Population‐wide dietary salt (sodium) reduction is recommended by a wide range of public health and scientific organizations based on systematic reviews demonstrating adverse health effects associated with excess salt consumption, in particular increased blood pressure (BP).1, 2, 3 The World Health Organization (WHO) recommends that adults consume less than 5 g of salt per day, with lower amounts for children aged 2 to 15 years.4 To minimize the economic burden of noncommunicable diseases, the United Nations has asked countries to reduce dietary salt intake by 30% by 2025.5 These public health efforts to reduce dietary salt have resulted in a large number of publications.6 This rapidly growing volume of literature makes it challenging, even for focused experts, to stay up to date.
As part of an initiative to assist scientific, clinical, and policy stakeholders to stay up to date in the science of salt, a periodic review of published studies will be reported in the Journal. As previously described,7 these summaries will be published six times per year with alternating reviews on studies relating to health outcomes and to salt reduction implementation programs. The objective of the current summary is to review the literature published in June and July 2015 about the effects of salt on health outcomes.
Methodology
A detailed description of the methodological approach used to identify and evaluate the literature in this review has been previously published.7 Briefly, articles were identified on a weekly basis through a MEDLINE search. The search strategy for the current review was based on that developed by the Cochrane Collaboration for systematic reviews on dietary salt and health, used in the development of the WHO dietary salt recommendations.1, 4 The studies of the effects of salt on health outcomes included in this review were identified from June 2, 2015, to July 28, 2015.
All identified studies were assessed using the Cochrane risk of bias tool.8, 9 Articles were reviewed, summarized, and appraised with reference to the risk of bias assessment findings. Information about both high‐ and low‐quality studies are captured in this review.
Results
The MEDLINE search identified 1171 possibly relevant studies that met the criteria for full review on the basis of their findings related to salt and health outcomes (Figure). Three studies evaluated the relationship between salt and BP, four studies assessed the associations between salt intake and surrogate outcomes for cardiovascular or kidney diseases, and one study examined the association between salt intake and mental distress. The risk of bias assessments for these studies can be found as part of the Appendix S1. Two studies10, 11 met the methodological standards applied to studies included in the Cochrane review (ie, randomized controlled trials of ≥4 weeks' duration, with no concurrent interventions, that achieved ≥40 mmol/d sodium reduction between intervention and control with sodium intake measured by 24‐hour urinary sodium excretion, and prospective cohort studies of ≥1 year duration that measured sodium intake in any way).1
Figure 1.
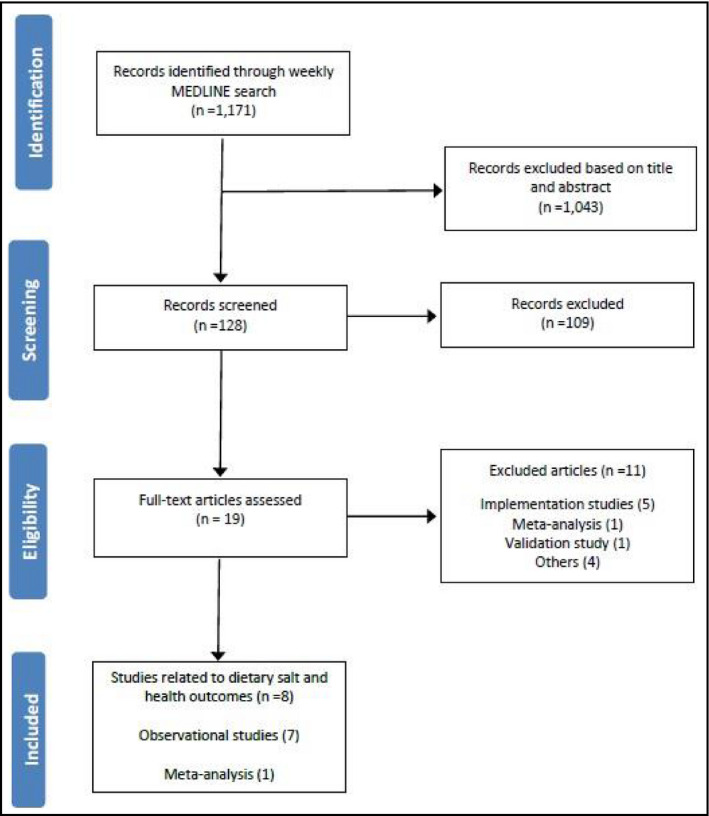
Studies included in the June to July 2015 review.
