Abstract
This paper examines blood pressure (BP) control after 6 months of an intensive pharmacist‐managed intervention in a mixed‐methods randomized controlled trial conducted at the Iowa City Veteran Affairs Health Care System and two community‐based outreach clinics. Patients received the pharmacist intervention for the first 6 months. The study coordinator conducted a summative evaluation with 37 patients 18 to 24 months following the initial 6‐month intervention period. BP was significantly reduced in diabetic patients following an intensive pharmacist intervention (−8.0/−4.0±14.4/9.1 mm Hg systolic/diastolic, P<.001 and P=.001, respectively). BP was reduced even more in nondiabetic patients (−14.0/−5.0±1.9/10.0 mm Hg, P<.001). Medication adherence significantly improved from baseline to 6 months (P=.017). BPs were significantly lower at 6 months following an intensive pharmacist intervention. Patients also expressed a high level of satisfaction with and preference for co‐management of their hypertension, as well as other chronic diseases.
Hypertension (HTN) affects more than 1 million US veterans and is a risk factor for heart disease, stroke, renal failure, peripheral vascular disease, and death. Every 3‐mm Hg reduction in systolic blood pressure (BP) could reduce cardiovascular mortality by 5% and stroke mortality by 8%.1 However, HTN remains inadequately treated.2, 3 Although older studies found that a majority of patients with HTN were poorly controlled, a recent Veterans Administration (VA) study found that only 23% of hypertensive patients had suboptimal BP control.4 One of the many ways the Veterans Health Administration (VHA) has been able to achieve these improved results is by implementing team‐based care utilizing pharmacists to manage hypertension. Even with these assertive efforts, there is still room for improvement for controlling hypertension. VA/Department of Defense (DoD) guidelines suggest that pharmacists be used to help with medication adjustments to improve BP control.5 Although clinical trials have found that higher BP control rates can be achieved, this requires increasing intensity of therapy over time to maintain long‐term BP control. Such aggressive management is not typical in practice where clinical inertia and patient nonadherence create barriers to achieving BP control.
Within the Iowa City VA Health Care System, pharmacists currently provide ad hoc consultation on patients with HTN with the ability to adjust dosages of primary care provider (PCP) prescribed medications. The Iowa City VA has a pharmacist‐run anticoagulation clinic and pharmacists independently manage patients referred to them by PCPs for hyperlipidemia, diabetes mellitus, and smoking cessation with the ability to prescribe and adjust medications and order appropriate laboratory tests. Within the health system, there is no guidance on how to use pharmacist interventions after BP has been controlled or if patients can be referred back to primary care.6, 7, 8, 9, 10, 11, 12
In this study, PCPs gave the pharmacist authority to prescribe BP medications and order appropriate laboratory tests to assess renal function and electrolytes. To date, few randomized studies have evaluated team‐based strategies to sustain the effect of such interventions. Thus, the primary goal of the main study is to evaluate how to sustain long‐term BP control in Veterans with HTN following a 6‐month intensive pharmacist intervention. As part of the parent study, all patients received the intervention for 6 months and then were subsequently randomized to either continued intervention or referral back to usual care following simple education. The final results of the randomized clinical trial will not be available until after 2014. The purpose of the present paper is to report BP control following the initial 6‐month intervention and to describe findings from a summative evaluation of the intervention that was conducted 18 to 24 months later (Figure).
Figure 1.
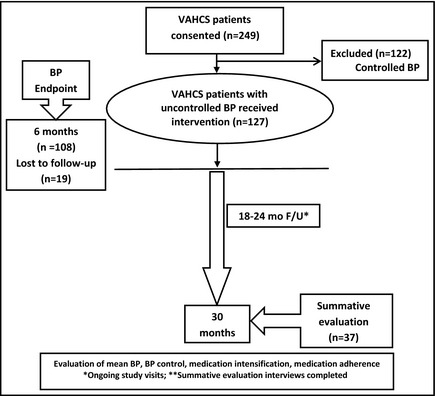
Study overview. VAHCS indicates Veteran Affairs Health Care System; BP, blood pressure; F/U, follow‐up.
Methods
Study Design and Patient Selection
This study was a randomized controlled trial conducted at the Iowa City Veteran Affairs Health Care System (VAHCS), and two community‐based outreach clinics (CBOCs) in Coralville and Cedar Rapids, IA. The study was approved by the University of Iowa's institutional review board and the Iowa City VA Research and Development Committee.
