The renal nerves contribute to hypertension through effects in the kidney that enhance sodium retention and renin secretion, and by effects in the central nervous system that increase systemic sympathetic activity. Therefore, targeting the renal nerves provides a logical basis for treating hypertension. Several trials of renal denervation––achieved by applying radiofrequency energy through catheters placed in the renal arteries––have been completed. Clinical results have been inconsistent, however, partly because of factors related to the ablation technique and partly because these studies have been performed in patients with the inadequately defined clinical condition of “treatment‐resistant hypertension.” This statement now explains our conclusion that future studies of renal denervation should be guided by the established randomized, controlled clinical trial designs used for studying antihypertensive drugs and other treatments for hypertension.
Background
Even before the treatment of hypertension became routine, it was understood that high levels of blood pressure (BP) were associated with premature death, stroke, cardiovascular events, and renal failure. One of the early and effective interventions for severe or symptomatic hypertension was the use of surgical sympathectomy.1 Although this technique was useful in reducing BP and improving survival, major side effects such as disabling postural hypotension and sexual dysfunction were important limiting factors. Later, many of the early drugs developed for treating hypertension were also based on interrupting the sympathetic nervous system, but, again, their side effects––although not as severe as those associated with surgical intervention––made these drugs difficult to use.
A More Selective Approach
Innovative physiologic research in animal models of hypertension made it clear that the renal sympathetic nerves play a critical role in BP regulation.2 It was demonstrated that the efferent fibers of these nerves play a major role in governing release of renin from the kidney as well as in increasing sodium reabsorption in the renal tubules and promoting renal vasoconstriction. Another critical role of the renal nerves, however, is mediated by the afferent fibers, which, by their actions in the central nervous system, have the effect of stimulating central sympathetic outflow. Taken together, these actions of the renal nerves––stimulating renin, enhancing sodium retention, and increasing systemic sympathetic activity––provide a tempting target for therapeutic intervention. In fact, studies in animal models of hypertension have revealed that severing the renal nerves can produce meaningful reductions in BP and even prevent the onset of hypertension.2
This knowledge has recently been applied to human hypertension. Because the renal nerve fibers are in close anatomical proximity to the renal arteries, most of the clinical methods for achieving renal denervation in humans have relied on ablative interventions applied through catheters inserted into the renal arteries.
Early Results
The first important published work on renal denervation as a treatment for hypertension was reported in patients with so‐called “treatment‐resistant hypertension.” These were patients whose BPs appeared to remain dangerously high despite multiple antihypertensive medications. The early trials reported encouraging results with reductions in systolic BPs averaging as high as 30 mm Hg. The two best known trials of denervation in this patient population were titled Symplicity Hypertension‐13 and Symplicity Hypertension‐2.4 These trials used a radiofrequency catheter (the Symplicity catheter) with a single electrode at its tip such that when the catheter was rotated during withdrawal through four 90° segments, so as to make contact with the artery wall and apply energy, it achieved a helical pattern of circumferential ablation of the renal nerve fibers lying in and around the artery's adventitia. The intention of the study protocols was to achieve bilateral ablation of the renal nerves.
To some observers it appeared unusual that early clinical trials of a new hypertension treatment in human subjects were conducted in the complicated and poorly defined condition of treatment‐resistant hypertension, but it was argued that the strong need to control BP in these uncontrolled hypertensive patients taking multiple medications could justify the testing of an intervention for which safety had not yet been fully established.
A Disappointing Outcome
Based on the promising results of the early experiences, a definitive registration study in the United States, the Symplicity Hypertension‐3 trial,5 was conducted using a more rigorous design than the earlier trials. In particular, patients with treatment‐resistant hypertension, with office systolic BPs ≥160 mm Hg despite taking an average of five antihypertensive drugs, were randomized to active treatment with the Symplicity catheter or to a “sham procedure” in which a renal angiogram was performed but no ablation of the renal nerves was undertaken.
