Abstract
We aimed to examine hypertension prevalence, awareness, treatment and control in a community sample and investigate the impact of using 24 hour ABPM. Office blood pressure (BP) was taken from the electronic health record. Study BP was measured by standardised methods. Participants were invited to undergo ABPM. Hypertension was defined by accepted thresholds or anti‐hypertensive use. Standardised questions assessed awareness and treatment. Control was defined as anti‐hypertensive use with BP below normal threshold. There were 931 (45%) participants with office BP, study BP and ABPM. By study BP, hypertension prevalence was 60%, awareness 59%, 60% were treated and 46% controlled. By daytime ABPM threshold, prevalence was 61%, awareness 59%, 59% were treated and 54% controlled. ABPM reclassified 13.5% from normotensive to hypertensive and 14.5% from hypertensive to normotensive. ABPM may not hugely impact population hypertension prevalence but at an individual level it reduces misclassification and facilitates more appropriate management.
Hypertension is a leading risk factor for cardiovascular mortality. In 2009 the World Health Organization estimated that raised blood pressure (BP) caused 51% of stroke deaths and 45% of coronary heart disease deaths worldwide.1 However, many people with hypertension are undiagnosed2 and of those who are diagnosed many have poorly controlled BP.3
Accurate measurement of BP is essential for the diagnosis and management of hypertension. Traditionally, measurements are carried out in a clinical setting and a diagnosis of hypertension is made based on this office reading. Ambulatory BP monitoring (ABPM) provides information over a 24‐ or 48‐hour period and in particular gives important information on nighttime BP. Ambulatory BP has been shown to be superior for the prediction of clinical events.4, 5
A systematic review and meta‐analysis on the relative effectiveness of clinic BP measurements and home BP monitoring (HBPM) compared with ABPM concluded that treatment decisions based on clinic BP or HBPM alone might result in overdiagnosis of hypertension.6 A subsequent United Kingdom study on the cost‐effectiveness of options for the diagnosis of hypertension in primary care reported that ABPM would reduce misdiagnosis and save costs. It was suggested that in the United Kingdom the increased costs related to ABPM would be counterbalanced by cost savings from better targeted therapy.7 The National Institute for Health and Care Excellence (NICE) in 2011 recommended that if office BP is 140/90 mm Hg or higher, ABPM should be offered to confirm the diagnosis of hypertension.8 The 2013 European Society of Hypertension (ESH) guidelines state that office BP remains the “gold standard” for screening, diagnosis and management of hypertension.9 They recommend HBPM or ABPM be carried out in certain clinically indicated situations including suspected white‐coat hypertension, drug resistance, and hypotensive symptoms. Assessment of nighttime BP is also a specific indication for ABPM.
Generally, studies examining hypertension prevalence have used clinic or home BP readings measured using standardized techniques.10 A recent study highlights the prevalence of masked uncontrolled hypertension among patients taking treatment for hypertension.11 While many studies have investigated masked hypertension and white‐coat hypertension diagnosed by ABPM,12, 13, 14 few population studies have compared the effect of different methods of measurement on prevalence rates of hypertension.15
The aim of this paper is to examine the prevalence, awareness, treatment, and control rates of hypertension in a community‐based sample and to investigate the impact of using ABPM on these rates. We also aim to examine the sensitivity and specificity of office BP measurements and the prevalence of white‐coat and masked hypertension in the sample.
Methods
Details of the Mitchelstown cohort have previously been described.16 In summary, patients were recruited from a single large primary care center, the LivingHealth Clinic in Mitchelstown, a town located in the south of Ireland. The practice serves a population catchment area of approximately 20,000. Those registered with the clinic in the 50‐ to 69‐year‐old age bracket were assigned a random number. Participants were invited based on this random number in batches of 150 until the target sample size of 2000 was achieved.
Participants self‐reported a history of doctor‐diagnosed hypertension and antihypertensive medication use by questionnaire.