A study coordinator and departmental research assistants (RAs) were trained to measure BP using standardized guidelines.13, 14 BP was measured 3 times using an automated device (HEM 907‐XL; Omron Corporation, Schaumburg, IL) at baseline and 6‐month time points. The second and third BP values at each study visit were averaged and used to determine the research BP value.
Patients followed by a VA PCP were identified from the VAHCS computerized patient record system (CPRS) and screened for inclusion by the study coordinator. The coordinator determined whether the patient had a diagnosis of HTN and reviewed clinic BP values over the previous 18 months. If the most recent clinic BP met study criteria or the average of the last 3 BP values met criteria, the coordinator then determined whether any exclusions (see below) were present. Eligible patients were mailed a letter inviting them to participate and then contacted via telephone by a member of the research team who explained the study. If the patient expressed interest, the 10‐item Pfeiffer Short Portable Mental Status Questionnaire was administered. If no impairment in cognitive function was noted, a baseline visit was scheduled.
Patients were eligible if they were enrolled in primary care through the VAHCS and met the following inclusion criteria: (1) diagnosis of HTN captured by the International Classification of Diseases, Ninth Revision, Clinical Modification codes from prior outpatient visits; (2) elevated BP (≥140/90 mm Hg among nondiabetics or ≥140/80 mm Hg among diabetics based on VA guidelines)5, 15 during the most recent VA clinic visit or based on the average from the last 3 visits; and (3) elevated BP measured by a member of the research team at the baseline visit. Patients were excluded if they had: (1) a prior history or current signs of hypertensive emergency including symptoms of angina, stroke, or acute renal failure; (2) severe HTN (systolic BP >200 mm Hg or diastolic BP >114 mm Hg); (3) a history of acute MI, stroke, or unstable angina in the prior 6 months; (4) congestive heart failure (CHF) caused by systolic dysfunction with a left ventricular ejection fraction <35% documented by echocardiography, nuclear medicine study, or ventriculography; (5) renal insufficiency, defined by a glomerular filtration rate <30 mL/min or previously documented proteinuria >1 g/d; (6) significant hepatic disease, including prior diagnoses of cirrhosis, hepatitis B or C infection, or laboratory abnormalities (serum alanine transaminase or aspartate transaminase >2 times control or total bilirubin > 1.5 mg/dL) in the prior 6 months; (7) pregnancy; (8) prior diagnoses of pulmonary hypertension or sleep apnea (unless treated by continuous positive pressure ventilation); (9) poor prognosis, with a life expectancy estimated to be <2 years; (10) residence in a nursing home or diagnosis of dementia; (11) inability to give informed consent or impaired cognitive function (defined as ≥3 errors on the 10‐item Pfeiffer Short Portable Mental Status Questionnaire; and (12) no telephone for follow‐up calls.
At the baseline visit, the study coordinator or RA reviewed the study with the patient and obtained informed consent. If the patient's BP did not meet the inclusion criteria or if their BP was systolic BP >200 mm Hg or <100 mm Hg; diastolic BP >114 mm Hg or <50 mm Hg, research staff informed the PCP, and the patient was not enrolled. If the BP still met the inclusion criteria, the following information was obtained: (1) the duration of HTN; (2) other cardiac risk factors (eg, smoking status and alcohol use); (3) diet, nutrition, activity, and exercise; (4) symptoms and adverse drug reactions; (5) medication17 and dietary adherence (eg, low sodium or reduced calorie); (6) sociodemographics; (7) comorbidities; and (8) current medications. The patient's height, weight, and pulse were also collected. We utilized two tools to measure adherence, the Morisky and Hill‐Bone questionnaires, in order to reduce bias from self‐report. Following the interview, patients were referred to the intervention pharmacist.
Intervention
All enrolled patients received the study intervention for a period of 6 months. This included structured visits with the pharmacist at baseline and 1, 2, 4, and 6 months and telephone calls at 2 weeks and between the in‐person visits as needed. The pharmacist reviewed the CPRS electronic medical record and performed a structured interview, including: (1) a detailed medication history of all prescription, nonprescription, and herbal therapies; (2) an assessment of patient knowledge of all medications (eg, the purpose of each, dosages and timing, and potential side effects (eg, orthostatic lightheadedness); (3) potential contraindications to specific medications (eg, renal insufficiency for thiazide diuretics and severe obstructive lung disease for β‐blockers); (4) expectations for future dosage changes and of the need for future monitoring; and (5) potential barriers to BP control (eg, side effects,16 nonadherence,17, 18 low self‐efficacy) (Table 1).19, 20, 21, 22 Three different pharmacists provided the intervention over the course of the study.