BP and Clinical Outcomes
How Long Does it Take to Achieve BP Reduction After Sodium Restriction, and is the Dose‐Response Relationship Between Dietary Sodium Intake and BP the Same in Hypertensives and Normotensives?
Graudal N, Hubeck‐Graudal T, Jürgens G, et al. The significance of duration and amount of sodium reduction intervention in normotensive and hypertensive individuals: a meta‐analysis. Adv Nutr. 2015;6(2):169–177. Erratum in: Adv Nutr. 2015;6:285.
Summary. In 2011, Graudal and colleagues conducted a systematic review and meta‐analysis of 167 randomized controlled trials to determine the effects of varying amounts of sodium intake on BP.12 They now present a supplementary analysis using a subset of the same data, examining: (1) the time to maximal BP response following initiation of dietary sodium reduction (“maximal efficacy analysis”), and (2) the dose‐response relationship between dietary sodium intake and BP.12 Of the pool of 167 studies, 15 trials were included in the maximal efficacy analysis. To examine changes in BP that occurred in the weeks following sodium reduction, serial comparisons were made for weeks 1 through 6. There were no statistically significant differences in systolic or diastolic BP detected from week to week. The effect of varying doses of sodium intake on BP was assessed in eight studies. Among hypertensive patients, higher sodium intake resulted in increased BP; however, no such relationship was observed among normotensive individuals.
Comment. The authors conclude that dietary sodium reduction decreases BP in hypertensive but not normotensive patients, and that maximal BP reduction occurs by the end of the first week of a sodium reduction intervention, based on the absence of changes in future weeks. The quantitative synthesis of the data in this study is a strength, but the design was weakened by the failure to search for and include possible new studies conducted since 2011. Publications related to dietary sodium are rising and it is possible that data have been missed.6 The present analysis also includes substantially fewer studies compared with the original analysis of 167 randomized controlled trials, based on the selected criteria to include trials with at least two follow‐up BP measurements between weeks 1 and 4 for the maximal efficiency analysis and three different dosages of sodium intake for the dose response analysis. These criteria potentially excluded a significant number of important studies. By comparison, a 2013 meta‐analysis by He and coworkers3 included many more studies (34 studies, 3230 participants) with a longer follow‐up period (≥4 weeks) and found that salt reduction was associated with significant reductions in BP in both hypertensive and normotensive individuals.3 Thus, the varying observations of Graudal and colleagues may be more reflective of inadequate statistical power than the absence of therapeutic effect. For example, Graudal and colleagues12 included five trials with 83 participants in the analysis of normotensives, whereas He and colleagues included 12 trials with 2240 participants.
In the dose‐response analysis, data were reported for the normotensive and hypertensive subgroups separately. However, meta‐regression was not performed to determine whether the apparent differences between the hypertensive and normotensive subgroups were statistically significant. In the time to maximal efficacy analyses, overestimation of the variances (and therefore wider confidence intervals) could occur because each time point was treated independently, rather than accounting for correlations between BPs across multiple time points within each study.13 Cross‐over trials that were included did not report paired data, and these studies were analyzed as if they were parallel‐group trials. This could also result in estimates having wider confidence intervals, which, in turn, could mask clinically important heterogeneity.14, 15 In addition, possible carryover effects in cross‐over trials without washout periods could result in underestimation of the treatment effect. The absence of a detectable difference in BP in pairwise comparisons during the first 6 weeks does not preclude the possibility that a significant difference in BP may be present and detectable with longer follow‐up.
We also noted possible discrepancies with respect to the accuracy of data extraction. For example, the included study by Gow and colleagues16 reported a mean systolic BP of 120 mm Hg in the normal sodium diet group, and Gradual and colleagues reports these data as missing. Table S4 contains errors in sodium intake and BP values for Burnier and colleagues17 and Heer and colleagues18 A detailed analysis of all the extracted data has not yet been conducted.