After the participant had been in a relaxed seated position for at least 5 minutes, three BP readings were taken on the right arm, 1 minute apart, using the Omron Model M7 digital automatic BP monitor (Omron Healthcare, Inc, Lake Forest, IL). The average of the second and third BP reading was defined as the study BP.
Participants were offered 24‐hour ABPM at the time of their study visit. ABPM measurements were performed using the Meditech ABPM‐05 (Medical Information Technology, Inc, Westwood, MA) and data were stored using the dabl ABPM system (dabl Ltd, Dublin, Ireland).
Consent was obtained to access the electronic patient record and, if available, the most recent BP recorded by the participant's general practitioner (GP) was documented as the office BP.
Participants whose electronic patient record included codes for elevated BP, uncomplicated hypertension, and complicated hypertension (International Classification of Primary Care, Second Edition [ICPC‐2] codes K85, K86, K87) were defined as having coded hypertension.
Hypertension was defined using the BP thresholds for the different measurement techniques or by current antihypertensive medication use. Study and office thresholds were systolic BP (SBP) ≥140 mm Hg and/or a diastolic BP (DBP) ≥90 mm Hg. For ABPM the daytime and nighttime windows were defined by diary records. [Correction added on March 21, 2016, after first online publication: The sentence was amended.] ABPM data were excluded if there were <14 daytime readings and/or <7 nighttime readings. The daytime thresholds were an SBP ≥135 mm Hg and/or a DBP ≥85 mm Hg. Nighttime thresholds were an SBP ≥120 mm Hg and/or a DBP ≥70 mm Hg. Twenty‐four–hour thresholds were an SBP ≥130 mm Hg and/or a DBP ≥80 mm Hg. Daytime and/or nighttime hypertension was defined by combining the daytime hypertension threshold with the nighttime hypertension threshold.
Participants were classified as being aware of their hypertension if they answered “yes” to the question “Have you ever been told by a doctor that you have, or have had, high blood pressure?” Participants were classified as treated if they answered “yes” to the question “Has your doctor given you a prescription for blood pressure tablets?”
Control of hypertension was defined by being on antihypertensive medications with a BP value of <140/90 mm Hg for the clinic and study BP measurements. For ABPM readings, controlled hypertension was defined by being on antihypertensive medications with BP values <135/85 mm Hg for daytime, <120/70 mm Hg for nighttime, and <130/80 mm Hg for 24‐hour BP.
Prevalence, awareness, treatment, and control rates of hypertension were calculated and compared using the different measurement techniques and thresholds. The sensitivity and specificity of the study and office BP measurements were calculated using the ABPM daytime threshold as the gold standard.
Ethical approval for the study was obtained from the Clinical Research Ethics Committee of the Cork Teaching Hospitals. All participants provided written informed consent. The study was carried out in accordance with the Declaration of Helsinki.
Results
Of 3051 invitees, 2047 (67%) participants were recruited into the study. Of these, 2042 (99.8%) had their BP measured for the study, 1723 (84.2%) had a previous BP documented by their GP, and 1207 (59.0%) underwent 24‐hour ABPM. We excluded 128 patients from the ABPM analysis because of incomplete data. A total of 931 (45%) study participants had satisfactory data for 24‐hour ABPM, study BP, and previous office BP. The baseline characteristics of the sample overall and those with all three measures are shown in Table 1. Those in the subsample were more likely to report a doctor diagnosis of hypertension and to report use of antihypertension medications, 36% vs 29% in the overall group for both. The groups were similar in other measured characteristics.
Table 1.
