Abstract
This randomized, double‐blind, placebo‐controlled study evaluated the early effects of canagliflozin on blood pressure (BP) in patients with type 2 diabetes mellitus (T2DM) and hypertension. Patients were randomized to canagliflozin 300 mg, canagliflozin 100 mg, or placebo for 6 weeks and underwent 24‐hour ambulatory BP monitoring before randomization, on day 1 of treatment, and after 6 weeks. The primary endpoint was change in mean 24‐hour systolic BP (SBP) from baseline to week 6. Overall, 169 patients were included (mean age, 58.6 years; glycated hemoglobin, 8.1%; seated BP 138.5/82.7 mm Hg). At week 6, canagliflozin 300 mg provided greater reductions in mean 24‐hour SBP than placebo (least squares mean −6.2 vs −1.2 mm Hg, respectively; P=.006). Numerical reductions in SBP were observed with canagliflozin 100 mg. Canagliflozin was generally well tolerated, with side effects similar to those reported in previous studies. These results suggest that canagliflozin rapidly reduces BP in patients with T2DM and hypertension.
Hypertension is a common comorbidity of diabetes mellitus, affecting up to 60% of patients.1, 2 Sodium‐glucose cotransporter 2 (SGLT2) inhibitors, which have been shown to improve glycemic control in patients with type 2 diabetes mellitus (T2DM), may also reduce blood pressure (BP).3, 4 In a meta‐analysis of 27 randomized trials (most studies with a follow‐up of 12–52 weeks), treatment with SGLT2 inhibitors was associated with significant reductions in systolic BP (SBP) and diastolic BP (DBP) from baseline.5 Similarly, a pooled analysis of four randomized trials (duration of follow‐up 26 weeks) showed significant placebo‐corrected reductions in SBP when canagliflozin was given at doses of 300 mg and 100 mg in patients with T2DM and elevated SBP at baseline.6
Most studies of SGLT2 inhibitors have evaluated changes in BP after 12, 26, or 52 weeks of therapy.5, 6, 7, 8, 9, 10, 11 The immediate effects (ie, less than 12 weeks) of SGLT2 inhibitors on BP have not been well characterized. The current 6‐week study12 was designed to evaluate the early effects of treatment with canagliflozin on BP, including a 24‐hour BP assessment after the first dose, using ambulatory BP monitoring (ABPM).
Methods
Study Design and Patient Population
This randomized, double‐blind, placebo‐controlled, parallel‐group, three‐arm, multicenter study consisted of three phases: (1) a pretreatment phase comprising a screening visit and a 2‐week single‐blind, placebo run‐in; (2) a double‐blind, 6‐week treatment phase; and (3) a follow‐up phase of 30 days after the last dose of the study drug. Protocol‐specified inclusion and exclusion criteria were assessed at the screening visit (day −21).
Patients aged from 18 years to less than 75 years were eligible for inclusion in the study if they had: (1) hypertension (defined as a seated office SBP ≥130 mm Hg and <160 mm Hg and seated office DBP ≥70 mm Hg) and were taking stable doses of one to three antihypertensive agents (including either an angiotensin‐converting enzyme [ACE] inhibitor or angiotensin II receptor blocker [ARB], with or without calcium channel blockers, β‐blockers, or diuretics other than loop diuretics; patients taking loop diuretics were excluded from the study) for ≥5 weeks before screening, and (2) inadequately controlled T2DM (glycated hemoglobin [HbA1c] test ≥7.0% to <10%) despite stable doses of one to three antihyperglycemic agents (including metformin at 2000 mg/d or a maximally tolerated dose, with or without sulfonylureas, thiazolidinediones, or dipeptidyl peptidase‐4 inhibitors; patients taking insulin were excluded from the study).
Patients were excluded if they had a diagnosis of type 1 diabetes mellitus, diabetic ketoacidosis, or diabetes secondary to pancreatitis or pancreatectomy; had repeated (ie, ≥2 over a 1‐week period) fasting self‐monitored blood glucose measurements ≥240 mg/dL (13.3 mmol/L) during the pretreatment phase, despite reinforcement of diet and exercise counselling; had uncontrolled hypertension (ie, with an average of three seated office BP readings with an SBP >160 mm Hg or a DBP >110 mm Hg) at screening; were taking SGLT2 inhibitor, insulin, or glucagon‐like peptide‐1 receptor agonist therapy in the 12 weeks prior to screening or in the 2‐week run‐in period; or had received antihypertensive therapy with ACE inhibitors, ARBs, loop diuretics, calcium channel blockers, or β‐blockers, not on a stable regimen (ie, same medications and doses) for at least 5 weeks before screening.
At the baseline visit (day 1), patients who met all enrollment criteria were stratified by use of β‐blockers and randomly assigned in a 1:1:1 ratio to receive canagliflozin 100 mg, canagliflozin 300 mg, or placebo once daily for the duration of the 6‐week treatment phase. Background therapies (stable doses of antihyperglycemic and antihypertensive agents) were continued throughout the run‐in and treatment phases.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice, and applicable regulations. Written informed consent was obtained from each patient before any study procedures were undertaken. Each study site's institutional review board approved the study protocol, the informed consent document, and updates of the document in advance of use.
Endpoints and Assessments
For each patient, BP recordings were collected over a 24‐hour period using an ABPM device (90207 ABP monitor; Spacelabs Healthcare, Snoqualmie, WA) every 20 minutes during the daytime and every 30 minutes during the nighttime at three time points during the study: baseline (from day −7 to day −6, or day −6 to day −5, or day −5 to day −4); from day 1 to day 2 (after randomization following the first dose of study medication); and at week 6 (from day 43 to day 44) (Figure 1). The primary and secondary BP‐related endpoints (related to the 24‐hour BP measurements) were defined based on these 24‐hour ABPM readings.