Finally, certain definitions were inconsistently applied. In the dose‐response analysis, sodium intake was classified according to four predefined categories (ie, low [<90 mmol/d], low usual [90–159 mmol/d], high usual [159–248 mmol/d], and high [>248 mmol/d]). However, if a trial examined two sodium dosages that were both within the same category, one of the interventions was reclassified into a neighboring one. In addition, one trial in the time to maximal efficacy analysis had BP ascertained at weeks 2, 4, 8, 12, and 1419; however, in the meta‐analysis, BP data for the week 8 measurement were reclassified to week 6, and data at weeks 12 and 14 were excluded from the final analysis. Consequently, both the exposures and outcomes of interest may have been subject to misclassification.
Overall, the multiple limitations impede the opportunity to make robust conclusions from this study and the results are inconsistent with other larger and more up‐to‐date meta‐analyses on the same topic.
Does Dietary Sodium Affect BP in Adolescent Girls?
Buendia JR, Bradlee ML, Daniels SR, et al. Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. JAMA Pediatrics. 2015;169(6):560–568.
Summary. This study examined the association between dietary sodium, potassium, and potassium‐to‐sodium ratio on BP in adolescent girls in a 10‐year longitudinal cohort study (National Heart, Lung, and Blood Institute's Growth and Health Study).10 In 1987 to 1988, the study enrolled 2185 girls between the ages of 9 and 10 years. Dietary sodium was assessed with 3‐day food records at baseline and in follow‐up years 1 to 5, 7, 8, and 10. After adjusting for multiple potential confounders, there was no association between sodium intake and BP either during adolescence or at the end of adolescence for participants who consumed >2500 mg/d (equivalent to 6.3 g/d salt). Higher levels of potassium intake were associated with lower systolic and diastolic BP. Likewise, a higher potassium‐to‐sodium ratio was associated with lower systolic BP but not diastolic BP.
Comment. The authors conclude that there is no association between sodium intake and BP in adolescent girls, but that there and is an association between potassium intake and BP and the potassium‐sodium ratio and BP. Since there are few prospective studies examining the relationship between dietary sodium and BP in children and adolescents, this study addresses an important evidence gap; however, the findings should be interpreted with care as they contradict clinical trial data showing BP reductions with reduced sodium intake.1, 20 Some methodological issues may also have influenced the findings, which are largely related to the observational study design. This includes residual confounding and the use of a “change‐in‐estimate” definition of ≥10% to identify confounders, which may lead to incorrect conclusions.21 Finally, dietary sodium intake estimates were based on self‐reported intakes via a 3‐day food record during 8 of the 10 study years; however, this may not be the most accurate way of assessing salt intake in an adolescent population.22 The study included only girls, thus the findings cannot be extrapolated to boys or younger children. The cardinal strengths of the study include its large sample size, long duration of follow‐up, detailed assessment of diet with multiple assessments of exposure over time, and the inclusion of a number of potential confounders in the analysis. The finding that the sodium‐potassium ratio is associated with BP for this population group is consistent with other studies; however, since the results related to sodium conflict with clinical trial data,1, 20 it cannot be concluded that sodium has no effect on the BP of adolescents based on the observational design.
How Much Sodium is Derived From Snack Foods Consumed by Adolescents and How Does This Affect Blood Pressure?
Ponzo V, Ganzit GP, Soldati L, et al. Blood pressure and sodium intake from snacks in adolescents. Eur J Clin Nutr. 2015;69(6):681–686.
Summary. Ponzo and colleagues23 examined the association between BP and overall sodium intake and sodium from processed snacks in a cross‐sectional study of 1200 Italian adolescents (aged 11–13 years) who were randomly selected from 6876 children participating in a school‐based health examination.23 All patients completed a 19‐item food frequency questionnaire (FFQ) that assessed snack consumption. To estimate total sodium intake, a food recall was administered among a randomly selected subsample of students (n=400). Approximately half of the average daily sodium consumed was from processed snack foods: average daily sodium intake was 3100±900 mg/d, with snacks contributing almost 1400±600 mg of sodium/d. Higher systolic BP and diastolic BP were associated with higher sodium consumption from snacks (FFQ, n=1200). An association was also found between BP and total sodium intake (food recall, n=400).