Baseline Characteristics
| Total Sample (N=2047) | Subsample With All Three Measures (n=931) | |
|---|---|---|
| Age, y | 60±6 | 60±5 |
| Men | 1008 (49) | 435 (47) |
| Smoking status | ||
| Nonsmoker | 1002 (51) | 470 (52) |
| Former smoker | 671 (34) | 283 (32) |
| Current smoker | 292 (15) | 146 (16) |
| Medical history | ||
| Hypertension | 567 (29) | 329 (36) |
| Myocardial Infarction | 49 (2) | 24 (2.6) |
| Stroke | 22 (1) | 11 (1.2) |
| Heart failure | 8 (0.4) | 5 (0.6) |
| Diabetes | 174 (9) | 82 (9) |
| Antihypertensive medication | 584 (29) | 329 (36) |
| Cholesterol‐lowering medication | 711 (36) | 352 (38) |
| BMI | 29±5 | 29±5 |
| Waist circumference | 97±13 | 97±13 |
| LDL | 3.2±0.9 | 3.2±0.9 |
| Creatinine | 71±16 | 71±15 |
| ACR | 0.7±2.1 | 0.7±1.9 |
| eGFR | 90±13 | 89±13 |
| Office systolic BP | 132±13 | 134±15 |
| Office diastolic BP | 78±9 | 79±9 |
| Study systolic BP | 130±17 | 134±15 |
| Study diastolic BP | 80±10 | 83±10 |
| Daytime systolic BP | 131±13 | |
| Daytime diastolic BP | 77±9 | |
| Nighttime systolic BP | 112±14 | |
| Nighttime diastolic BP | 63±8 | |
| 24‐h systolic BP | 124±13 | |
| 24‐h diastolic BP | 72±8 | |
ACR, albumin/creatinine ratio; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; LDL, low‐density lipoprotein. Values are expressed as number (percentage) or mean±standard deviation.
The mean office BP was 134/79 mm Hg and the study BP was 134/83 mm Hg. The daytime BP was 131/77 mm Hg, with a nighttime BP of 112/63 mm Hg. The prevalence of hypertension ranged from 50% to 64% depending on the measurement method and threshold used (Table 2 and Figure). For ABPM, the chosen threshold impacted rates, with the 24‐hour threshold resulting in lower prevalence rates and higher awareness, treatment, and control rates compared with the other thresholds. Of those who were hypertensive by office BP, 31% (161/521) were coded as hypertensive in the electronic health record. Of these individuals, 80% (120/150) were aware and 81% (128/159) were treated, while 49% (63/128) were controlled based on office BP. (Data not shown, some missing data for awareness and treatment questions.)
Table 2.
Hypertension Prevalence, Awareness, Treatment, and Control
| Total (N=931) | Prevalence, n/N (%) | Awareness, n/N (%) | Treatment, n/N (%) | Control, n/N (%) |
|---|---|---|---|---|
| Study hypertension | 557/931 (60) | 314/528 (59) | 329/549 (60) | 151/329 (46) |
| Office hypertension | 521/931 (56) | 313/492 (64) | 329/512 (64) | 161/329 (49) |
| Daytime hypertension | 568/931 (61) | 316/535 (59) | 329/553 (59) | 176/329 (54) |
| Nighttime hypertension | 479/931 (51) | 311/450 (69) | 329/469 (70) | 205/329 (62) |
| 24‐h hypertension | 462/931 (50) | 309/435 (71) | 329/452 (73) | 225/329 (68) |
| Daytime and/or nighttime hypertension | 593/931 (64) | 317/559 (57) | 329/577 (57) | 151/329 (46) |
Due to missing data, N in columns 2 and 3 do not represent n in column 1.
Figure 1.
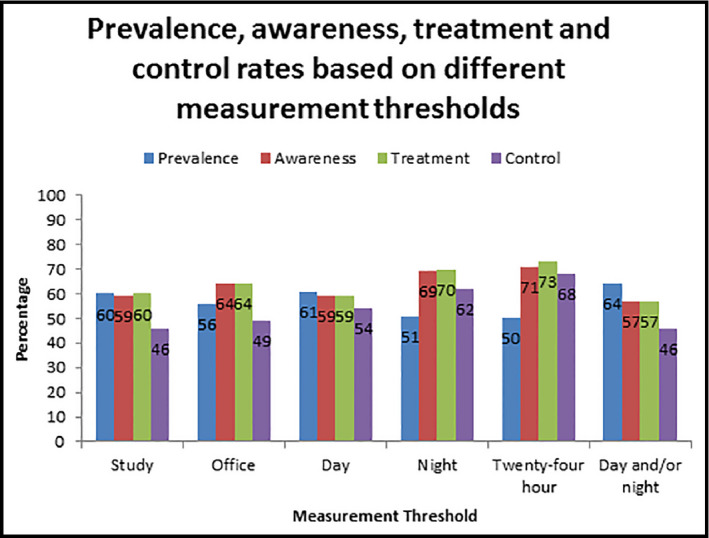
Prevalence, awareness, treatment, and control rates of hypertension by different measurement thresholds.