Figure 1.
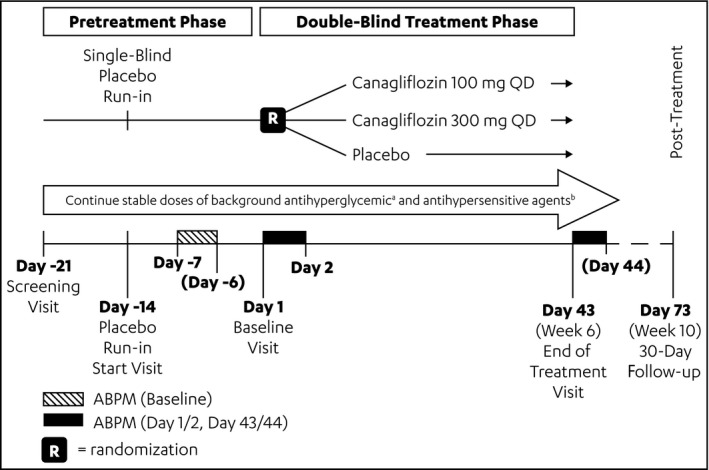
Study design. ABPM indicates ambulatory blood pressure monitoring; QD, once daily. aOne to three antihyperglycemic agents (metformin with or without sulphonyureas, thiazolidinediones, or dipeptidyl peptidase‐4 inhibitors). bOne to three antihypertensive agents (angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker with or without diuretics [other than loop diuretics], calcium channel blockers, or β‐blockers).
On day 1 (randomization) and day 2 and in week 3 and week 6 (end of treatment), patients had their seated office BP, seated heart rate (HR), standing office BP, and standing HR measured at clinician visits. Seated and standing BP assessments were measured in the same arm. For seated BP, three readings were taken at least 1 minute apart, after the patient rested for 5 minutes. Following the triple seated BP measurement, standing BP was measured after 2 minutes.
Time 0 was defined as the time of the first valid ABPM measurement immediately following the morning dose of the study medication. Daytime and nighttime were defined for each individual based on diary entries. For example, for a patient who recorded in the diary the bedtime as 11 pm on the day of ABPM assessment and awakening time as 7 am the following day, the nighttime would be defined as time from 11 pm to 7 am and the daytime would then be defined as the time when the patient was awake (ie, from the start of ABPM on the day of assessment through 24 hours the following day but excluding the hours from 11 pm through 7 am when the patient was asleep). All assessments were completed prior to intake of study drug.
The primary efficacy endpoint was the change from baseline to week 6 in the mean 24‐hour SBP. Key secondary endpoints included change from baseline to week 6 in mean 24‐hour DBP, change in mean daytime SBP and DBP, and change in mean nighttime SBP and DBP. In addition, changes in these parameters and mean 24‐hour SBP were also assessed from baseline to day 2. Day 2 ABPM measurements were conducted from day 1 to day 2 during initial dosing after randomization. Additional efficacy endpoints included changes from baseline (measured on day 1) to week 6 in fasting plasma glucose (FPG) and body weight.
Safety
Safety evaluations included the collection of adverse event (AE) information (including volume‐depletion‐related AEs, osmotic‐diuresis‐related AEs and renal‐related AEs), laboratory tests, vital signs, assessment of orthostasis (significant orthostasis is defined as symptoms on standing [eg, dizziness and lightheadedness] or a reduction in office SBP of ≥20 mm Hg or in office DBP of ≥15 mm Hg after 2 minutes of standing). Hypoglycemia was considered to be an AE reported in the safety results, and classified as either biochemically documented (ie, a hypoglycemia episode with a concurrent reported glucose value of ≤70 mg/dL [≤3.9 mmol/L]) and/or severe (an event requiring the assistance of another person to actively administer a carbohydrate or glucagon, or if a patient lost consciousness or experienced a seizure during an episode). Serious AEs that occurred during the 30‐day post‐treatment phase were also recorded.
Statistical Analyses
Initially, a sample size of 56 patients in each treatment group was planned to have a 90% power to perform a two‐sided test of the mean 24‐hour SBP change of −4.0 mm Hg, assuming a common standard deviation (SD) of 6.5 mm Hg and type I error rate of 5%. After taking into account a 10% dropout rate, a total of 189 patients were planned to be included in this study. However, because of slow trial accrual, the sample size and power calculations were reassessed. Based on the revised sample size estimate, a sample size of at least 46 patients completing in each group (total 138 patients across the three study groups) would have 84% power. Taking into account the 10% dropout rate, the sample size was adjusted upwards to at least 153 patients (51 per study group).
The efficacy and safety analyses were performed using the full analysis set, which included all patients assigned randomly into the study who took at least one dose of the study drug. Supportive analyses were performed based on the 6‐week completer dataset (patients who completed the trial). For the main efficacy and safety analyses, data were pooled across the two strata created by the stratification variable (defined as using, or not using, β‐blockers as background antihypertensive medication) at baseline. Descriptive statistics were used to summarize patient baseline characteristics and endpoints. Missing efficacy data were imputed using the last observation carried forward method.
Based on the full analysis set, an analysis of covariance (ANCOVA) model (with treatment and stratification factor as fixed effects and corresponding baseline value as covariates) was used. All pairwise treatment differences in the least squares (LS) means of the change from baseline values and their two‐sided 95% confidence intervals (CIs) were estimated based on this ANCOVA model. A hierarchical testing sequence was used to adjust for type I error at a prespecified level of 5% (P=.05). For day 2, a separate ANCOVA analysis was performed, and associated CIs for difference in means were reported for the relevant endpoints.