Comment. This study shows that a high proportion of dietary sodium was derived from processed snack foods in Italian adolescents. The authors conclude that dietary sodium is positively associated with BP in adolescents; however, the study has several methodological limitations. The cross‐sectional design of this study cannot demonstrate causation. Snack foods containing high amounts of sodium also tend to be calorically dense (and associated with adiposity) and low in potassium; hence, the association with higher BP may actually be the result of residual confounding through adiposity and low dietary potassium. Furthermore, the total sodium from snacks may be underestimated, as the administered FFQ may not have captured a complete estimate of all sources of sodium from snacks. The design weaknesses are duly acknowledged by the authors. Despite these limitations, the study results are consistent with extensive data from randomized controlled trials and epidemiological studies that increased dietary sodium increases BP in adults and children.1
Surrogate Outcomes
What is the Relationship Between Sodium Intake and Cardiovascular Structure and Function?
Haring B, Wang W, Lee ET et al. Effect of Dietary Sodium and Potassium Intake on Left Ventricular Diastolic Function and Mass in Adults ≤40 Years (from the Strong Heart Study). Am J Cardiol. 2015;115(9):1244–1248.
Summary. This 4‐year longitudinal cohort study (Strong Heart Study) included 1065 American Indians aged 18 to 39 years and assessed the relationship between sodium and potassium intake and changes in cardiac structure and function.11 Dietary sodium intake was assessed using a 119‐item FFQ. Sodium intake was not associated with any changes in cardiac function or geometry in normotensive patients; however, an increase in the sodium‐to‐potassium ratio was positively associated with arterial filling fraction. In hypertensive patients, dietary sodium was positively associated with atrial filling fraction, and an increase in the sodium‐to‐potassium ratio was related to left ventricular mass index.
Comment. The authors conclude that there is an association between sodium intake and atrial filling fraction in hypertensive patients, but no association was found with 11 other echocardiographic parameters. Significant changes in left ventricular structure and function may not have occurred given the young age of this population, as the myocardium may have a greater ability to adapt to a higher preload, particularly among normotensive individuals. This study has limited generalizability because it included only American Indians. In addition, sodium intake was estimated from an FFQ, which does not usually correlate well with objective measures of sodium intake.24, 25 Moreover, it is unknown whether the questions included on the FFQ were validated or adapted to appropriately capture the cultural foods and patterns of an American Indian population. Despite the strength of this study in assessing longitudinal changes in cardiac structure and function over time, there is a high risk of type I error as a result of multiple testing without statistical correction, and therefore their results may be due to chance.
What is the Relationship Between Sodium Intake and Cardiovascular Structure and Function?
Lee SK, Kim JS, Kim SH, et al. Sodium Excretion and Cardiovascular Structure and Function in the Nonhypertensive Population: The Korean Genome and Epidemiology Study. Am J Hypertens. 2015;28(8):1010–1016.
Summary. This cross‐sectional study assessed the relationship between sodium intake and several makers of cardiac structure and function in 1586 people 70 years and older with normal BP and no history of cardiovascular disease.26 Results from spot urine collection and Tanaka equation were used to estimate dietary sodium intake. After adjusting for confounders, sodium intake was not associated with any markers of left ventricular structure or function. Brachial ankle pulse wave velocity and carotid intima‐media thickness (IMT) were inversely associated with estimated sodium intake. Systolic and diastolic BP positively correlated with estimated sodium intake.
Comment. The authors conclude that higher sodium intake is associated with higher systolic and diastolic BP in normotensive individuals, but with decreased arterial stiffness and better arterial wall structure. The cross‐sectional design does not provide an indication of longitudinal changes in these cardiovascular endpoints with prolonged sodium exposure. Confounding, reverse causality, or the method of dietary sodium assessment may explain these divergent findings. A major limitation of the study is the use of a spot urine collection to estimate sodium intake, for reasons described in more detail in the Discussion. The use of a spot urine collection may explain why these findings contradict other studies that have shown positive linear relationships between sodium intake and IMT, pulse wave velocity, augmentation index, or central pulse pressure27, 28, 29; all of which used 24‐hour urine collections to estimate sodium intake. The authors have acknowledged this limitation. The findings should be interpreted with care.
Does Sodium Intake Affect the Relationship Between Blood Pressure and Vascular Damage?