Table 3 compares ABPM with study BP. The prevalence of white‐coat hypertension was 14.5% (133/931) and masked hypertension was 13.5% (125/931) using the daytime ABPM threshold. Variations are seen depending on the chosen threshold and treatment status, with a higher rate of white‐coat hypertension (24%, 225/931) and a lower rate of masked hypertension (6%, 56/931) when the 24‐hour threshold was chosen.
Table 3.
Impact of ABPM on Blood Pressure Classification
| Hypertensive by Study BP and ABPM, n/N (%) | Normotensive by Study BP and ABPM, n/N (%) | White‐Coat Hypertension (Study Hypertension and ABPM Normotension), n/N (%) | Masked Hypertension (Study Normotension and ABPM Hypertension), n/N (%) | |
|---|---|---|---|---|
| Daytime threshold | ||||
| All | 273/931 (29) | 400/931 (43) | 133/931 (14.5) | 125/931 (13.5) |
| Treated | 108/329 (33) | 106/329 (32) | 70/329 (21) | 45/329 (14) |
| Untreated | 156/577 (27) | 286/577 (49) | 61/577 (11) | 74/577 (13) |
| 24‐h threshold | ||||
| All | 181/931 (19) | 469/931 (50) | 225/931 (24) | 56/931 (6) |
| Treated | 81/329 (25) | 128/329 (39) | 97/329 (29) | 23/329 (7) |
| Untreated | 93/577 (16) | 332/577 (58) | 124/577 (21) | 28/577 (5) |
| Nighttime threshold | ||||
| All | 194/931 (21) | 444/931 (48) | 212/931 (23) | 81/931 (9) |
| Treated | 91/329 (28) | 118/329 (36) | 87/329 (26) | 33/329 (10) |
| Untreated | 97/577 (17) | 316/577 (55) | 120/577 (21) | 44/577 (8) |
| Combined day and night thresholds | ||||
| All | 300/931 (32) | 383/931 (41) | 106/931 (11) | 142/931 (15) |
| Treated | 126/329 (38) | 99/329 (30) | 52/329 (16) | 52/329 (16) |
| Untreated | 164/577 (28) | 279/577 (48) | 53/577 (9) | 81/577 (14) |
The total sample was N=931 and n=577 were treated and n=329 untreated. Information on antihypertensive medication was missing for 25 patients.
Using daytime BP measured by ABPM as the gold standard, the sensitivity and specificity of the study BP was 70% and 76%, respectively, vs 56% and 74%, respectively, for office BP.