The results proved surprising and discouraging. Reductions in BP in the patients receiving denervation were not different from those in the sham‐treated arm, even though the changes within each group were significant.5 This study included ambulatory BP monitoring (ABPM) as well as office measures of BP, but the findings with ABPM also failed to show a significant difference between the two treatment arms. Why, after the clear successes of the Symplicity HTN‐1 and HTN‐2 studies, did this large randomized clinical trial with a blinded control arm––a sham procedure––fail to confirm the earlier results?
Lessons Learned From Symplicity HTN‐3
For all the initial disappointment, Symplicity HTN‐3 was a valuable exercise that has provided information and direction for future work. Importantly, observers interpreted the trial's results as strongly confirming the necessity for sham controls in denervation trials in the same way that studies of new drugs typically include a placebo arm. Beyond this, other important issues were clarified.
A major lesson was that treatment‐resistant hypertension is an ill‐defined and uncertain term that cannot always stand up to scrutiny. One problem with establishing the diagnosis is the white‐coat effect––a high BP in the clinic that is not observed elsewhere. ABPM can mitigate this diagnostic error, although in HTN‐3, where ABPM measurements were included in patient enrollment procedures, the ABPM diagnostic criteria used may not have been sufficiently rigorous to guarantee that the office BP entry criteria were fully met.
Various factors can explain inadequate BP responses to antihypertensive drug therapy, including the BP‐raising effects of unrelated drugs used for treating comorbidities, dietary factors such as excessive salt or alcohol use, underlying forms of secondary hypertension––sleep apnea and aldosterone excess being two relatively common explanations––and, most of all, poor adherence by patients to their prescribed drug regimens. In HTN‐3, treatment compliance was assessed only by patient self‐reporting, so there was no certainty that they were actually taking their prescribed drugs when their BPs met the threshold for study entry. Beyond that, during the trial there were increases or decreases in antihypertensive drug use documented in about 40% of patients despite a protocol mandate to maintain a stable dosing regimen for 6 months post‐randomization, and it was not possible to confirm the adherence of the other 60% of patients to their complex multidrug regimens. For these reasons, a study relying on community‐based treatment‐resistant hypertension includes patients with widely disparate reasons for their poor BP control and thus may not be a dependable basis for evaluating a new therapy.
Beyond its value in ruling out white‐coat hypertension, the importance of ABPM as a measure of outcomes has become more apparent. Despite the various explanations for the HTN‐3 results, it was difficult to understand why the earlier trials, HTN‐1 and HTN‐2, had achieved clinic BP reductions that were of far greater amplitude than those reported in HTN‐3. This difference, however, may not have been quite as great as supposed, because HTN‐2,4 which performed ABPM in a subset of patients, reported a reduction in systolic BP that was considerably less than the fall observed in clinic BP, and was not greatly different from the ABPM results observed in HTN‐3. At least one important lesson has emerged from this consideration and will influence future research: ABPM will become the primary endpoint in new trials of renal denervation for the treatment of hypertension.
HTN‐3 was commendable in being a large trial with substantial patient numbers. Unfortunately, virtually all of the procedures performed in this first major US trial were by operators who were experts in endovascular procedures but did not have previous renal denervation experience. Moreover, there was incomplete knowledge regarding what should constitute an “adequate” or “complete” series of bilateral ablations. In fact, post‐hoc analysis has indicated that fewer than 25% of patients received complete bilateral treatment with ablations in a spiral pattern, with at least one ablation in each quadrant, as specified in the protocol.6
Another consequence of HTN‐3, as experts pondered the unexpected result, was the growing realization that our previous understanding of the anatomy of the renal nerves might not have been entirely accurate. Indeed, further careful evaluation done largely since the trial was completed has established that many of the critical nerve fibers––including the afferent fibers that conduct messages to the central nervous system––come into close proximity to the renal arteries mainly at distal sites, often in the vicinity of the bifurcation of the arteries.7 As well, the potential importance of performing nerve ablations in accessory renal arteries is now becoming appreciated. Clearly, a more thorough ablation protocol must be tested in future trials.