Jankowski P, Stolarz‐Skrzypek K, Kawecka‐Jaszcz K, et al. Does sodium intake affect the relationship between blood pressure and vascular damage? Pol Arch Med Wewn. 2015;125(5):347–357.
Summary. This cross‐sectional study of 182 participants (37±14 years), who were randomly selected from the European Project On Genes in Hypertension cohort, assessed the relationship between carotid IMT and central and peripheral BP, and the effect of sodium intake on this relationship.30 Sodium intake and excretion was assessed with a 24‐hour urine collection. The correlation between sodium excretion and IMT was not statistically significant. When the participants were divided according to median sodium excretion (≤ or >232.6 mmol/d, equivalent to 13.4 g/d salt and 5.4 g/d sodium), IMT was associated with central systolic BP and pulse pressure in the patients with high sodium excretion. IMT was related only to central pulse pressure in patients with low sodium excretion.
Comment. This study identified a positive association between central pulse pressure and IMT, a marker of arterial wall structure, which may be modulated by dietary sodium. The analysis in this study is limited since a subgroup analysis based on sodium intake was performed, rather than including sodium intake as a covariate in the regression models. A strength of this study was the use of a 24‐hour urine collection to assess sodium intake, the gold standard assessment method; however, there was only 1 day of assessment, which is inadequate to assess usual intake in an individual. There is also no reported assessment regarding the completeness of the urine collections, potentially producing biased underestimates of sodium through the inclusion of patients who provided incomplete urine collections. The authors acknowledge the limitations of their study and it is difficult to determine from the research whether dietary sodium modulates the relationship between IMT and BP.
What is the Association Between Sodium Intake and Biochemical Risk Factors Associated With Chronic Kidney Disease Progression?
Nerbass FB, Pecoits‐Filho R, McIntyre NJ, et al. High sodium intake is associated with important risk factors in a large cohort of chronic kidney disease patients. Eur J Clin Nutr. 2015;69(7):786–790.
Summary. This cross‐sectional study of the Renal Risk in Derby cohort included 1733 patients from England with stage 3 chronic kidney disease (73±9 years) to determine the relationship between sodium intake and biochemical markers of cardiovascular disease and chronic kidney disease progression.31 Sodium intake was estimated with the average urinary sodium concentration in spot urine samples from 3 consecutive days and a predictive formula developed for the chronic kidney disease population. Those consuming >100 mmol of sodium per day (equivalent to 5.8 g/d salt and 2.3 g/d sodium) had significantly higher diastolic BP, mean arterial pressure, urine albumin‐to‐creatinine ratio, high‐sensitivity C‐reactive protein, and uric acid and used a greater number of antihypertensive drugs, compared with those with those who consumed <100 mmol of sodium per day. In multivariable regression analysis, sodium intake >100 mmol/d was an independent predictor of mean arterial pressure and albuminuria.
Comment. The authors conclude that higher dietary sodium is associated with markers of kidney disease and cardiovascular disease. The strength of the study is the large group of patients studied and detailed analyses performed. The major limitation is the use of a cross‐sectional design and spot urine collections to estimate sodium intake. The use of spot urine collections in healthy individuals is controversial; however, in a chronic kidney disease population it is likely even more troublesome based on the disturbed renal sodium handling that occurs with reduced renal blood flow, neurohumoral activation, and medical therapies that alter sodium homeostasis.
Despite these limitations, the findings are relatively consistent with randomized clinical trials in patients with chronic kidney disease.32
Other Outcomes
What is the Association Between Salt Intake and Mental Distress?
Shimizu Y, Kadota K, Koyamatsu J, et al. Salt intake and mental distress among rural community‐dwelling Japanese men. J Physiol Anthropol. 2015;34(1):26.
Summary: A cross‐sectional study examined the association between salt intake and mental distress among 1014 rural‐dwelling Japanese men.33 Salt intake was determined by a spot urine collection, which was used with the Tanaka equation to estimate 24‐hour urinary sodium. A Kessler 6 (K6) scale score ≥5 was used to identify mental distress. Approximately 10% of participants were identified as having mental distress. In a multivariable analysis, the lowest salt intake tertile (median 7.0 g salt per day, equivalent to 2.8 g sodium per day), compared with the highest salt intake tertile (10.8 g salt per day, equivalent to 4.3 g sodium per day), was associated with a K6 score of ≥5 in the overall group and in people with hypertension.
