. Second, only one spot urine and one 24‐hour urine collection sample was taken, which does not reflect normal urinary excretion given daily and longer‐term variations in dietary Na and K at an individual level. Lastly, the study did not go into detail on the potential participation of individuals with chronic kidney, heart, or liver disorders.
Comparison of Results With the Literature
Because we limited the study to the correlation coefficient between two measurements, our results are comparable with those previously described in the literature,10, 11, 12, 13, 14, 15 but for clinical practice, only results using the Bland‐Altman method are acceptable. Work carried out by Doenyas‐Barak and colleagues19 and Han and colleagues20 fit these criteria.
Doenyas‐Barak and colleagues19 validated the use of a mean from four urinary spot samples taken at precise times in the day as an alternative for the estimation of NaCl urinary excretion in an Israeli population without cardiovascular or kidney disease. For NaCl, the validity limits of the Bland‐Altman plots were acceptable at d=−0.6 g, d±2SD=3.12, and −4.3 g.19 Their limits were much lower than ours and this could be explained by the much larger quantity of samples taken during their study, which took place over a 2‐day period for a 24‐hour time frame. It therefore took into account variation between days, which our study did not. However, our Bland‐Altman results for KCl are similar to those found by these authors. Their agreement limits for KCl were d=0.8 g, d±2SD=4.3, and −2.6 g. These results validate the use of spot urine to determine urinary excretions of K. Han and colleagues20 validated the use of spot urine samples taken during the second urination of the day to determine urinary Na excretion in an Asian population with hypertension. They showed Bland‐Altman agreement limits of d=0 g, d±2SD at 6.3, and −6.6 g/24 h of NaCl using the Kawasaki method.20 However, their confidence interval for the mean difference seems unacceptable from a clinical standpoint. This suggests a looser interpretation of the Bland‐Altman results. Furthermore, our results were for healthy patients.
Clinically, the differences in the concordance between our estimates of Na and K intakes may relate to dietary intake differences induced by the study design, ie, participants would eat less salt (Na) but not less fruit (K) along some social desirability bias, or by differences in metabolism or excretion of these electrolytes. Participants were not informed of the focus on salt and K but were informed that their diet would be assessed and were advised to adhere to their usual diet. In addition, the intake of Na varies more over time than K because of the ubiquitous composition of K in many foods, while Na tends to be concentrated in only a few foods (and added salt at the table). This may partly explain the good agreement between estimates of K in spot urine and 24‐hour urine and the lower agreement in Na. This differential variability in dietary intake of Na and K further justifies the use of repeat measurements of either spot or 24‐hour urine over several days to reliably assess intake at the individual level.
Conclusions
This study comparing Na and K excretion based on spot urine vs 24‐hour urine collection suggests that spot samples are useful to estimate daily Na and K intake. However, estimates differ substantially for Na. Again, one must contrast the purpose of urine analysis for salt intake. If analysis is performed to assess population levels, then spot urine results are unbiased and valid. If the purpose is to assess an individual's level (eg, in order to advise this person), then estimates based on one spot urine is unreliable and likely not useful (and should rely on repeat spot urine and/or repeat 24‐hour urine collection to improve accuracy). As shown, even 24‐hour collections are quite unreliable at the individual level and should be repeated.37 As the purpose of our study was to assess population salt intake estimates, spot urine is and remains a valid approach and can be used in similar epidemiological surveys. However, if the purpose of the study was to assess the association between salt intake at the individual level and subsequent health outcomes, then repeat 24‐hour urine collection should be performed.
Disclosure
The authors declare no conflicts of interest.
Acknowledgments and funding
We thank Mr William Francis who translated this manuscript, the National Non‐Communicable Diseases Control Program of Benin Health Ministry for contribution in collecting data, and the participants who gave their consent for this study. The study benefited from unconditional seed funding from PepsiCo (United States) through the African Institute for Health & Development, Nairobi, Kenya. Additional funding was provided by the World Health Organization (Benin) and INSERM UMR_S 1094, Limoges, France.
J Clin Hypertens (Greenwich). 2016;18:634–640. DOI: 10.1111/jch.12722 © 2015 Wiley Periodicals, Inc.
