In most hypertensive patients, left ventricular (LV) wall thickness is normal or only mildly increased (≤13 mm).1 A minority of patients may have more substantial hypertrophy (up to 16 mm) and fall into a “gray zone” in which electrocardiographic and echocardiographic features are indistinguishable from those of hypertrophic cardiomyopathy (HCM).1, 2, 3, 4 Clinical evaluation after appropriate antihypertensive treatment may help define the correct diagnosis.
A 51‐year‐old man was referred to our center after an incidental finding of high blood pressure (220/120 mm Hg). At first clinical presentation, the patient was asymptomatic and took no drugs. Physical examination showed a loud second heart sound and no murmurs. His blood pressure was 190/115 mm Hg. Findings from electrocardiography exhibited signs of LV hypertrophy (LVH) (RV5+SV1 = 56 mm), ST‐segment abnormalities, and negative T waves in anterolateral leads (Figure 1A). Echocardiography showed marked symmetric LVH (septal thickness, 18 mm; posterior wall thickness, 16 mm; LV mass/height2.7, 73 g/m2.7),5 normal LV cavity (LV diastolic diameter/body surface area, 2.7 cm/m2), mildly enlarged left atrium (LA volume/body surface area, 30 mL/m2), and normal ejection fraction (62%). Aortic root was mildly dilated. Diastolic filling showed impaired relaxation (Figure 2A). The patient had no family history of HCM or sudden death.
Figure 1.
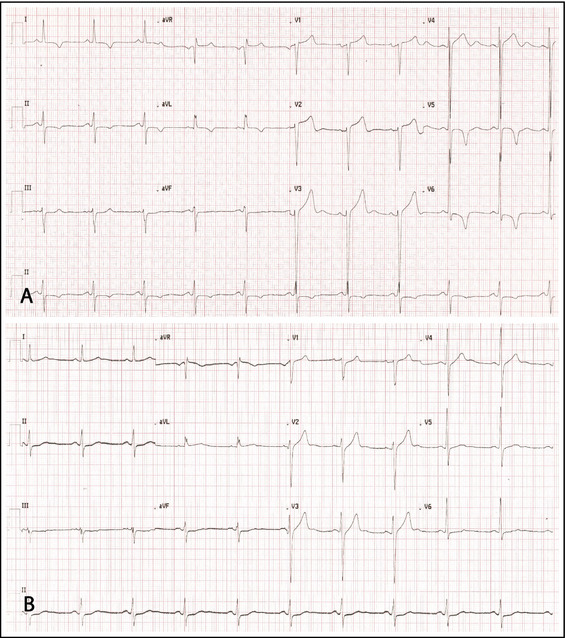
Electrocardiogram in December 2011 (A). Electrocardiogram in March 2012 (B).
Figure 2.
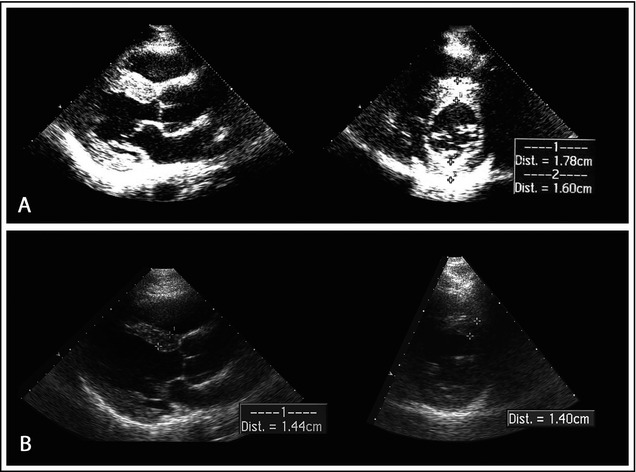
Echocardiography in December 2011 (A). Echocardiography in March 2012 (B). Parasternal long‐axis (leftward) and short‐axis (rightward) view at end diastole. Maximum end‐diastolic thickness of the anterior wall decreased from 18 mm to 14 mm in 3 months. Dist indicates distance.
LVH was likely to be secondary to hypertension, but overlapping hypertension on HCM could not be excluded. We prescribed an antihypertensive treatment with ramipril 10 mg and amlodipine 10 mg, but blood pressure was not adequately controlled. Two weeks later, we substituted valsartan/hydrochlorothiazide 320/12.5 mg for ramipril 10 mg.6 β‐Blockers were not prescribed because of bradycardia. Secondary causes of hypertension were excluded.