Table 1.
Typical Pharmacist Interventions to Improve Blood Pressure Control
| Suboptimal regimens |
| Thiazide diuretics added to the regimen if not contraindicated. |
| Medication at low dosages increased to at least moderate dosages. |
| Medications known to be important for coexisting conditions added if not already in the regimen (eg, angiotensin‐converting enzyme inhibitors for patients with diabetes). |
| Since most patients require at least 2 antihypertensive agents, combinations known to be additive or synergistic were employed when necessary. |
| Patient factors |
| Provide the patient with the goal blood pressure. |
| Patients with poor adherence assessed to determine whether it is intentional or unintentional. |
| If unintentional (eg, patient forgets doses), the pharmacist provided adherence aids (eg, pill boxes), simplified the regimen, and solicited the assistance of family members when possible. |
| If intentional, the pharmacist determined whether this was caused by adverse reactions, misconceptions about hypertension, cost, or other factors and worked to change the regimen if there were adverse reactions or cost issues. Patients were educated about the risks of hypertension and the basic mechanisms of medications. |
| Educate the patient about diet, exercise, and other lifestyle modifications. |
Summative Evaluation
A summative evaluation based on semistructured patient interviews was used to assess the impact of the intervention during the initial 6‐month and subsequent 18‐ to 24‐month period. The evaluation examined: (1) patient recall of pharmacist recommendations (quitting smoking, Dietary Approaches to Stop Hypertension (DASH) eating plan, exercise, reduced sodium intake, and reduced caffeine intake); (2) calibration and proper use of BP machine; (3) education about BP medications; (4) patient perception of pharmacist‐managed chronic disease; (5) preferences for physician‐pharmacist collaborative management vs usual VA care; and (6) patients' overall study perceptions.
The coordinator conducted the interviews 18 to 24 months after the initial 6‐month intervention period. Detailed notes were taken and later entered into the study database. Data collection ended once the interviews had reached saturation (ie, until no new information was generated), which occurred after interviewing 37 patients.
Data Analysis
Quantitative Data
This study used an intent‐to‐treat analysis in which all 127 enrolled and eligible patients were included regardless of treatment engagement, adherence, and completion of follow‐up visits. Nineteen patients had missing BP data at 6 months. We examined changes in BP from baseline to 6 months using the last‐observation‐carried‐forward (LOCF) method. As sensitivity analysis, we imputed values for all variables with missing data and created 5 data sets using multiple imputation.23 The 5 imputed data sets were analyzed simultaneously using the SAS MIANALYZE procedure (SAS Institute, Cary, NC).23 We also performed a complete case analysis in which those with missing 6‐month data were excluded from the analysis. The direction and magnitude of the changes in BP were similar for the 3 methods. Therefore, we chose to utilize the LOCF for all analyses reported in the text since it was considered to be the most conservative approach in that it assumes no improvement in BP among patients with missing data. (Results based on all 3 approaches to analysis are, however, presented in Table 2) We compared baseline and 6‐month BP data (Table 2) using paired t tests. These comparisons were broken down into subgroups based on diabetes status. BP data, dichotomized as controlled vs uncontrolled were compared (Table 3) for patient medication changes and adherence using chi‐square statistics for categorical variables and independent sample t tests for continuously measured variables.
Table 2.
Sensitivity Analysis of Baseline vs 6‐Month Systolic and Diastolic Blood Pressures
| Blood Pressure | Baseline (Mean/SD) | 6‐Month | Change | SD of Change | P Value |
|---|---|---|---|---|---|
| Systolic: Diabetic | |||||
| n=54 | 140 (10.2) | 132 (13.3) a | −8.0 a | 14.4 a | <.001 a |
| 132 (13.8) b | −8.0 b | 16.1 b | .001 b | ||
| n=45 c | 130 (14.1) c | −10.0 c | 16.0 c | <.001 c | |
| Systolic: nondiabetic | |||||
| n=73 | 153 (11.6) | 139 (15.8) a | −14.0 a | 16.4 a | <.001 a |
| 137 (15.0) b | −16.0 b | 16.7 b | <.001 b | ||
| n=63 c | 135 (15.1) c | −17.0 c | 16.8 c | <.001 c | |
| Diastolic: diabetic | |||||
| n=54 | 76 (9.8) | 72 (11.6) a | −4.0 a | 9.1 a | .001 a |
| 72 (11.4) b | −4.0 b | 9.8 b | .001 b | ||
| n=45 c | 70 (11.9) c | −6.0 c | 10.0 c | <.001 c | |
| Diastolic: nondiabetic | |||||
| n=73 | 82 (11.4) | 77 (11.2) a | −5.0 a | 10.0 a | <.001 a |
| 76 (10.7) b | −6.0 b | 10.4 b | <.001 b | ||
| n=63 c | 75 (10.9) c | −7.0 c | 10.8 c | <.001 c | |
Abbreviation: SD, standard deviation. Three different methods to evaluate the 6‐month data considering missing data. aLast‐observation‐carried‐forward method. bUsing imputed data. cComplete case analyses.