A sequential testing procedure (for superiority of canagliflozin 300 mg or 100 mg vs placebo relating to mean 24‐hour SBP, mean 24‐hour DBP, FPG, body weight, and mean daytime and nighttime SBP and DBP) was applied in a prespecified sequence to control the familywise error rate at 5%. Statistical testing of an endpoint was performed only if all preceding tests in the sequence were rejected. Testing of subsequent endpoints was also performed; however, the P values for these endpoints were considered strictly nominal.
Results
Patient Disposition and Baseline Characteristics
A total of 171 patients were randomized from 41 sites in the United States. Of these patients, 169 received one or more dose of the study medication and comprised the full analysis set (Figure 2). Overall, 90.6% of patients completed the 6‐week double‐blind treatment period, forming the completer dataset: canagliflozin 300 mg (n=54); canagliflozin 100 mg (n=54); and placebo (n=47).
Figure 2.
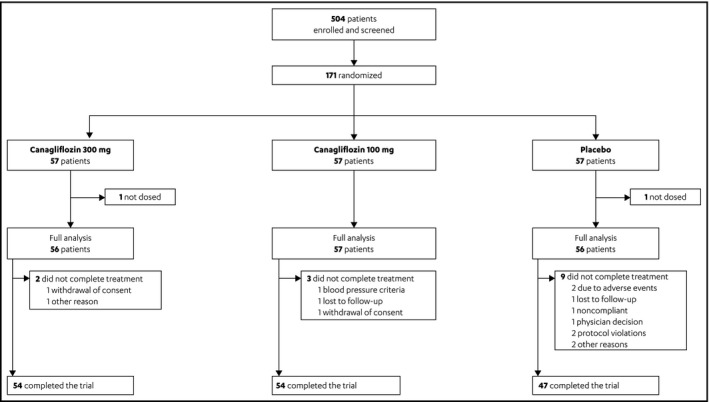
Patient disposition.
Baseline characteristics were generally balanced across treatment groups (Table 1). Overall, 58.0% of patients were men, mean age was 58.6 years, mean body weight was 94.3 kg, mean body mass index was 33.3 kg/m2, and 55.6% of patients described their ethnicity as Hispanic/Latino. Seated BP at baseline was 138.5/82.7 mm Hg and mean 24‐hour ambulatory BP was 137.6/78.6 mm Hg. Mean baseline HbA1c was 8.1%, mean FPG was 173.6 mg/dL (9.6 mmol/L), and median duration of diabetes was 9 years. The majority (80.5%) of the patients were not taking β‐blockers at the time of randomization.
Table 1.
Baseline Demographic Characteristics
| Canagliflozin 300 mg (n=56) | Canagliflozin 100 mg (n=57) | Placebo (n=56) | Canagliflozin Total (n=113) | Total (N=169) | |
|---|---|---|---|---|---|
| Male, No. (%) | 31 (55.4) | 34 (59.6) | 33 (58.9) | 65 (57.5) | 98 (58.0) |
| Age, y | 58.3 (6.9) | 57.8 (8.7) | 59.6 (9.5) | 58.1 (7.8) | 58.6 (8.4) |
| Race, No. (%) | |||||
| White | 43 (76.8) | 45 (78.9) | 46 (82.1) | 88 (77.9) | 134 (79.3) |
| Black or African American | 12 (21.4) | 10 (17.5) | 9 (16.1) | 22 (19.5) | 31 (18.3) |
| Asian | 1 (1.8) | 1 (1.8) | 0 | 2 (1.8) | 2 (1.2) |
| Other | 0 | 1 (1.8) | 0 | 1 (0.9) | 1 (0.6) |
| Unknown | 0 | 0 | 1 (1.8) | 0 | 1 (0.6) |
| Ethnicity, No. (%) | |||||
| Hispanic or Latino/Latina | 26 (46.4) | 35 (61.4) | 33 (58.9) | 61 (54.0) | 94 (55.6) |
| Not Hispanic or Latino/Latina | 30 (53.6) | 22 (38.6) | 23 (41.1) | 52 (46.0) | 75 (44.4) |
| Body weight, kg | 96.1 (20.2) | 95.3 (22.2) | 91.7 (17.5) | 95.7 (21.2) | 94.3 (20.1) |
| BMI, kg/m² | 34.1 (6.8) | 33.0 (6.0) | 32.9 (5.7) | 33.6 (6.4) | 33.3 (6.2) |
| Glycated hemoglobin, % | 8.0 (0.8) | 8.1 (0.9) | 8.2 (0.9) | 8.0 (0.8) | 8.1 (0.9) |
| eGFR, mL/min/1.73 m2 | 85.6 (19.7) | 87.2 (20.3) | 87.9 (18.3) | 86.4 (19.9) | 86.9 (19.4) |
| FPG, mmol/L | 9.4 (2.0) | 9.7 (2.1) | 9.8 (2.4) | 9.5 (2.0) | 9.6 (2.2) |
| Seated SBP, mm Hg | 139.2 (8.8) | 138.5 (11.1) | 137.7 (8.6) | 138.9 (10.0) | 138.5 (9.6) |
| Seated DBP, mm Hg | 83.0 (8.2) | 82.4 (7.7) | 82.7 (8.6) | 82.7 (7.9) | 82.7 (8.1) |
| Mean 24‐h ABPM SBP, mm Hg | 139.6 (10.9) | 136.5 (11.5) | 136.7 (10.3) | 138.0 (11.3) | 137.6 (11.0) |
| Mean 24‐h ABPM DBP, mm Hg | 79.3 (7.9) | 78.0 (8.1) | 78.4 (7.3) | 78.7 (8.0) | 78.6 (8.0) |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; SBP, systolic blood pressure. Data are represented as mean (standard deviation) unless otherwise indicated.