An additional factor that might have prevented optimal therapy in HTN‐3 was the use of a single electrode catheter––described earlier––that required considerable manipulation to achieve a complete circumferential ablation. It is probable that a large fraction of patients in the trial received less‐than‐complete bilateral renal denervation. Fortunately, newer catheters designed to apply radiofrequency ablation may have solved this problem. For instance, the new Medtronic Symplicity Spyral catheter and the Boston Scientific Vessix catheter are equipped with an array of electrodes so there is no longer a need to manipulate the catheter tip to achieve circumferential denervation. In addition, catheters such as the ReCor Medical Paradise catheter that depend on ultrasound energy to interrupt renal nerve traffic have been demonstrated to provide full circumferential effects. As well, catheters that create nerve ablation by the local microinjection of pharmacologic agents (eg, dehydrated alcohol) through the renal artery wall have been shown to be effective. Studies with these differing catheter treatments are now under way, or will commence soon, and the results will be eagerly awaited.
Importantly, Simplicity HTN‐3 has provided valuable insights into the safety of renal denervation therapies. There were no significant (and less‐than‐expected) associated systemic complications or vascular injury effects that would discourage further or expanded use of this interventional procedure in the future. Consequently, clinical trialists can take the position that this procedure‐based therapy need not be restricted to “refractory” clinical conditions, but can also be studied in a broader range of hypertension settings.
Heterogeneity of Results
Another characteristic of clinical trials of renal denervation is the heterogeneity of results among patients. Some patients experience remarkable reductions in BP––both by office readings and ABPM––whereas others have no reductions or even increases in BPs.3, 8 So far, there has been no good explanation for heterogeneity other than inconsistent medication‐taking by patients. One somewhat more tangible suggestion has been that older patients have not responded as well as younger patients, supposedly because the isolated systolic hypertension associated with aging is more likely to depend on intrinsic arterial stiffness and is thus less amenable to sympathetic denervation.9 This argument, however, may not be entirely valid. We know from pivotal studies in older people with predominant systolic hypertension that differing pharmacologic agents, including diuretics, calcium channel blockers, and angiotensin‐converting enzyme inhibitors, have very effectively reduced BP and prevented major cardiovascular outcomes in people aged well into their 80s.10, 11 In the renal denervation studies performed until now, older patients with treatment‐resistant hypertension were already taking these types of drugs and so may have represented an atypical subgroup of patients with isolated systolic hypertension. Clarifying the effects of renal denervation in this important form of hypertension will require studies in patients not receiving concurrent drug treatment.
Starting Over and Getting It Right
In planning the next steps for evaluating renal denervation, it has become obvious that we should avoid the uncertainties associated with treatment‐resistant hypertension and instead be guided by the well‐established methods that have been successfully used during recent years for the development of new antihypertensive drugs. There are obvious differences, of course, in testing drugs and devices. For instance, unlike with drugs, renal denervation––at least, as we currently perform it––does not have dosing steps and so does not require initial clinical studies to elaborate this information. In fact, it could be argued that data from all formal trials performed with this intervention could represent the basis for regulatory approval. In essence, this new treatment should be shown to be effective in reducing BP in hypertensive patients when used as a single entity, and, once that has been established, the next major step should be to demonstrate that this new treatment has additive effects when used in combination with drug therapies. Clearly, drug development strategies cannot set an exact paradigm for the development of device‐based therapies, but the general principles should be similar and apply.
The following clinical trial outlines are intended as guides to the evaluation of renal denervation as a treatment for hypertension. Because there has not yet been any experience with this therapy in these types of trials, any details we offer are for illustrative purposes. Even so, we hope that we can provide the basis for clinical studies that will start defining the place of renal denervation in the treatment of hypertension.
First Step: Is Renal Denervation Effective as a Single Therapy?
The fundamental evaluation of renal denervation must be to test whether its BP‐lowering efficacy in hypertensive patients is significantly greater than that of placebo––or, in this case, a sham procedure. Just as important, a trial design must take into account the safety of the procedure, particularly as renal denervation entails a vascular intervention.