References
- 1. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. [DOI] [PubMed] [Google Scholar]
- 2. WHO . Effect of Reduced Sodium Intake on Cardiovascular Disease, Coronary Heart Disease and Stroke. Geneva: WHO; 2012. [Google Scholar]
- 3. WHO . Effect of Increased Potassium Intake on Cardiovascular Disease, Coronary Heart Disease and Stroke. Geneva: WHO; 2012. [Google Scholar]
- 4. WHO . Less Salt, Less Risk of Heart Disease and Stroke. Reducing Salt Intake in Populations. Report of a WHO Forum and Technical Meeting 5‐7 October 2006, Paris, France. Geneva: WHO; 2007. [Google Scholar]
- 5. Bentley B. A review of methods to measure dietary sodium intake. J Cardiovasc Nurs. 2006;21:63–67. [DOI] [PubMed] [Google Scholar]
- 6. Stamler J. The INTERSALT Study: background, methods, findings, and implications. Am J Clin Nutr. 1997;65(Suppl 2):626s–642s. [DOI] [PubMed] [Google Scholar]
- 7. Holbrook JT, Patterson KY, Bodner JE, et al. Sodium and potassium intake and balance in adults consuming self‐selected diets. Am J Clin Nutr. 1984;40:786–793. [DOI] [PubMed] [Google Scholar]
- 8. Schachter J, Harper PH, Radin ME, et al. Comparison of sodium and potassium intake with excretion. Hypertension. 1980;2:695–699. [DOI] [PubMed] [Google Scholar]
- 9. Ji C, Sykes L, Paul C, et al. Systematic review of studies comparing 24‐hour and spot urine collections for estimating population salt intake. Pan Am J Public Health. 2012;32:307–315. [DOI] [PubMed] [Google Scholar]
- 10. Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20:7–14. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24‐h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. [DOI] [PubMed] [Google Scholar]
- 12. Mann SJ, Gerber LM. Estimation of 24‐hour sodium excretion from spot urine samples. J Clin Hypertens (Greenwich). 2010;12:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mann SJ, Gerber LM. Estimation of 24‐h sodium excretion from a spot urine sample using chloride and creatinine dipsticks. Am J Hypertens. 2010;23:743–748. [DOI] [PubMed] [Google Scholar]
- 14. Kang SS, Kang EH, Kim SO, et al. Use of mean spot urine sodium concentrations to estimate daily sodium intake in patients with chronic kidney disease. Nutrition. 2012;28:256–261. [DOI] [PubMed] [Google Scholar]
- 15. Kawamura M, Ohmoto A, Hashimoto T, et al. Second morning urine method is superior to the casual urine method for estimating daily salt intake in patients with hypertension. Hypertens Res. 2012;35:611–616. [DOI] [PubMed] [Google Scholar]
- 16. Fuhrman C, Chouaïd C. Concordance between two variables: numerical approaches (qualitative observations ‐ the kappa coefficient‐; quantitative measures. Rev Mal Respir. 2004;21:123–125. [DOI] [PubMed] [Google Scholar]
- 17. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 18. Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. [DOI] [PubMed] [Google Scholar]
- 19. Doenyas‐Barak K, Beberashvili I, Bar‐Chaim A, et al. Daily sodium and potassium excretion can be estimated by scheduled spot urine collections. Nephron. 2015;130:35–40. [DOI] [PubMed] [Google Scholar]
- 20. Han W, Sun N, Chen Y, et al. Validation of the spot urine in evaluating 24‐hour sodium excretion in Chinese hypertension patients. Am J Hypertens. 2015;28:1368–1375. [DOI] [PubMed] [Google Scholar]
- 21. Chronic diseases and health promotion . Manuel STEPS: Using the STEPS Manual. World Health Organization. http://www.who.int/chp/steps/manual/fr/. Accessed April 28, 2015.
- 22. Henderson H, Sundaresan T. Cluster sampling to assess immunization coverage: a review of experience with a simplified sampling method. Bull World Health Organ. 1982;60:253–260. [PMC free article] [PubMed] [Google Scholar]
- 23. Lemeshow S, Robinson D. Surveys to measure programme coverage and impact: a review of the methodology used by the expanded programme on immunization. World Health Stat Q. 1985;38:65–75. [PubMed] [Google Scholar]
- 24. Bennett S, Woods T, Liyanage WM, Smith DL. A simplified general method for cluster‐sample surveys of health in developing countries. World Health Stat Q. 1991;44:98–106. [PubMed] [Google Scholar]
- 25. Institut National de la Statistique et de l'Analyse Economique (INSAE) . Cahier des villages et quartiers de ville du département du Zou [Internet]. INSAE; 2004. http://www.ancb-benin.org/pdc-sdac-monographies/monographies_communales/Monographie_Bohicon.pdf. Accessed April 28, 2015.
- 26. Rhodes DG, Murayi T, Clemens JC, et al. The USDA Automated Multiple‐Pass Method accurately assesses population sodium intakes. Am J Clin Nutr. 2013;97:958–964. [DOI] [PubMed] [Google Scholar]
- 27. Arcand J, Floras JS, Azevedo E, et al. Evaluation of 2 methods for sodium intake assessment in cardiac patients with and without heart failure: the confounding effect of loop diuretics. Am J Clin Nutr. 2011;93:535–541. [DOI] [PubMed] [Google Scholar]
- 28. WHO . Sodium Intake for Adults and Children. Geneva: WHO; 2012. [PubMed] [Google Scholar]
- 29. WHO . Potassium Intake for Adults and Children. Geneva: WHO; 2012. [PubMed] [Google Scholar]
- 30. Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bingham SA. Biomarkers in nutritional epidemiology. Public Health Nutr. 2002;5:821–827. [DOI] [PubMed] [Google Scholar]
- 32. Bingham S, Cummings JH. The use of 4‐aminobenzoic acid as a marker to validate the completeness of 24 h urine collections in man. Clin Sci. 1983;64:629–635. [DOI] [PubMed] [Google Scholar]
- 33. Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813. [DOI] [PubMed] [Google Scholar]
- 34. Webster J, Garrow JS. Creatinine excretion over 24 hours as a measure of body composition or of completeness of urine collection. Hum Nutr Clin Nutr. 1985;39:101–106. [PubMed] [Google Scholar]
- 35. Bingham SA, Cummings JH. The use of creatinine output as a check on the completeness of 24‐hour urine collections. Hum Nutr Clin Nutr. 1985;39:343–353. [PubMed] [Google Scholar]
- 36. Division de la nutrition et de la protection des Consommateurs . Profil nutritionnel du Bénin [Internet]. FAO; 2011. ftp://ftp.fao.org/ag/agn/nutrition/ncp/ben.pdf. Accessed April 28, 2015.
- 37. Lerchl K, Rakova N, Dahlmann A, et al. Agreement between 24‐hour salt ingestion and sodium excretion in a controlled environment. Hypertension 2015;66:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]