Efficacy Results
LS mean and placebo‐subtracted LS mean changes from baseline to week 6 for the primary and secondary endpoints, including full and completer analyses, are shown in Table 2. For the primary efficacy endpoint in the full analysis set, LS mean change from baseline to week 6 in mean 24‐hour ABPM SBP was −6.2 mm Hg for canagliflozin 300 mg, −4.5 mm Hg for canagliflozin 100 mg, and −1.2 for placebo. Placebo‐subtracted LS mean changes were therefore −4.9 mm Hg (95% CI, −8.4 to −1.5; P=.006) for canagliflozin 300 mg and −3.3 (95% CI, −6.7 to 0.2; P=.062) for canagliflozin 100 mg. Among completers, LS mean change from baseline to week 6 in mean 24‐hour ABPM SBP was −6.6 mm Hg for canagliflozin 300 mg, −5.0 mm Hg for canagliflozin 100 mg, and −1.4 mm Hg for placebo. Placebo‐subtracted LS mean changes were −5.2 mm Hg (95% CI, −8.9 to −1.6; P=.006) for canagliflozin 300 mg and −3.7 mm Hg (95% CI, −7.4 to −0.0; P=.049) for canagliflozin 100 mg.
Table 2.
LS Mean and Placebo‐Subtracted LS Mean Change From Baseline to Week 6 (Last Observation Carried Forward)
| Endpoint | Canagliflozin 300 mg (n=56) | Canagliflozin 100 mg (n=57) | Placebo (n=56) | ||
|---|---|---|---|---|---|
| P Value (Minus Placebo)a | P Valuea | ||||
| Mean 24‐hour ABPM SBP change, mm Hg | |||||
| LS mean (SE) | −6.2 (1.4) | −4.5 (1.4) | −1.2 (1.4) | ||
| PBO‐subtracted LS mean (95% CI) | −4.9 (−8.4 to −1.5) | .006 b | −3.3 (−6.7 to 0.2) | .062 | |
| LS mean (SE)b | −6.6 (1.4) | −5.0 (1.4) | −1.4 (1.5) | ||
| PBO‐subtracted LS mean (95% CI)b | −5.2 (−8.9 to −1.6) | .006 | −3.77 (−7.4 to −0.0c) | .049 | |
| Mean 24‐hour ABPM DBP change, mm Hg | |||||
| LS mean (SE) | −3.2 (0.8) | −2.2 (0.8) | −0.3 (0.8) | ||
| PBO‐subtracted LS mean (95% CI) | −2.9 (−5.0 to −0.9) | .005 | −1.9 (−4.0 to 0.1) | .062 | |
| LS mean (SE)b | −3.4 (0.8) | −2.3 (0.8) | −0.4 (0.9) | ||
| PBO‐subtracted LS mean (95% CI)b | −3.0 (−5.2 to −0.9) | .006 | −2.0 (−4.1 to 0.2) | .071 | |
| Mean daytime ABPM SBP change, mm Hg | |||||
| LS mean (SE) | −6.2 (1.4) | −4.8 (1.4) | −0.8 (1.4) | ||
| PBO‐subtracted LS mean (95% CI) | −5.4 (−8.9 to −1.9) | .003 | −4.0 (−7.5 to −0.5) | .025 | |
| Mean daytime ABPM DBP change, mm Hg | |||||
| LS mean (SE) | −3.1 (0.8) | −2.4 (0.8) | −0.2 (0.8) | ||
| PBO‐subtracted LS mean (95% CI) | −3.0 (−5.0 to −0.9) | .006 | −2.2 (−4.3 to −0.1) | .039 | |
| Mean nighttime ABPM SBP change, mm Hg | |||||
| LS mean (SE) | −6.2 (1.7) | −4.0 (1.6) | −3.2 (1.7) | ||
| PBO‐subtracted LS mean (95% CI) | −3.0 (−7.2 to 1.1) | .152 | −0.9 (−5.1 to 3.3) | .676 | |
| Mean nighttime ABPM DBP change, mm Hg | |||||
| LS mean (SE) | −3.6 (1.0) | −2.0 (1.0) | −1.2 (1.0) | ||
| PBO‐subtracted LS mean (95% CI) | −2.4 (−4.9 to 0.2) | .071 | −0.8 (−3.4 to 1.8) | .557 | |
| Seated office SBP, mm Hg | |||||
| LS mean (SE) | −7.5 (2.0) | −5.3 (2.0) | −3.9 (2.0) | ||
| PBO‐subtracted LS mean (95% CI) | −3.6 (−8.7 to 1.6) | .173 | −1.3 (−6.4 to 3.8) | .604 | |
| Seated office DBP, mm Hg | |||||
| LS mean (SE) | −2.8 (1.1) | −2.1 (1.1) | −2.2 (1.1) | ||
| PBO‐subtracted LS mean (95% CI) | −0.6 (−3.4 to 2.2) | .688 | 0.2 (−2.6 to 3.0) | .898 | |
| FPG change, mmol/L | |||||
| LS mean (SE) | −1.6 (0.3) | −0.2 (0.3) | −0.4 (0.3) | ||
| PBO‐subtracted LS mean (95% CI) | −1.2 (−2.0 to −0.3) | .009 | 0.2 (−0.7 to 1.1) | .636 | |
| Body weight change, % | |||||
| LS mean (SE) | −1.5 (0.4) | −1.1 (0.4) | 0.2 (0.4) | ||
| PBO‐subtracted LS mean (95% CI) | −1.7 (−2.7 to −0.7) | <.001 | −1.3 (−2.3 to −0.4) | .008 | |
Abbreviations: ABPM, ambulatory blood pressure monitoring; ANCOVA, analysis of covariance; CI, confidence interval; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; LS, least squares; PBO, placebo; SBP, systolic blood pressure; SE, standard error. Note: The LS mean is presented with associated P values and 95% CI based on ANCOVA models with terms for treatment, the use of β‐blockers at baseline strata as factors, and adjusting for the baseline value as a covariate. Based on the prespecified hierarchical testing sequence (which included testing the mean 24‐hour ambulatory SBP, mean 24‐hour ambulatory DBP, FPG, body weight, and mean daytime and mean nighttime SBP and DBP), the change from baseline in the mean 24‐hour ambulatory SBP for canagliflozin 100 mg was compared with the PBO group following the statistically significant comparison of canagliflozin 300 mg vs PBO. Although the canagliflozin 100‐mg dose showed greater numerical reductions in the mean 24‐hour ambulatory SBP compared with PBO, it did not achieve statistical significance. Due to the lack of statistical significance for this comparison, P values reported for subsequent endpoints are considered to be strictly nominal with no inference regarding their statistical significance. aNominal P value. bCompleter analysis set: canagliflozin 300 mg (n=54), canagliflozin 100 mg (n=54), placebo (n=47). cValue: −.003. The bold value indicates statistical significance.