The patient was reevaluated after 3 months. Blood pressure was 110/70 mm Hg. Findings from electrocardiography were strikingly different: voltages did not meet anymore Sokolow‐Lyon criterion for LVH (RV5+SV1 = 29 mm), ST‐segment abnormalities had normalized, and anterolateral negative T waves had reverted to positive (Figure 1B). Findings from echocardiography showed significant reduction in LV wall thickness (septum, 14 mm; posterior wall, 10 mm; LV mass/height2.7, 48 g/m2.7) (Figure 2B). Three months later, amlodipine was discontinued. After 2 years of optimal blood pressure control with valsartan and hydrochlorothiazide, the patient underwent magnetic resonance imaging (MRI). The left ventricle had normal wall thickness (maximum 11 mm), cavity dimension (end‐diastolic LV volume index, 71 mL/m2), and ejection fraction (68%). LV mass/height2.7 was 30 g/m2.7. Post‐contrast images revealed no delayed enhancement (Figure 3).
Figure 3.
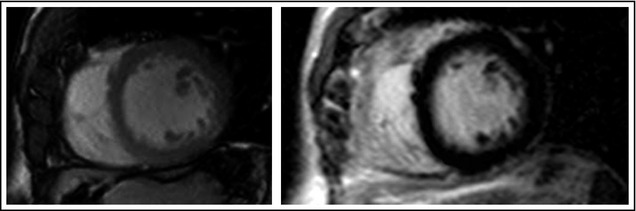
Magnetic resonance imaging (MRI) in November 2013. On the left, cine MRI using balanced steady state free precession technique (short axis) shows normal left ventricular wall thickness at end diastole. On the right, contrast‐enhanced inversion recovery imaging (short axis) shows the absence of myocardial enhancement.
Making the Correct Diagnosis
The finding of severe LVH in hypertensive patients may sometimes create a clinical dilemma: Is a coexistent cardiomyopathy present? Or is LVH a secondary consequence of severe chronic hypertension? A definitive diagnosis may be difficult to resolve.
It is quite uncommon to find severe LVH in a hypertensive 50‐year‐old patient. Diagnostic difficulties generally arise in elderly patients and, in the past, led to the definition of a distinct entity termed hypertensive hypertrophic cardiomyopathy of the elderly, characterized by severe concentric cardiac hypertrophy (wall thickness ≥16 mm; septal:posterior wall‐thickness ratio, 1:1), small LV cavity, supernormal indexes of systolic function, impaired diastolic function, and enlarged left atrium.7
At first clinical presentation, the clinical and morphological features of our patient were compatible with the diagnosis of overlapping hypertension on HCM. Three‐month treatment with antihypertensive drugs, though, was sufficient to reverse LVH. LVH in patients with HCM is caused by mutations in sarcomere genes and is not reversible. Conversely, LVH in hypertensive patients is secondary to increased afterload and may regress during antihypertensive treatment. Angiotensin receptor blockers seem to be particularly effective for this purpose.6 Regression of LVH in our patient led us to exclude HCM and lean toward the diagnosis of LVH secondary to hypertension. The absence of delayed gadolinium enhancement on MRI supported the latter diagnosis.8
Conclusions
A minority of hypertensive patients fall into the morphological gray zone between secondary LVH and HCM. Regression of LVH after antihypertensive treatment reveals hypertension as the underlying cause of LVH, excluding HCM.
Disclosure
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
References
- 1. Savage DD, Drayer JI, Henry WL, et al. Echocardiographic assessment of cardiac anatomy and function in hypertensive subjects. Circulation. 1979;59:623–632. [DOI] [PubMed] [Google Scholar]
- 2. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:e212–e260. [DOI] [PubMed] [Google Scholar]
- 3. Gradman AH1, Alfayoumi F. From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Prog Cardiovasc Dis. 2006;48:326–341. [DOI] [PubMed] [Google Scholar]
- 4. Lewis JF, Maron BJ. Diversity of patterns of hypertrophy in patients with systemic hypertension and marked left ventricular wall thickening. Am J Cardiol. 1990;65:874–881. [DOI] [PubMed] [Google Scholar]
- 5. de Simone G1, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. [DOI] [PubMed] [Google Scholar]
- 6. Thürmann PA1, Kenedi P, Schmidt A, et al. Influence of the angiotensin II antagonist valsartan on left ventricular hypertrophy in patients with essential hypertension. Circulation. 1998;98:2037–2042. [DOI] [PubMed] [Google Scholar]
- 7. Topol EJ, Traill TA, Fortuin NJ. Hypertensive hypertrophic cardiomyopathy of the elderly. N Engl J Med. 1985;312:277–283. [DOI] [PubMed] [Google Scholar]
- 8. Puntmann VO, Jahnke C, Gebker R, et al. Usefulness of magnetic resonance imaging to distinguish hypertensive and hypertrophic cardiomyopathy. Am J Cardiol. 2010;106:1016–1022. [DOI] [PubMed] [Google Scholar]