Figure 1 shows the overall design of a straightforward initial trial. Because it is anticipated that renal denervation might produce major changes in BP, it would be most appropriate to study hypertensive patients whose BPs, off any previous treatment, are in a relatively high range, eg, systolic values between 150 mm Hg and 180 mm Hg. Because patients should be randomized in this double‐blind trial design to a control (sham) procedure as well as to the active intervention, there should be a limited duration of this study period to minimize cardiovascular risk to sham‐group patients whose BPs would probably remain relatively high. There are precedents for placebo‐treated periods for up to at least 2 months with patients in this BP range.12 In addition, a 2‐month treatment period appears realistic, because previous studies with renal denervation have demonstrated BP‐lowering efficacy as early as 1 month following the procedure.3, 4, 8 The primary endpoint of the trial would be the difference in achieved systolic BPs between the intervention and control groups, measured by APBM after 2 months. Of course, office BP, as well as diastolic BP and heart rate, will also be of substantial interest.
Figure 1.
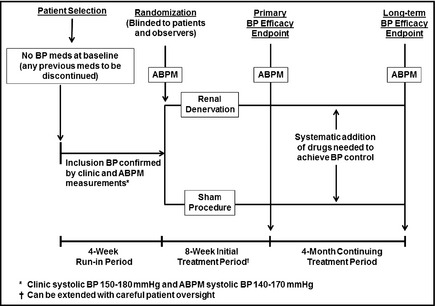
Renal Denervation Alone. This is a three‐period trial design. Period 1: Potentially eligible hypertensive patients are screened into a 4‐week run‐in period during which any previous blood pressure (BP) drugs are discontinued and stable BP is achieved. Patients meeting inclusion criteria, including clinic BP and ambulatory BP monitoring (ABPM) values, are randomized to active renal denervation or a sham procedure. Other than the intervention team, who has no patient contact after the procedure, all other staff members as well as patients are blinded to the assignment. Period 2: After an 8‐week initial treatment period, during which no BP medications can be added (except for emergencies), systolic BP by ABPM is used as the primary study endpoint; clinic BPs are a secondary endpoint. Period 3: Double‐blind status is maintained during a 4‐month period during which investigators systematically add medications or adjust doses according to a strict protocol, at 2‐ to 3‐week intervals, until BP control (clinic systolic BP <140 mm Hg) is achieved. ABPM and clinic BPs at the end of this period are secondary endpoints, as is the medication score (derived from the number of treatment steps at which drugs are added or doses increased). Safety endpoints: These include evidence for renal artery damage, decreases in renal function, or any other adverse findings possibly resulting from the procedure. Safety will continue to be monitored for up to 2 years following this trial.
After the primary endpoint is reached, the trial ideally should continue, still blinded, for another 3 to 4 months. The reasons for this additional time period are twofold: first, to extend the period of observation in order to increase the ability to observe any safety issues; and second, to allow a structured antihypertensive regimen to be applied to patients whose BPs are not yet controlled (systolic BP <140 mm Hg). This strategy creates an important secondary endpoint for the trial, namely a comparison of the intensity of antihypertensive drug regimens required to achieve BP control within the two patient groups. It would be of interest, for example, to test whether patients receiving renal denervation not only have a greater BP reduction than the control group but also have a reduced need for additional antihypertensive drugs to achieve optimal treatment targets.
An example of a drug titration scheme that could be used in this trial design (as well as the trial design that follows) is shown in the Table. This systematic therapeutic approach allows a treatment score to be established for each patient, thus enabling a statistical comparison of treatment intensity to be made between the two study arms. The sequence of drugs suggested in the Table is intended to minimize differences in responses among patients of different ethnicities, ages, and dietary intake but can be modified as necessary.
Table 1.