In the full analysis set, the mean 24‐hour SBP in the canagliflozin groups showed small numeric improvements compared with placebo at day 2 (Figure 3). Day 2 results indicated a reduction in LS mean 24‐hour ABPM SBP with canagliflozin 300 mg (−1.1 mm Hg) and canagliflozin 100 mg (−1.4 mm Hg) and an increase with placebo (0.7 mm Hg). The placebo‐subtracted LS mean for canagliflozin 300 mg and 100 mg were −1.7 (95% CI, −4.7 to 1.2) and −2.0 (95% CI, −5.0 to 0.9), respectively. Treatment with canagliflozin was associated with improvements in several other secondary ABPM endpoints (Table S1).
Figure 3.
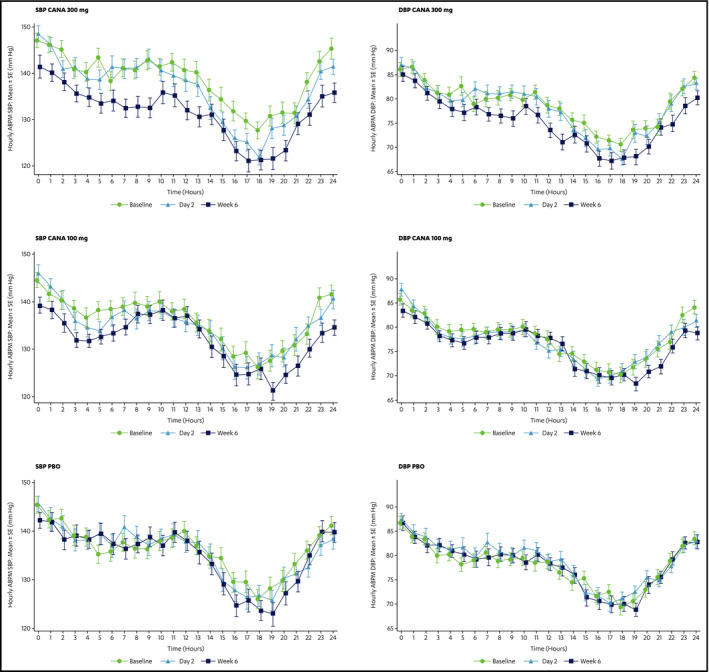
Hourly mean 24‐hour ambulatory blood pressure (BP) monitoring (ABPM) systolic BP (SBP) and diastolic BP (DBP) for canagliflozin (CANA) 300 mg, 100 mg, and placebo at baseline, day 2, and week 6 (last observation carried forward). The x axis indicates the hourly time after the morning dose of the study medication. SE indicates standard error.
Reductions in many of the secondary efficacy endpoint measures were also observed over the 6‐week study period. Placebo‐subtracted LS mean changes in 24‐hour ABPM DBP from baseline to week 6 were −2.9 mm Hg (95% CI, −5.0 to −0.9; P=.005) for canagliflozin 300 mg and −1.9 mm Hg (95% CI, −4.0 to 0.1; P=.062) for canagliflozin 100 mg. Among completers, LS mean change from baseline to week 6 in mean 24‐hour ABPM DBP was −3.4 mm Hg for canagliflozin 300 mg, −2.3 mm Hg for canagliflozin 100 mg, and −0.4 mm Hg for placebo (P=.006 for canagliflozin 300 mg vs placebo, P=.071 for canagliflozin 100 mg vs placebo).
Canagliflozin 300 mg and 100 mg both resulted in greater reductions in mean daytime ABPM SBP, compared with placebo, and greater reductions in mean daytime ABPM DBP, compared with placebo (Table 2). Numerical reductions in LS mean nighttime ABPM SBP and DBP and seated office SBP were also seen with canagliflozin 300 mg and 100 mg compared with placebo; treatment with canagliflozin 300 mg, but not canagliflozin 100 mg also reduced seated office DBP (Table 2).
FPG was reduced over the 6‐week study period by canagliflozin 300 mg, but not by canagliflozin 100 mg (Table 2). The LS mean change was −28.0 mg/dL (−1.6 mmol/L) for canagliflozin 300 mg, compared with −7.3 mg/dL (−0.4 mmol/L) for placebo (P=.009). Body weight was also reduced at week 6 with both canagliflozin doses, compared with placebo (LS mean percentage change −1.5% for canagliflozin 300 mg, −1.1% for canagliflozin 100 mg, and 0.2% for placebo; P<.001 for canagliflozin 300 mg vs placebo, P=.008 for canagliflozin 100 mg vs placebo).