Comment. The authors of this study conclude that lower salt intake is associated with mental distress. Although not cited by the authors, other research has not found an association between mental disorders and salt intake.34 The findings of this new study need to be taken in the context of the cross‐sectional study design, which may be subject to reverse causation, ie, people with high psychological distress might eat less and thus have lower salt intakes. In addition, a single spot urine sample to estimate usual salt intake in individuals has been critiqued as inadequate, as described in the Discussion. Furthermore, the Centers for Disease Control and Prevention in the United States indicates that the K6 is a standardized and validated measure of nonspecific psychological distress and uses a K6 score of ≥10 to estimate serious psychological distress in the Behavioral Risk Factor Surveillance System (United States).35 Based on these important methodological limitations, the findings should be considered with caution. Nevertheless, the hypothesis that lower intake of dietary salt is associated with mental distress is testable and reason for including psychosocial outcomes in high‐quality dietary salt studies.
Discussion
This updated periodic review identified more than 1000 articles on dietary sodium, published in June and July 2015. This was distilled to eight studies relating dietary sodium to health outcomes. As it relates to the primary outcomes of each study, four of the eight studies found harm from excess dietary salt, two were neutral, and two found health benefits associated with high dietary salt. The approach of this review is to examine both high‐ and low‐quality research. If quality filters were applied to ensure adequate study design, reduction in dietary sodium, and study duration, such as those used in Cochrane reviews (ie, randomized controlled trials of ≥4 weeks' duration, with no concurrent interventions, that achieved ≥40 mmol/d sodium reduction between intervention and control with sodium intake measured by 24‐hour urinary sodium excretion; and prospective cohort studies of ≥1 year duration that measured sodium intake in any way),1 only two of the eight studies in this review would have been included.10, 11 Furthermore, low‐quality research has the potential to generate unreliable findings and influence the field in exactly the same way as high‐quality research. As such, all published work is equally requiring of review regardless of its quality.
One area that impacts the study quality is the methodology used to measure dietary salt. There is an increasing trend towards the application of the spot urine collection that is apparent in the studies identified in this systematic review, which is likely influenced by the ease and feasibility of collecting a spot urine sample as compared to more burdensome methods such as 24‐hour urine collections or validated food intake methods such as food records. However, there are significant concerns with spot urine collections. A systematic review of the relationship of salt intake estimated from spot urine samples to 24‐hour urine samples found a wide range of correlations from very weak to strong.36 Further, the formula used for estimating sodium intake often incorporates age, sex, urinary creatinine, height, and weight and hence the estimate of salt intake is confounded by those factors that are also associated with many common health issues.37 Many factors impact short‐term sodium and creatinine excretion, apart from dietary salt, and hence estimates from a spot urine sample are a complex reflection of many inputs. This makes spot urine samples particularly unsuited to studies seeking to determine the effects of salt intake on health outcomes where a precise and reliable estimate of an individual's sodium intake is required.36, 38, 39, 40 It remains possible that the spot urine test may be a reliable means of assessing mean population salt intake, but that is an entirely different application of the method.
A consortium of international health and scientific organizations is developing recommended approaches for conducting etiological research on dietary salt. These recommendations will address the role of dietary assessment methods and spot urine samples to estimate usual salt intake in a range of different research settings. Until that time, investigators studying the effects of salt on health outcomes are encouraged to assess multiple days of sodium intake using 24‐hour urine collections.
Conclusions
This review of eight studies from June and July 2015 found varying effects of dietary salt on several different health outcomes, including BP, measures of cardiac structure and function, and mental health. There is high likelihood that many of the findings are attributable to weaknesses in the study designs rather than real effects of sodium on health outcomes.
Supporting information
Appendix S1. Risk of bias tables for included studies.
Acknowledgments
The process to provide regular updates on the science of sodium is supported by the World Hypertension League, WHO Collaborating Centre on Population Salt Reduction (George Institute for Global Health), Pan American Health Organization/WHO Technical Advisory Group on Cardiovascular Disease Prevention through Dietary Sodium, and World Action on Salt and Health.