Table 3.
Comparing Patients Characteristics for Those With Controlled vs Uncontrolled BP at 6 Months a
| Variable Name | Controlled BP (n=69) | Uncontrolled BP (n=58) | P Value |
|---|---|---|---|
| Medication change | |||
| Increased | 60.9 (43) | 41.1 (23) | .04 |
| Decreased | 8.6 (6) | 14.0 (8) | .37 |
| Other change | 10.0 (7) | 21.1 (12) | .10 |
| No change | 20.5 (14) | 23.8 (14) | .71 |
| Baseline thiazides | 42.9 (30) | 42.1 (24) | .93 |
| 6‐Month thiazides | 64.9 (45) | 46.7 (27) | .04 |
| Baseline morisky score | |||
| Mean (SD) | 1.0 (1.1) | 1.0 (1.1) | .87 |
| IQR | 0–5 | 0–4 | |
| 6‐Month Morisky score | |||
| Mean (SD) | 0.8 (1.1) | 0.6 (0.9) | .51 |
| IQR | 0–4 | 0–3 | |
| Baseline Hill‐Bone | |||
| Mean (SD) | 9.3 (2.0) | 9.3 (2.3) | .87 |
| IQR | 8–19 | 8–19 | |
| 6‐Month Hill‐Bone | |||
| Mean (SD) | 9.0 (1.5) | 8.6 (1.6) | .18 |
| IQR | 8–13 | 1–13 | |
Abbreviations: BP, blood pressure; IQR, interquartile range; SD, standard deviation. aThese numbers are generated using imputed data.
In order to measure medication changes made by the intervention pharmacist, we used medication information at both baseline and 6 months to create a medication change variable classifying patients as: (1) increased medication between baseline and 6 months, (2) decreased medication, (3) some other medication change, or (4) no change. A patient was identified as having increased their medications if they had an increase in the dose of at least 1 medication or had an increase in the total number of medications they were taking. A patient decreased medications if they had a decrease in the dosage of any medication or a reduction in the total number of medications taken. Other medication changes included replacing one medication for another (with dosages and the total number of medications staying the same). There was no medication change if the patient was taking the exact same medications (medication name, number, and dosage for all medications) at baseline and 6 months. If a patient had a mix of increases and decreases of medication, the final determination was based on difference in the total number of medications taken. In other words, if the pharmacist added some medications and stopped some medications or increased the dose of some medications and decreased the dose for some medications but the total number of medications increased from baseline to 6 months, the patient was coded as having an increase in medication. The reverse is true for patients who had a mix of increases and decreases in dosages and number of medications but had a decrease in the total number of medications between baseline and 6 months. A patient is only coded as “other change” if the total number of medications did not change from baseline to 6 months but some other change did occur.
Qualitative Data
A codebook for the summative evaluation was created by the primary interviewer (CC), intervention pharmacist (CP), and a senior member of the research team (MVW). Once the codebook was established, a subset of transcripts was randomly chosen (n=5) and independently coded by 3 members of the research team (CP, CC, and MVW) and compared for reliability. Discrepancies were resolved through discussion and consensus, and the codebook was modified to facilitate subsequent coding. All transcripts were then coded independently by two members of the research team (CC and CP) and compared for reliability. Discrepancies were resolved by MVW. Patterns in patient responses were identified and summarized using thematic analysis and frequency calculations.