An Example of Structured Drug Titration During Renal Denervation Trials
| Step (Target Systolic BP <140 mm Hg) | Drug | Treatment Score |
|---|---|---|
| 0 (not needed) | None | 0 |
| 1 (if needed) | CCB: mid‐dose | 1 |
| 2 (if needed) | ACE inhibitor or ARB: full dose | 2 |
| 3 (if needed) | Hydrochlorothiazide 12.5 mg | 3 |
| 4 (if needed) | Hydrochlorothiazide 25 mg | 4 |
| 5 (if needed) | CCB: increase to full dose | 5 |
| 6 (if needed) | Spironolactone or β‐blocker or clonidine | 6 |
| 7 (if needed) | Spironolactone or β‐blocker or clonidine | 7 |
| 8 (if needed) | Spironolactone or β‐blocker or clonidine | 8 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker. Usually 2 to 3 weeks between steps. If target reached, no further steps even if BP fluctuates above target. For steps 6, 7, and 8, choice of drug and dose at investigator's discretion. If initial systolic BP ≥160 mm Hg, steps 1 and 2 can be combined. Fixed‐combination drug products can be used to decrease pill burden. In protocols where patients already receive drugs, step sequence will begin between steps 2 and 6 depending on number of drugs in the ongoing regimen.
After the important secondary endpoints are reached at about 6 months of blinded follow‐up, the study can be unblinded, but patients who have received active denervation must be followed for a considerable time thereafter to evaluate long‐term safety.
Because this trial could be described as a “proof of principle” study––albeit clinically relevant––it would be useful to gather data to help provide reassurance that renal denervation, not confounded by the effects of concomitant drug therapies, produces its anticipated physiologic effects. In particular, the studies should document the effects of denervation on plasma renin activity and aldosterone as well as on office BP and ABPM changes in heart rate (a rough index of sympathetic activity, although in general estimates of sympathetic activity do not correlate closely with BP changes). There is probably no convenient way in a simple clinical trial to measure whether renal denervation increases natriuresis, although changes in serum concentrations of sodium and potassium could be of interest.
Second Step: Studies of Renal Denervation in Combination With Antihypertensive Drugs
A straightforward approach to testing whether denervation provides additive BP‐lowering effects in patients whose BPs remain elevated despite receiving strictly specified antihypertensive medications is shown in Figure 2. This design is similar to the one for testing renal denervation as a single therapy. Very simply, in this double‐blind study, renal denervation would be compared with a sham control in patients whose BPs remain elevated while receiving a standardized drug regimen. (Details of possible regimens are discussed below.) The baseline drug regimen would be maintained during the first part of the trial (2–3 months) until the primary endpoint. After that, just as in the previous trial design (Figure 1), further drugs could be added as needed (see the Table) to achieve BP control and provide further information on the interaction between renal denervation and drug therapy.
Figure 2.
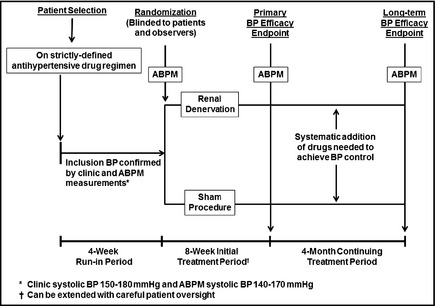
Renal Denervation Added to Antihypertensive Drug Therapy. This is a three‐part trial design. Period 1: Potentially eligible hypertensive patients are screened into a 4‐week run‐in period during which any previous blood pressure (BP) drugs are discontinued and replaced by a specified drug regimen (options are discussed in the text) and stable BP is achieved. Patients meeting inclusion criteria, including clinic BP and ambulatory BP monitoring (ABPM) values, are randomized to active renal denervation or a sham procedure. Other than the intervention team, who will have no patient contact after the procedure, all other staff members as well as patients are blinded to the assignment. Period 2: After an 8‐week initial treatment period, during which no BP medications can be added (except for emergencies), systolic BP by ABPM is used as the primary study endpoint; clinic BPs are a secondary endpoint. Period 3: Double‐blind status is maintained during a 4‐month period during which investigators systematically add medications or adjust doses according to a strict protocol, at 2‐ to 3‐week intervals, until BP control (clinic systolic BP <140 mm Hg) is achieved. ABPM and clinic BPs at the end of this period are secondary endpoints, as is the medication score (derived from the number of treatment steps at which drugs are added or doses increased). Safety endpoints: These include evidence for renal artery damage, decreases in renal function, or any other adverse findings possibly resulting from the procedure. Safety will continue to be monitored for up to 2 years following this trial.