Discussion
Our study demonstrates a high prevalence of hypertension ranging from 50% to 64% depending on the measurement method and BP threshold used. The awareness rate varied from 57% to 71%, while 57% to 73% were treated and 46% to 68% were controlled. These figures are similar to recent data from a nationally representative sample of community‐dwelling older Irish adults. The prevalence rate was 63.7% and awareness rate was 54.5%, with 58.9% taking antihypertensive medication and 51.6% with controlled BP.17 While these figures are suboptimal, they do compare favorably internationally. A cross‐sectional analysis from the Prospective Urban and Rural Epidemiological (PURE) study18 demonstrated an awareness rate of 46.5%, with 40.6% receiving treatment and 32.5% of those on treatment controlled across high‐, middle‐, and low‐income countries. The rates for high‐income countries alone were 49%, 46.7%, and 40.7%. A study carried out in Spain demonstrated achieved BP targets in 46.3% of those in the Presión Arterial en la Población Española en Los Centros de Atención Primaria (PRESCAP) 2010 cross‐sectional study.19 Other work from the same study highlights therapeutic inertia wherein healthcare providers often do not initiate or intensify therapy appropriately during visits. This has improved from 2002 to 2010 but remains an issue. Treatment was modified in just 41.4% of those with uncontrolled BP in 2010, an improvement from 18.3% in 2002.20
The prognostic importance of 24‐hour ambulatory BP is well‐known.4, 5 It is important to examine the day and night BP window as the nighttime BP is recognized to be of greater prognostic significance.21 Our findings confirm the clinical utility of ABPM in the management of patients with hypertension. A large proportion of apparently normotensive participants were hypertensive by ABPM and vice versa. Gijon‐Conde and colleagues22 recently demonstrated that a half of older apparently uncontrolled hypertensive patients were normotensive by ABPM. In our study, patients taking treatment were more likely to have white‐coat hypertension (21% vs 11% based on the daytime threshold). Masked hypertension was also common in both treated and untreated individuals. Therefore, ABPM would potentially allow for more appropriate management of treatment. This is in keeping with the study by Lovibond and colleagues7 which highlighted the cost savings with the use of ABPM due to more appropriately targeted therapy. In addition, the use of 24‐hour ABPM may help to overcome therapeutic inertia. The Rambler study23 demonstrated that the use of 24‐hour ABPM impacted prescribing of antihypertensive medication by GPs in Ireland.
The method of BP measurement and threshold level chosen had an impact on prevalence rates in this study. The higher sensitivity of the study BP over the office BP likely reflects the use of standardized methods and highlights the importance of measuring office BP according to guidelines to maximize accuracy.9 Using the recommended 24‐hour threshold resulted in more people being categorized as normotensive than using the daytime threshold level or a combination of both the daytime and nighttime thresholds. There has been considerable debate over diagnostic thresholds for ABPM.24 BP and its relationship with cardiovascular disease is continuous and the use of diagnostic thresholds therefore has limitations, which is highlighted by our study.25 However, clinicians do require diagnostic thresholds of normality for 24‐hour ABPM when treating patients, but they also need to be aware of the limitations of these thresholds.
This study offers an opportunity to reflect on the use of health information technology and electronic health records in particular in the management of hypertension. Accurate information is essential to facilitate optimal patient care, clinical governance, and healthcare planning. This is important given recently highlighted barriers to medical coding26 and present financial constraints within healthcare systems. While the impact of health information technology on time utilization is mixed, it has been shown to improve the delivery of preventative care, improve clinical monitoring, and to reduce medication errors.27 GPs generally carry out more coding than their hospital colleagues.28 In the Irish healthcare setting, individual GPs carry out their own coding with no healthcare policy incentives or support to do so. In our study, patients who were coded as hypertensive in the electronic health record were more likely to be aware of and to be on treatment for their hypertension, while control rates were similar to the overall office hypertension group. The increased awareness and improved treatment rates need to be translated into better control rates. In the United Kingdom, the introduction of incentivized care did result in improved recording and documentation of health indicators.29, 30, 31 Policy makers need to realize the importance of investment in information technology and support to facilitate coding in primary and secondary care, which, in turn, should impact hypertension awareness, treatment, and control rates.
Study Limitations and Strengths
Our study is limited by selection bias, with patients with a previous diagnosis of hypertension or taking antihypertensive medication more likely to agree to undergo 24‐hour ABPM. Of the total sample, 29% self‐reported a previous doctor diagnosis of hypertension vs 36% of those included in this subsample. Therefore, our findings may not be generalizable to the general population and the ABPM results may be an overestimate of actual prevalence. However, Table 3 highlights the potential impact of ABPM on the management of both treated and untreated individuals. Our results are based on one ABPM recording and it is recognized that nighttime BP profiles in particular are not fully reproducible, which may again have an impact on prevalence when BP is measured by ABPM.32 The fact that BP was measured using standardized methods in a large community‐based sample with the availability of previously documented office BP and 24‐hour ABPM are major strengths of this study. The availability of hypertension coding data is a further strength of the study, as it gives deeper insight into the challenges faced in the management of hypertension in daily clinical practice.