Results
A total of 249 patients with uncontrolled BP as documented in CPRS were initially consented for the study. However, when the BP was properly measured by the research staff, 122 (49%) had controlled BP and were therefore excluded. The remaining 127 patients entered the study and completed the baseline data collection visit. The study sample had a mean age of 64.5±10.9 years and was predominantly male (n=124; 98%) and white (n=121; 95%). Over half (54%) had more than a high school education, were married (61%), and had an annual income ≥$25,000 (58%). Private insurance or “other” was used by 42% of patients, with fewer receiving Medicare (32%), free care or self‐pay (21%), or Medicaid (4%). Fifty‐four patients (42.5%) had diabetes (Table 4).
Table 4.
Demographic Variables and Blood Pressure for Entire Sample (N=127) at Baseline and 6 Months a
| Variable | Percentage (No.) b |
|---|---|
| Sex | |
| Male | 97.6 (124) |
| Female | 2.4 (3) |
| Race/ethnicity | |
| Non‐Hispanic Caucasian | 92.9 (118) |
| Minority | 4.7 (6) |
| Declined to answer | 2.4 (3) |
| Education, y | |
| ≤12 | 43.3 (55) |
| >12 | 54.3 (69) |
| Missing | 2.4 (3) |
| Marital status | |
| Married | 61.4 (78) |
| Not married | 35.4 (45) |
| Missing | 3.2 (4) |
| Insurance coverage | |
| Medicare | 31.5 (40) |
| Private and other | 41.7 (53) |
| Medicaid | 3.9 (5) |
| Free and none/self‐pay | 21.3 (27) |
| Missing | 1.6 (2) |
| Annual income, $ | |
| <25,000 | 34.6 (44) |
| ≥25,000 | 58.3 (74) |
| Missing | 7.1 (9) |
| Smoking status | |
| Current | 15.0 (19) |
| Former smoker | 42.5 (54) |
| Never smoker | 40.9 (52) |
| Missing | 1.6 (2) |
| Alcohol intake | |
| No alcohol intake | 44.9 (57) |
| Any alcohol intake | 53.5 (68) |
| Missing | 1.6 (2) |
| Age, y | |
| Mean (standard deviation) | 64.6 (10.8) |
| Min–max | 27–94 |
| No. of antihypertensive medications (baseline n=112)[Link] | |
| Mean (standard deviation) | 2.3 (1.1) |
| Min–max | 1–6 |
| No. of antihypertensive medications (6 months n=122)[Link] | |
| Mean (standard deviation) | 2.5 (1.1) |
| Min–max | 1–6 |
| Medication change (n=123) | |
| Increase | 52.0 (66) |
| Decrease | 11.0 (14) |
| Other change | 15.0 (19) |
| No change | 22.1 (28) |
| Diabetic | 42.5 (54) |
| Quit smoking (n=22) | |
| Yes | 9.1 (2) |
| No | 63.6 (14) |
| Missing | 27.3 (6) |
aThere were no missing data unless otherwise listed. bUnless otherwise indicated. cNumber of patients taking antihypertensive agents at baseline and 6 months.
BP was significantly reduced in diabetic patients. Mean changes were −8.0±14.4 mm Hg for systolic BP (P<.001) and −4.0±9.1 mm Hg for diastolic BP (P=.001). BP was reduced even more in nondiabetic patients (−14.0±1.9 mm Hg and −5.0±10.0 mm Hg for systolic and diastolic BP, respectively, P<.001 for each) (Table 2). There were significant differences in medication changes between the controlled and uncontrolled groups such that the number of medications was increased more frequently in the controlled group (P=.04) (Table 2).
Thiazide diuretics, including chlorthalidone and hydrochlorothiazide (HCTZ), were used more frequently at 6 months by patients with controlled BP (64.9%; n=45) vs those with uncontrolled BP (46.7%; n=27). This difference was found to be significantly significant (P=.04).
Adherence significantly improved from baseline to 6 months with a decrease in the Morisky score but not the Hill‐Bone score (P=.002 vs P=.12). The improvement in adherence measured by the Hill‐Bone questionnaire trended in the same direction as Morisky, but patients' initial Hill‐Bone adherence scores left little room for improvement (ceiling effect). The correlation coefficient for the Morisky and Hill‐Bone scores at baseline and at 6 months were 0.67 and 0.447 (P<.0001), respectively. This trend suggests that both adherence tools support each other.
Qualitative Results
Five primary themes emerged from the in‐depth patient interviews: (1) A desire for physician‐pharmacist co‐managed care for chronic disease states in addition to HTN; (2) Greater perceived access to the pharmacist for education and questions about their chronic disease as a result of the intervention; (3) Patients preferred the more aggressive approach provided by the pharmacist vs usual care, (4) Patients reported the pharmacist helped lower their BP better than usual care; (5) Patients were able to recall lifestyle changes suggested by the pharmacist. Each of these themes are discussed in more detail below using representative quotes provided by patients.