Safety Results
The overall incidence of treatment‐emergent AEs was higher in the canagliflozin 300 mg and 100 mg groups (26.8% and 26.3%, respectively) than in the placebo group (19.6%) (Table 3). Two patients (3.6%), both in the canagliflozin 300 mg group, experienced volume‐depletion‐related AEs. Five patients (8.9%) in the canagliflozin 300 mg and two (3.5%) in the canagliflozin 100 mg groups experienced osmotic diuresis–related AEs compared with three (5.4%) in the placebo group. One renal‐related AE (an increase in serum creatinine) was reported in the canagliflozin 100 mg group. Significant orthostasis was reported at day 2 in 3.6% (n=2) of the canagliflozin 300 mg group, 3.5% (n=2) of the canagliflozin 100 mg group, and 0 patients in the placebo group. At week 3, the rates were 1.8% (n=1), 3.5% (n=2), and 1.9% (n=1), and at week 6 the rates were 7.1% (n=4), 3.8% (n=2), and 3.9% (n=2), respectively. There were no urinary tract infections or genital mycotic infections reported in this study. The percentage of patients who experienced hypoglycemia was higher in the canagliflozin 300 mg group (five patients [8.9%]) compared with the canagliflozin 100 mg (three patients [5.3%]) or placebo (four patients [7.1%]) groups. All 12 patients experiencing a hypoglycemic event were being treated with sulfonylurea in their background antihyperglycemic therapy. There were no instances of severe hypoglycemic events. None of the AEs related to the study drug led to discontinuation.
Table 3.
Summary of Treatment‐Emergent Adverse Events
| Canagliflozin 300 mg (n=56) | Canagliflozin 100 mg (n=57) | Placebo (n=56) | |
|---|---|---|---|
| Any AE | 15 (26.8) | 15 (26.3) | 11 (19.6) |
| AEs related to study druga | 10 (17.9) | 4 (7.0) | 3 (5.4) |
| AEs of special interest | |||
| Significant orthostasis at week 6b | 4 (7.1) | 2 (3.8) | 2 (3.9) |
| Volume depletionc | 2 (3.6) | 0 | 0 |
| Osmotic diuresisd | 5 (8.9) | 2 (3.5) | 3 (5.4) |
| Renale | 0 | 1 (1.8) | 0 |
| Genital mycotic infection | 0 | 0 | 0 |
| Urinary tract infection | 0 | 0 | 0 |
| Documented hypoglycemia | 5 (8.9) | 3 (5.3) | 4 (7.1) |
| AEs leading to discontinuation | 0 | 0 | 2 (3.6) |
| AEs related to study druga leading to discontinuation | 0 | 0 | 0 |
| Serious AEs | 0 | 0 | 1 (1.8) |
| Deaths | 0 | 0 | 0 |
Abbreviations: AE, adverse event; DBP, diastolic blood pressure; SBP, systolic blood pressure. Data are represented as number (percentage). Note: Percentages calculated with the number of patients in each group as denominator. aRelated to study drug includes relationship determined by investigators: possibly related, probably related, and very likely related. AEs related to canagliflozin 300 mg included fatigue, hypoglycemia, postural dizziness, nocturia, pollakiuria (n=3), polyuria, penile erythema, genital pruritus, and orthostatic hypotension; AEs related to canagliflozin 100 mg included diarrhea, increase in serum creatinine, hypoglycemia, micturition urgency, and pollakiuria. AEs related to placebo included dry mouth, hypoglycemia, dizziness, and nocturia. bSignificant orthostasis is defined as symptoms on standing (eg, dizziness, lightheadedness) or a reduction in office SBP ≥20 mm Hg or reduction in office DBP ≥15 mm Hg 2 minutes after standing. Sample sizes: placebo (n=51); canagliflozin 100 mg (n=53); canagliflozin 300 mg (n=56). cVolume depletion includes the following AEs: postural dizziness, dehydration, and/or orthostatic hypotension. dOsmotic diuresis includes the following AEs: dry mouth, micturition urgency, nocturia, pollakiuria (increased urinary frequency), and/or polyuria (increased urinary volume). eRenal related includes an increase in serum creatinine.
Discussion
To our knowledge, this is the first study to evaluate the acute effects of an approved SGLT2 inhibitor therapy on BP using 24‐hour ABPM to capture orthostatic effects over a period of 6 weeks and as early as day 2 after randomization (day 1 on treatment). The results with both doses of canagliflozin indicate a rapid reduction in BP after treatment initiation. These findings are consistent with data from a previous 12‐week study7 and several longer‐term studies (26–52 weeks) of canagliflozin showing positive effects on BP,6, 9, 10, 11 including one study showing an improvement in hypertension with canagliflozin regardless of the presence or absence of concomitant antihypertensive treatment (ACE inhibitor, ARB, and/or diuretics).6 These observations may have important clinical implications, because strategies that control both hyperglycemia and hypertension have been shown to significantly reduce the risk of CV complications and mortality in high‐risk patients with T2DM.13
Few other studies have focused on the effects of SGLT2 inhibition on hypertension at later time points. In a 12‐week study, empagliflozin significantly reduced ABPM‐assessed SBP (adjusted mean difference vs placebo in change from baseline: −3.44 mm Hg with empagliflozin 10 mg and −4.16 mm Hg with empagliflozin 25 mg; both P<.001),14 and pooled data from four 24‐week studies showed that empagliflozin reduced SBP by −3.6 mm Hg and DBP by −1.3 mm Hg compared with placebo.15 Dapagliflozin 10 mg led to baseline‐adjusted reductions in SBP of −3.3 mm Hg, as measured by ABPM in a 12‐week study in patients with T2DM,16 and pooled data from 13 placebo‐controlled clinical trials in patients with T2DM and hypertension showed that dapagliflozin 10 mg was associated with modest reductions in BP over 24 weeks (placebo‐subtracted changes from baseline in SBP and DBP were −3.6 and −1.2 mm Hg, respectively).17 Lastly, a BP‐lowering effect was seen in a 4‐week ABPM study of ertugliflozin: significant reductions in placebo‐adjusted 24‐hour mean SBP of −3.0 to −4.0 mm Hg were seen for all doses (1 mg, 5 mg, 25 mg).18