Conclusions
This study highlights a number of important points. Firstly, hypertension remains a public health priority. It is highly prevalent, and treatment and control rates are suboptimal. Secondly, the use of 24‐hour ABPM may not hugely impact overall population prevalence rates but it reduces misclassification at an individual level and therefore refines diagnosis and management of hypertension and should be considered a vital tool in the provision of care. Finally, healthcare information technology should be fully utilized so patients can receive best practice care and the burden of hypertension and its public health consequences can be reduced.
Funding Sources
Dr Anne‐Marie O'Flynn is funded by a Health Research Board Ireland Research Training Fellowship (reference HPF/2012/14) and has also received the John Feely research bursary from the Irish Heart Foundation to support this work. She has also received payment unrelated to the submitted work through her institution for the development of the European Society of Cardiology e‐learning platform. Dr Ronan Curtin has received funding and subsistence support for lectures and activities outside of the submitted work from A. Menarini, Daichi Sankyo, Astra Zeneca, Bayer, Bristol Myers Squibb, Pfizer, and Servier pharmaceutical companies. Professor Ivan Perry received funding through his institution from the Health Research Board Ireland to fund the initial data collection for the submitted work (reference HRC/2007/13). Professor Patricia Kearney has received grants from the Health Research Board Ireland and the European Union FP7 for work outside of the submitted work.
Acknowledgments
The authors give sincere thanks to the study participants, staff of the LivingHealth Clinic, the survey team, nurses, and administrators without whom this work would not be possible.
J Clin Hypertens (Greenwich). 2016;18:697–702. DOI: 10.1111/jch.12737 © 2015 Wiley Periodicals, Inc.
References
- 1. WHO. Global health risks: morality and burden of disease attributable to selected major risks. Geneva: World Health Organization, 2009. http://www.who.int/gho/publications/world_health_statistics/2012/en/index.html. Accessed September 15, 2015.
- 2. Yoon SS, Burt V, Louis T, Carroll MD. Hypertension among adults in the United States, 2009–2010. NCHS Data Brief. 2012;107:1–8. [PubMed] [Google Scholar]
- 3. Prugger C, Keil U, Wellmann J, et al. Blood pressure control and knowledge of target blood pressure in coronary patients across Europe: results from the EUROASPIRE III survey. J Hypertens. 2011;29:1641–1648. [DOI] [PubMed] [Google Scholar]
- 4. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin Outcome Study. Hypertension. 2005;46:156–161. [DOI] [PubMed] [Google Scholar]
- 5. Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood‐pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. [DOI] [PubMed] [Google Scholar]
- 6. Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lovibond K, Jowett S, Barton P, et al. Cost‐effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet. 2011;378:1219–1230. [DOI] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence . Hypertension in adults: diagnosis and management. http://www.nice.org.uk/guidance/CG127. Accessed September 15, 2015. [PubMed]
- 9. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 10. Wolf‐Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. [DOI] [PubMed] [Google Scholar]
- 11. Banegas JR, Ruilope LM, de la Sierra A, et al. High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur Heart J. 2014;35:3304–3312. [DOI] [PubMed] [Google Scholar]
- 12. Mancia G, Facchetti R, Bombelli M, et al. Long‐term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–853. [DOI] [PubMed] [Google Scholar]
- 13. Trudel X, Brisson C, Larocque B, Milot A. Masked hypertension: different blood pressure measurement methodology and risk factors in a working population. J Hypertens. 2009;27:1560–1567. [DOI] [PubMed] [Google Scholar]