In designing this trial, a major question arises: What is the ideal baseline (prerandomization) drug regimen? Single‐drug therapy, which is typical for combination studies with new antihypertensive drugs; a two‐drug combination, which would focus on patients with more challenging forms of hypertension; or a three‐agent combination, which, in essence, would pull the study into the domain of treatment‐resistant hypertension.
Choosing an Appropriate Baseline Drug Regimen
Here are some considerations regarding possible drug regimens:
A one‐drug regimen: This is obviously the simplest approach. Patients taking a single effective antihypertensive agent, but whose systolic BPs remain between 150 mm Hg and 180 mm Hg, could be regarded as having at least “moderate” hypertension. This approach is often used in developing new antihypertensive agents.
A two‐drug regimen: If we were to select this regimen according to common contemporary drug usage, it would probably comprise either an ACE inhibitor or an angiotensin receptor blocker (ARB) in combination with a thiazide (or a calcium channel blocker). This two‐drug regimen should achieve control of BP in most people with hypertension, including those with stage 2 hypertension. Thus, patients whose systolic BPs remain in the range of 150 mm Hg to 180 mm Hg on this regimen could be associated with legitimate challenges in hypertensive management and might be an appropriate target for this type of trial. It is critical to emphasize that the trial protocol should specify exactly what the two‐drug regimen should be and, preferably, this therapy should be provided by the trial sponsor to investigators and their patients to ensure treatment consistency and to help keep patients adherent to their treatment.
A three‐drug regimen: This regimen should normally comprise an ACE inhibitor or ARB combined with both a thiazide diuretic and a calcium channel blocker in maximum doses. Quite clearly, patients who are taking such a regimen, and whose systolic BPs remain significantly elevated (in the range of 150–180 mm Hg), satisfy the conventional criteria for treatment‐resistant hypertension.13 Studies of three‐drug regimens have demonstrated that these combinations produce substantial reductions in systolic pressure averaging around 40 mm Hg,14 such that those patients whose systolic BPs remain >150 mm Hg would represent only a small fraction (<10%) of the total hypertension population, and the complexities discussed earlier with studying treatment‐resistant hypertension might come into play. Finding patients taking three‐drug regimens in the community who satisfy the entry criteria for such a trial could prove difficult. It would be far better, as discussed for the two‐drug combination, to adjust whatever multidrug regimens patient candidates might have been taking previously and replace them with a standardized three‐drug protocol, preferably contained within a single tablet. Recently, an open‐label renal denervation trial in France demonstrated the feasibility of studying patients receiving a standardized three‐drug regimen at baseline, although patient enrollment was enhanced by using the range of 140 mm Hg to 180 mm Hg rather than 150 mm Hg to 180 mm Hg as the office systolic BP entry criterion.15
Pros and Cons of the Combined Drug/Denervation Protocol
This trial has two clinically relevant benefits. First, it replicates a likely clinical setting where denervation might be considered in patients not readily controlled on drugs. Second, it might address the question of whether denervation in such patients is more likely to achieve BP control than intensifying a multidrug regimen. But there are limitations to this trial design. Using patients whose BPs remain high despite well‐constructed drug regimens creates a focus on patients who, for whatever reasons, are drug nonresponders, and at this early stage of assessing the efficacy of denervation, may limit the assessment of combined drug/denervation treatment. A second limitation to this design is that it would not be certain that BP reductions produced by renal denervation were additive to the drug effects or whether denervation as a single treatment might have achieved the same result. These limitations would be addressed by the following trial protocol.