The exact mechanism by which SGLT2 inhibitors reduce BP in patients with T2DM has not been established. Several possible mechanisms have been suggested, including osmotic diuresis, weight loss, mild natriuresis, nephron remodeling, and/or reduction in arterial stiffness.19, 20 Osmotic diuresis and reduced intravascular volume caused by increased glucose excretion may account for the rapid reductions in BP. However, these volume‐related changes typically resolve. Sha and colleagues7 noted that changes in plasma and urine volume present at week 1 returned to baseline by week 12, despite improvements in BP that were generally similar at both weeks 1 and 12. Weight loss associated with canagliflozin may also contribute to its BP‐lowering effects. In a pooled analysis of data from four 26‐week, placebo‐controlled trials, weight loss at 26 weeks (mainly derived by loss of fat mass) was determined to account for approximately 40% of the BP response to canagliflozin.21 It is also possible that SGLT2 inhibitors have a direct influence on the vasculature that is dependent on the type of artery, duration of treatment, and health status of the patient; studies have recently shown that acute treatment with canagliflozin inhibits vasodilation in pulmonary arteries,22 whereas chronic administration of canagliflozin led to relaxation of coronary arteries in diabetic mice. Taken together, these observations suggest that the BP‐lowering effect of canagliflozin is multifactorial: volume depletion and natriuresis may rapidly reduce BP in the early stages of treatment, while other factors such as weight loss or changes in the renin‐angiotensin system caused by intrarenal feedback may contribute to the sustained reductions in BP.6, 19, 20
As mentioned previously, the sample size and power calculations were reassessed during the study because of slow accrual. Although the calculated sample size was deemed adequate in this study, the observed variability of the overall treatment effect was somehow higher than anticipated. A larger study would likely provide more consistent dose‐related effects of canagliflozin for parameters of interest. There was discordance between the office BP readings and BP readings using ABPM, and a sizeable office‐related placebo effect was observed at week 6, with mean office seated SBP reduced by −3.9 mm Hg in the placebo group compared with baseline (these were −7.5 and −5.3 mm Hg with canagliflozin 300 mg and 100 mg, respectively). A placebo effect clearly exists in office BP measurements, but this effect is typically lessened when using ABPM;23 this was also reflected in our experience of using ABPM in the office setting. It should be noted that this study was not designed and powered to evaluate the attainment of the guideline‐based BP goal; however, a large pooled data analysis showed that canagliflozin treatment led a greater proportion of patients to achieve their BP goal compared with placebo.6 The use of ABPM also avoids the “white‐coat” effect often observed with office BP measurement, and meta‐analyses have shown that ABPM is a more sensitive predictor of clinical CV outcomes, such as coronary events and stroke, than conventional clinic‐based measurements.24, 25 We chose SBP as our primary endpoint, rather than DBP, because SBP measurements are better predictors of CV risk and future events than DBP measurements.26, 27, 28 It should also be noted that FPG was reduced over the 6‐week study period by canagliflozin 300 mg, but not 100 mg, which is inconsistent with previous studies showing early reductions in FPG with canagliflozin 100 mg.29 The reason for this inconsistency is unknown.
The findings of this study may have some important implications for clinical practice. Current evidence supports the later use of SGLT2 inhibitors as add‐on therapy for patients not achieving HbA1c targets.8, 30 The positive effects of SGLT2 inhibitors on BP and body weight have led some to suggest a role for these agents earlier in the disease course to help control these risk factors.31 The acute reductions in BP observed in the current study raise the issue of whether SGLT2 inhibition may increase the risk of hypotension, which has been documented in clinical trials of SGLT2 inhibitors.3, 4, 5, 7, 9 Caution is therefore warranted, particularly when initiating SGLT2 inhibition therapy while simultaneously intensifying antihypertensive therapies. For canagliflozin, it is recommended to assess volume status and correct hypovolemia before starting therapy in patients with advanced age, renal impairment, and low SBP and those taking diuretics, ACE inhibitors, or ARBs.
Lastly, the impact of the BP‐lowering effects of SGLT2 inhibition on clinical outcomes remains to be determined. Treatment with the SGLT2 inhibitor empagliflozin was recently shown to significantly reduce the rate of death from cardiovascular causes compared with placebo in patients with T2DM at high risk for CV events.32 In addition, empagliflozin reduced the risk of death from any cause and hospitalization for heart failure. The authors cited BP‐lowering effects as one of many possible mechanisms by which SGLT2 inhibition may improve CV outcomes. However, a direct link between these effects and long‐term outcomes has not been established. These CV results do, however, provide the first evidence that SGLT2 inhibition may reduce CV risk in patients with T2DM, and similar studies evaluating the effects of canagliflozin on CV outcomes are underway.33, 34
Conclusions
Treatment with canagliflozin 300 mg significantly reduced SBP, as measured by mean 24‐hour ABPM compared with placebo, after only 6 weeks of treatment in patients with T2DM and hypertension. In this study, the safety profile of canagliflozin was consistent with findings from previous large phase 3 studies. Ongoing studies will help further characterize the impact of SGLT2 inhibition on CV outcomes in patients with T2DM.32, 33, 34
Financial Disclosure
This study was funded by Janssen Research & Development, LLC.
Editorial assistance was provided by Adriana Stan, PhD, of Excerpta Medica, funded by Janssen Scientific Affairs, LLC. Statistical programming and validation work was performed by Holly Wang and Weiping Li, Janssen Research & Development, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
Disclosures
RT: consultant/advisory board––Medtronic, Janssen, GSK, Merck; IM: none; AT: previous employment at Janssen; MK: honoraria––Mitsubishi Tanabe Pharmaceutical Company; JR, UJ, CVD, and MP: employment at Janssen.