- 14. Franklin SS, Thijs L, Hansen TW, et al. Significance of white‐coat hypertension in older persons with isolated systolic hypertension: a meta‐analysis using the International Database on ambulatory blood pressure monitoring in relation to cardiovascular outcomes population. Hypertension. 2012;59:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banegas JR, de la Cruz JJ, Graciani A, et al. Impact of ambulatory blood pressure monitoring on reclassification of hypertension prevalence and control in older people in Spain. J Clin Hypertens (Greenwich). 2015;17:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kearney PM, Harrington JM, Mc Carthy VJ, et al. Cohort profile: the Cork and Kerry Diabetes and Heart Disease Study. Int J Epidemiol. 2013;42:1253–1262. [DOI] [PubMed] [Google Scholar]
- 17. Murphy CM, Kearney PM, Shelley EB, et al. Hypertension prevalence, awareness, treatment and control in the over 50s in Ireland: evidence from The Irish Longitudinal Study on Ageing. J Public Health (Oxf). 2015. Apr 28 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310:959–968. [DOI] [PubMed] [Google Scholar]
- 19. Llisterri JL, Rodriguez‐Roca GC, Escobar C, et al. Treatment and blood pressure control in Spain during 2002‐2010. J Hypertens. 2012;30:2425–2431. [DOI] [PubMed] [Google Scholar]
- 20. Escobar C, Barrios V, Alonso‐Moreno FJ, et al. Evolution of therapy inertia in primary care setting in Spain during 2002‐2010. J Hypertens. 2014;32:1138–1145. [DOI] [PubMed] [Google Scholar]
- 21. Hansen TW, Li Y, Boggia J, et al. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. [DOI] [PubMed] [Google Scholar]
- 22. Gijon‐Conde T, Graciani A, Lopez‐Garcia E, et al. Impact of ambulatory blood pressure monitoring on control of untreated, undertreated, and resistant hypertension in older people in Spain. J Am Med Dir Assoc. 2015;16:668–673. [DOI] [PubMed] [Google Scholar]
- 23. Uallachain GN, Murphy G, Avalos G. The RAMBLER study: the role of ambulatory blood pressure measurement in routine clinical practice: a cross‐sectional study. Ir Med J. 2006;99:276–279. [PubMed] [Google Scholar]
- 24. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 25. Kikuya M, Hansen TW, Thijs L, et al. Diagnostic thresholds for ambulatory blood pressure monitoring based on 10‐year cardiovascular risk. Circulation. 2007;115:2145–2152. [DOI] [PubMed] [Google Scholar]
- 26. Atoyebi T. What are the barriers to E‐coding of quality clinical data in Irish hospitals from a coder's perspective? https://www.scss.tcd.ie/postgraduate/health-informatics/assets/pdfs/Toyosi%20Atoyebi%202012%20Msc%20Dissertation%20(10261072).pdf. Accessed September 15, 2015.
- 27. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical Care. Ann Intern Med. 2006;144:742–752. [DOI] [PubMed] [Google Scholar]
- 28. Kljakovic M, Abernethy D, de Ruiter I. Quality of diagnostic coding and information flow from hospital to general practice. Inform Prim Care. 2004;12:227–234. [PubMed] [Google Scholar]
- 29. Simpson CR, Hannaford PC, Lefevre K, Williams D. Effect of the UK incentive‐based contract on the management of patients with stroke in primary care. Stroke. 2006;37:2354–2360. [DOI] [PubMed] [Google Scholar]
- 30. McGovern MP, Boroujerdi MA, Taylor MW, et al. The effect of the UK incentive‐based contract on the management of patients with coronary heart disease in primary care. Fam Pract. 2008;25:33–39. [DOI] [PubMed] [Google Scholar]
- 31. McGovern MP, Williams DJ, Hannaford PC, et al. Introduction of a new incentive and target‐based contract for family physicians in the UK: good for older patients with diabetes but less good for women? Diabet Med. 2008;25:1083–1089. [DOI] [PubMed] [Google Scholar]
- 32. Cuspidi C, Meani S, Salerno M, et al. Reproducibility of nocturnal blood pressure fall in early phases of untreated essential hypertension: a prospective observational study. J Hum Hypertens. 2004;18:503–509. [DOI] [PubMed] [Google Scholar]