An Alternative Combination Study Design
A trial design adapted from that traditionally used for evaluating combination antihypertensive drug therapies is shown in Figure 3. This randomized, double‐blind three‐arm study is designed to test whether the combination of denervation plus medication is superior to denervation alone as well as to medication alone. This approach would provide strong evidence regarding the legitimacy of this type of combined therapy. In this design, the drug therapy would be either a single agent or possibly a combination, although if a two‐drug combination were to be used it would be prudent to restrict patient enrollment to those with off‐treatment stage 2 hypertension (systolic BP ≥160 mm Hg) so as to better discriminate any additive BP‐lowering effects of denervation. This efficacy segment of this trial could be shorter in duration than the earlier designs because add‐on drug therapy following the primary endpoint would probably not be undertaken as part of the trial. Finally, it should be noted that undertaking a pivotal trial such as this would be contingent on positive results from the initial proof‐of‐principle study.
Figure 3.
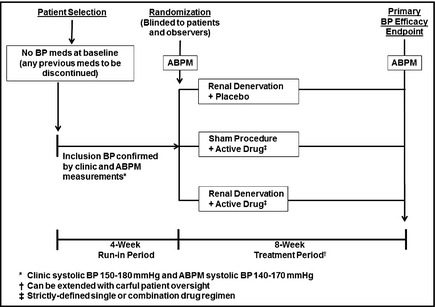
A Definitive Combination Therapy Protocol. This is a two‐part trial design. Period 1: Potentially eligible hypertensive patients are screened into a 4‐week run‐in period during which any previous blood pressure (BP) drugs are discontinued and stable BP is achieved; a single‐blind placebo could be administered during this run‐in period. Patients meeting inclusion criteria, including clinic BP and ambulatory BP monitoring (ABPM) values, are randomized to one of three treatment arms: active renal denervation alone (plus drug placebo), active drug therapy alone (plus sham denervation), or the combination of denervation plus active drug therapy. Other than the intervention team, who will have no patient contact after the procedure, all other staff members as well as patients are blinded to the assignment. Period 2: After an 8‐week treatment period, during which no BP medications can be added (except for emergencies), systolic BP by ABPM is used as the primary study endpoint; clinic BPs are a secondary endpoint. It is assumed that the active drug therapy in this trial will generally be a single agent. It would also be possible to use a two‐drug combination, but it would be recommended that the clinic systolic BP criterion for randomization be ≥160 mm Hg and the ABPM be ≥145 mm Hg to allow a BP range sufficiently high to adequately assess the additive effects of denervation when combined with such a drug regimen. Safety endpoints: These include evidence for renal artery damage, decreases in renal function, or any other adverse findings possibly resulting from the procedure. Safety will continue to be monitored for up to 2 years following this study.
Some General Considerations
In view of the complexities already discussed, it is essential that investigators and their staff selected to conduct these trials be experienced in hypertension research. Choosing patients whose BPs are likely to be in the desired range, typically systolic BPs of 150 mm Hg to 180 mm Hg, whether not taking current treatment or taking a carefully structured drug regimen, will require experts experienced in hypertension trials.
A similar argument should be made for the selection of the interventionalists to conduct the denervation procedures in study patients. Even though these professionals almost certainly will have had considerable experience performing coronary and peripheral artery interventions, there must be clear pre‐study agreement and training regarding the techniques to be used in the trials. The big issues include how aggressive operators should be in seeking to produce distal ablation of the renal nerves and, overall, how many ablations should be attempted. There should also be clear agreement on whether to treat accessory renal arteries, branch arteries, and other such details. These decisions should also take into account the potentially differing ablation characteristics of the catheters being tested.
Renal Denervation: What Is Its Future?