Supporting information
Table S1. LS Mean and PBO‐Subtracted LS Mean Change From Baseline to Day 2 (LOCF).
J Clin Hypertens (Greenwich). 2016;18:43–52. DOI: 10.1111/jch.12747. © 2015 Wiley Periodicals, Inc.
References
- 1. Kabakov E, Norymberg C, Osher E, et al. Prevalence of hypertension in type 2 diabetes mellitus: impact of the tightening definition of high blood pressure and association with confounding risk factors. J Cardiometab Syndr. 2006;1:95–101. [DOI] [PubMed] [Google Scholar]
- 2. Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380:601–610. [DOI] [PubMed] [Google Scholar]
- 3. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium–glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med. 2013;159:262–274. [DOI] [PubMed] [Google Scholar]
- 4. Rosenthal N, Meininger G, Ways K, et al. Canagliflozin: a sodium glucose co‐transporter 2 inhibitor for the treatment of type 2 diabetes mellitus. Ann N Y Acad Sci. 2015; doi: 10.1111/nyas.12852 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5. Baker WL, Smyth LR, Riche DM, et al. Effects of sodium–glucose co‐transporter 2 inhibitors on blood pressure: a systematic review and meta‐analysis. J Am Soc Hypertens. 2014;8:262–275. [DOI] [PubMed] [Google Scholar]
- 6. Weir MR, Januszewicz A, Gilbert RE, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich). 2014;16:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sha S, Polidori D, Heise T, et al. Effect of the sodium glucose co‐transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16:1087–1095. [DOI] [PubMed] [Google Scholar]
- 8. Saulsberry WJ, Coleman CI, Mearns ES, et al. Comparative efficacy and safety of antidiabetic drug regimens added to stable and inadequate metformin and thiazolidinedione therapy in type 2 diabetes. Int J Clin Pract. 2015;69:1221–1235. [DOI] [PubMed] [Google Scholar]
- 9. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet. 2013;382:941–950. [DOI] [PubMed] [Google Scholar]
- 10. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. NCT01939496 . Evaluation of blood pressure reduction, safety, and tolerability of canagliflozin in patients with hypertension and type 2 diabetes mellitus on stable doses of anti‐hyperglycemic and anti‐hypertensive agents. http://www.clinicaltrials.gov. Accessed September 21, 2015. [Google Scholar]
- 13. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. [DOI] [PubMed] [Google Scholar]
- 14. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. [DOI] [PubMed] [Google Scholar]
- 15. Chilton R, Tikkanen I, Cannon CP, et al. The sodium glucose cotransporter 2 inhibitor empagliflozin reduces blood pressure and markers of arterial stiffness and vascular resistance in type 2 diabetes. J Hypertens. 2015;33(suppl 1):e53. [Google Scholar]
- 16. Heerspink HJ, de Zeeuw D, Wie L, et al. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sjöström CD, Johansson P, Ptaszynska A, et al. Dapagliflozin lowers blood pressure in hypertensive and non‐hypertensive patients with type 2 diabetes. Diab Vasc Dis Res. 2015;12:352–358. [DOI] [PubMed] [Google Scholar]
- 18. Amin NB, Wang X, Mitchell JR, et al. Blood pressure‐lowering effect of the sodium glucose co‐transporter‐2 inhibitor ertugliflozin, assessed via ambulatory blood pressure monitoring in patients with type 2 diabetes and hypertension. Diabetes Obes Metab. 2015;17:805–808. [DOI] [PubMed] [Google Scholar]
- 19. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens. 2015;9:48–53. [DOI] [PubMed] [Google Scholar]
- 20. Majewski C, Bakris G. Blood pressure reduction: an added benefit of sodium–glucose cotransporter 2 inhibitors in patients with type 2 diabetes. Diabetes Care. 2015;38:429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58:1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han Y, Cho Y‐E, Ayon R. SGLT inhibitors attenuate NO‐dependent vascular relaxation in the pulmonary artery but not in the coronary artery. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1027–L1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asmar R, Safar M, Queneau P. Evaluation of the placebo effect and reproducibility of blood pressure measurement in hypertension. Am J Hypertens. 2001;14:546–552. [DOI] [PubMed] [Google Scholar]
- 24. Pickering TG, Shimbo D, Haas D. Ambulatory blood‐pressure monitoring. N Engl J Med. 2006;354:2368–2374. [DOI] [PubMed] [Google Scholar]
- 25. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 26. Black HR. The paradigm has shifted, to systolic blood pressure. Hypertension. 1999;34:386–387. [DOI] [PubMed] [Google Scholar]
- 27. Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. [DOI] [PubMed] [Google Scholar]
- 28. Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr Opin Nephrol Hypertens. 2003;12:293–297. [DOI] [PubMed] [Google Scholar]
- 29. Devineni D, Morrow L, Hompesch M, et al. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14:539–545. [DOI] [PubMed] [Google Scholar]
- 30. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. [DOI] [PubMed] [Google Scholar]
- 31. Brunton SA. The potential role of sodium glucose co‐transporter 2 inhibitors in the early treatment of type 2 diabetes mellitus. Int J Clin Pract. 2015;69:1071–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; doi 10.1056/NEJMoa1504720 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33. Neal B, Perkovic V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium–glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–411. [DOI] [PubMed] [Google Scholar]
- 34. A study of the effects of canagliflozin (JNJ‐28431754) on renal endpoints in adult participants with type 2 diabetes mellitus (CANVAS‐R). Study protocol NCT01989754. http://www.clinicaltrials.gov. Accessed September 21, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. LS Mean and PBO‐Subtracted LS Mean Change From Baseline to Day 2 (LOCF).