Disclosures
NC is a member of World Action on Salt and Health (a dietary salt reduction organization) but has no financial interests to disclose. JA, CJ, TSR, RM, KT, and AAL have no conflicts of interest to disclose. JW is Director of the WHO Collaborating Centre on Population Salt Reduction and is supported by a National Health and Medical Research Council/National Heart Foundation Career Development Fellowship on international strategies to reduce salt. BN is Chair of the Australian Division of World Action on Salt and Health. BN and JW have funding from WHO, VicHealth, and the Australian National Health and Medical Research Council of Australia for research on salt reduction. MMYW is a research consultant with Renal Research Institute and Arbor Research Collaborative for Health.
References
- 1. Aburto NJ, Ziolkovska A, Hooper L, et al. Effect of lower sodium intake on health: systematic review and meta‐analyses. BMJ. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta‐analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: cochrane systematic review and meta‐analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 4. WHO . Guideline: Sodium Intake for Adults and Children. Geneva: World Health Organization (WHO); 2012. [PubMed] [Google Scholar]
- 5. World Health Organization . Report of the Formal Meeting of Member States to Conclude the Work on the Comprehensive Global Monitoring Framework, Including Indicators, and a set of Voluntary Global Targets for the Prevention and Control of Noncommunicable Diseases. Report, 1–6. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 6. Johnson C, Raj TS, Trudeau L, et al. The science of salt: a systematic review of clinical salt studies 2013 to 2014. J Clin Hypertens (Greenwich). 2015;17:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arcand J, Webster J, Johnson C, et al. Announcing “Up to Date in the Science of Sodium.” J Clin Hypertens (Greenwich). 2016;18:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cochrane Methods . Cochrane Handbook: Chapter 8: Assessing Risk of Bias in Included Studies. http://bmg.cochrane.org/assessing-risk-bias-included-studies. Accessed August 19, 2015.
- 9. McLaren L, Sumar N, Lorenzetti DL, et al. Population‐level interventions in government jurisdictions for dietary sodium reduction (Protocol). Cochrane Database Syst Rev. 2012, Art. No.: CD010166. DOI: 10.1002/14651858.CD010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buendia JR, Bradlee ML, Daniels SR, et al. Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. JAMA Pediatr. 2015;169:560–568. [DOI] [PubMed] [Google Scholar]
- 11. Haring B, Wang W, Lee ET, et al. Effect of dietary sodium and potassium intake on left ventricular diastolic function and mass in adults ≤40 years (from the Strong Heart Study). Am J Cardiol. 2015;115:1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graudal N, Hubeck‐Graudal T, Jurgens G, McCarron DA. The significance of duration and amount of sodium reduction intervention in normotensive and hypertensive individuals: a meta‐analysis. Adv Nutr. 2015;6:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis, Part 5: Complex Data Structures. Hoboken, NJ: John Wiley & Sons, 2009. [Google Scholar]
- 14. Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org. Accessed October 7, 2015. [Google Scholar]
- 15. Elbourne DR, Altman DG, Higgins JP, et al. Meta‐analyses involving cross‐over trials: methodological issues. Int J Epidemiol. 2002;31:140–149. [DOI] [PubMed] [Google Scholar]
- 16. Gow IF, Dockrell M, Edwards CR, et al. The sensitivity of human blood platelets to the aggregating agent ADP during different dietary sodium intakes in healthy men. Eur J Clin Pharmacol. 1992;43:635–638. [DOI] [PubMed] [Google Scholar]
- 17. Burnier M, Monod ML, Chiolero A, et al. Renal sodium handling in acute and chronic salt loading/depletion protocols: the confounding influence of acute water loading. J Hypertens. 2000;18:1657–1664. [DOI] [PubMed] [Google Scholar]
- 18. Heer M, Baisch F, Kropp J, et al. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol. 2000;278:F585–F595. [DOI] [PubMed] [Google Scholar]
- 19. Nowson CA, Wattanapenpaiboon N, Pachett A. Low‐sodium Dietary Approaches to Stop Hypertension‐type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res. 2009;29:8–18. [DOI] [PubMed] [Google Scholar]