It is still too early in the development of this technology to define where it might fit into the overall management of hypertension. We have already learned that renal denervation has produced inconsistent results in treatment‐resistant hypertension. Understandably, resistant hypertension was selected as a starting point because––however inexactly and inadequately it has been defined––this condition would appear to justify a procedure that is interventional and may carry a greater initial financial cost than usually associated with hypertension therapy. Even so, it may well be a reasonable proposition that renal denervation could also be of value in other settings of hypertension, especially as extensive experience until now has not raised major safety concerns. Still, bearing in mind the mixed results so far, our first task will be to resolve the uncertainties regarding the BP effects of renal denervation and, if appropriate, to then move forward with clinical trials aimed at defining its place in clinical practice.
Disclosures
MAW: Consultant: Medtronic, Boston Scientific, ReCor, Ablative Solutions, Lilly, Novartis; Speaker: Arbor. AK: Grants to institution: Medtronic, Boston Scientific, St Jude, Abiomed, Vascular Dynamics, Abbott Vascular, Lilly. LM: Consultant: Medtronic, ReCor, St Jude, Biotronic, Eli Lilly; Grants to institution: Abbott, Boston Scientific, Medtronic, Cordis, Bristol Myers Squibb, Sanofi‐Aventis, Lilly, Daiichi Sankyo, Biotronik; RRT: Consultant: Medtronic, Janssen, GSK; Royalties: UpToDate; Grants: NIH, Fukuda Denshi; DEK: Research/grant support, consulting: Medtronic, Boston Scientific MBL: Scientific advisory board and clinical research support: Abbott Vascular, Boston Scientific, Medtronic, Edwards Lifescience; Grants to institution: Medtronic, Boston Scientific, St Jude, Abiomed, Vascular Dynamics, Abbott Vascular, Lilly.
Acknowledgments
The authors received no external funding for this work.
This editorial is being co‐published in The Journal of Clinical Hypertension (DOI: 10.1111/jch.12590), Clinical Cardiology (DOI: 10.1002/clc.22424), and Catheterization and Cardiovascular Interventions (DOI: 10.1002/ccd.26028).
References
- 1. Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension: results in 1,266 cases. JAMA. 1953;152:1501–1504. [DOI] [PubMed] [Google Scholar]
- 2. DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. [DOI] [PubMed] [Google Scholar]
- 3. Krum H, Schlaich M, Whitbourn R. Catheter‐based renal sympathetic denervation for resistant hypertension: a multi‐centre safety and proof‐of‐principle cohort study. Lancet. 2009;373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 4. Esler MD, Krum H, Sobotka PA, et al; SYMPLICITY HTN‐2 Investigators . Renal sympathetic denervation in patients with treatment‐resistant hypertension (the SYMPLICITY HTN‐2 Trial): a randomized controlled trial. Lancet. 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 5. Bhatt DL, Kandzari DE, O'Neill WW, et al; SYMPLICITY HTN‐3 Investigators . A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 6. Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the SYMPLICITY HTN‐3 trial. Eur Heart J. 2015;36:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atherton DS, Deep NL, Mendelsohn FO. Microanatomy of the renal sympathetic nervous system: a human post‐mortem histologic study. Clin Anat. 2012;25:628–633. [DOI] [PubMed] [Google Scholar]
- 8. Schwerg M, Heutel C, Strajnic D. Renal sympathetic denervation: early impact on ambulatory resistant hypertension. J Clin Hypertens (Greenwich). 2014;16:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ewen S, Ukena C, Linz D, et al. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension. 2015;65:193–199. [DOI] [PubMed] [Google Scholar]
- 10. The SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 11. Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group . Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 12. White WB, Weber MA, Sica D, et al. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57:413–420. [DOI] [PubMed] [Google Scholar]
- 13. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. [DOI] [PubMed] [Google Scholar]
- 14. Lacourciere Y, Crikelair N, Glazer RD, et al. 24‐hour ambulatory blood pressure control with triple‐therapy amlodipine, valsartan and hydrochlorothiazide in patients with moderate to severe hypertension. J Hum Hypertens. 2011;25:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open‐label, randomised controlled trial. Lancet. 2015. Jan 23; DOI: 10.1016/S0140-6736(14)61942-5. [DOI] [PubMed] [Google Scholar]