- 20. He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: meta‐analysis of controlled trials. Hypertension. 2006;48:861–869. [DOI] [PubMed] [Google Scholar]
- 21. Miettinen OS, Cook EF. Confounding: essence and detection. Am J Epidemiol. 1981;114:593–603. [DOI] [PubMed] [Google Scholar]
- 22. Micheli ET, Rosa AA. Estimation of sodium intake by urinary excretion and dietary records in children and adolescents from Porto Alegre, Brazil: a comparision of two methods. Nutr Rev. 2003;23:1477–1487. [Google Scholar]
- 23. Ponzo V, Ganzit GP, Soldati L, et al. Blood pressure and sodium intake from snacks in adolescents. Eur J Clin Nutr. 2015;69:681–686. [DOI] [PubMed] [Google Scholar]
- 24. McLean RM. Measuring population sodium intake: a review of methods. Nutrients. 2014;6:4651–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. [DOI] [PubMed] [Google Scholar]
- 26. Lee SK, Kim JS, Kim SH, et al. Sodium excretion and cardiovascular structure and function in the nonhypertensive population: the Korean Genome and Epidemiology Study. Am J Hypertens. 2015;28:1010–1016. [DOI] [PubMed] [Google Scholar]
- 27. Polonia J, Maldonado J, Ramos R, et al. Estimation of salt intake by urinary sodium excretion in a Portuguese adult population and its relationship to arterial stiffness. Rev Port Cardiol. 2006;25:801–817. [PubMed] [Google Scholar]
- 28. Njoroge JN, El Khoudary SR, Fried LF, et al. High urinary sodium is associated with increased carotid intima‐media thickness in normotensive overweight and obese adults. Am J Hypertens. 2011;24:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park S, Park JB, Lakatta EG. Association of central hemodynamics with estimated 24‐h urinary sodium in patients with hypertension. J Hypertens. 2011;29:1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jankowski P, Stolarz‐Skrzypek K, Kawecka‐Jaszcz K, et al. Does sodium intake affect the relationship between blood pressure and vascular damage? Pol Arch Med Wewn. 2015;125:347–357. [DOI] [PubMed] [Google Scholar]
- 31. Nerbass FB, Pecoits‐Filho R, McIntyre NJ, et al. High sodium intake is associated with important risk factors in a large cohort of chronic kidney disease patients. Eur J Clin Nutr. 2015;69:786–790. [DOI] [PubMed] [Google Scholar]
- 32. McMahon EJ, Campbell KL, Bauer JD, Mudge DW. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev. 2015;2:CD010070. [DOI] [PubMed] [Google Scholar]
- 33. Shimizu Y, Kadota K, Koyamatsu J, et al. Salt intake and mental distress among rural community‐dwelling Japanese men. J Physiol Anthropol. 2015;34:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davison KM, Kaplan BJ. Nutrient intakes are correlated with overall psychiatric functioning in adults with mood disorders. Can J Psychiatry. 2012;57:85–92. [DOI] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention . Surveillance Data Sources Health‐Related Quality of Life—Behavioral Risk Factor Surveillance System (BRFSS). http://www.cdc.gov/mentalhealth/data_stats/nspd.htm, Accessed August 12, 2015.
- 36. Ji C, Sykes L, Paul C, et al. Systematic review of studies comparing 24‐hour and spot urine collections for estimating population salt intake. Rev Panam Salud Publica. 2012;32:307–315. [DOI] [PubMed] [Google Scholar]
- 37. Cogswell ME, Elliott P, Wang CY, et al. Assessing U.S. sodium intake through dietary data and urine biomarkers. Adv Nutr. 2013;4:560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cogswell ME, Wang CY, Chen TC, et al. Validity of predictive equations for 24‐h urinary sodium excretion in adults aged 18‐39 y. Am J Clin Nutr. 2013;98:1502–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pan American Health Organization . Salt Smart Americas. Report, 1‐140. 2013. Washington, DC: Pan American Health Organization. [Google Scholar]
- 40. Campbell NR, Lackland DT, Niebylski ML, Nilsson PM. Is reducing dietary sodium controversial? Is it the conduct of studies with flawed research methods that is controversial? A perspective from the World Hypertension League Executive Committee. J Clin Hypertens (Greenwich). 2015;17:85–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Risk of bias tables for included studies.