Theme 1: A Desire for Physician‐Pharmacist Co‐Managed Care of Chronic Disease States
When asked whether they were to have a choice about who managed their BP and whether they would stay with their usual VA care or meet with a pharmacist on a regular basis, patients' responses suggested they preferred a co‐managed, team‐based approach. Representative comments included: “I never thought about having a pharmacist to meet with. Generally I just go and get meds, never sat down with one (pharmacist). It was nice. Both (physician and pharmacist) are the best of both worlds.”
Another patient responded: “They (physician and pharmacist) both need to work together; they need to work in conjunction. One without the other wouldn't work as well. They are a good team.”
A third patient responded: “… two heads are better than one.”
Theme 2: Increased Access to the Pharmacist for Education and Questions
When inquiring about what patients liked most about having the pharmacist manage their BP, patients reported feeling that they had more access to the pharmacist than their PCP. “… he (pharmacist) was very flexible and said if I couldn't make an appointment, I could call and reschedule. It usually worked out pretty good.”
Another patient responded: “Wish I could see the pharmacist because doctors are so busy.”
A third patient responded: “He (pharmacist) gave me suggestions all the time that I wouldn't have thought of alone with my physician….”
Theme 3: Patients Preferred the More Aggressive Approach Provided by the Pharmacist vs Usual Care
When asked whether they thought the pharmacist helped lower their BP better than their usual care prior to the study, patients seemed to favor a more aggressive approach that obtained quicker results. Many patients who were assigned to the control group for the second phase of the trial indicated that they would have preferred having the pharmacist continue to manage their BP. One patient responded: “Got me back on track. I didn't realize I was that far off track; that my BPs were that high.”
Another patient responded: “Quick results. Kinda went with some drastic stuff right away and it worked.”
A third patient responded: “… More personalized and got to solving the problem quicker.”
Theme 4: Patients Reported the Pharmacist Helped Lower Their BP Better Than Usual Care
When asked how they liked having the pharmacist manage their BP, several patients commented on how the pharmacist lowered their BP more effectively than usual VA care prior to enrolling in the study. One patient responded: “Tried for a couple of years before that and got nowhere.”
Another patient responded: “He must have because they were running the same for a while. When I started the study they (pharmacist) increased my medication, then changed to a new medication. Once I got that I was doing better.”
A third patient responded: “I think so; the things I learned helped because I never really talked it over with a doctor before.”
Theme 5: Patients Were Able to Recall Lifestyle Changes Suggested by the Pharmacist
Many patients were able to recall, without prompting, lifestyle changes that the pharmacist discussed with them, including suggesting smoking cessation, implementing the DASH eating plan, increasing their exercise, and reducing their salt and caffeine intake. One patient said, “Made me aware of it. When I reach for the salt shaker I think about it; what he (pharmacist) said.” Another patient responded: “It (the intervention) changed the way I eat and thought about salt, for one thing.”
When examining the associations between the responses to the summative evaluation and BP control rates at 6 months, more patients with controlled BP were able to recall lifestyle changes suggested by the pharmacist to help lower their BP. This included the DASH eating plan (51.7% vs 37.5%), reducing salt intake (34.5% vs 25.0%), and reducing caffeine intake (13.8% vs 0%). More patients with controlled BP preferred co‐management of their hypertension (41.4% vs 12.5%).
Because we were concerned that one of the unintended consequences of co‐managed care is that patients could be uncertain as to who is in charge of their treatment, we also asked whether they were ever confused about who was managing their BP. The majority of patients with both controlled and uncontrolled hypertension at 6 months did not report confusion about who was managing their hypertension (82.8% and 87.5%, respectively).
Discussion
This study found that both systolic and diastolic BP decreased when patients were managed using a proven physician‐pharmacist collaborative model.24, 25 In addition to experiencing a significant mean reduction in BP, the summative evaluation found that patients liked having a pharmacist manage their chronic disease and in many cases preferred a physician‐pharmacist collaborative model to physician‐only care. Patients expressed a desire to have pharmacists co‐manage more of their chronic diseases, such as hyperlipidemia and diabetes mellitus.
The findings from this study are similar to those of previous studies in which pharmacists independently managed patients with hypertension. One study conducted at a tertiary care VA medical center and affiliated primary care clinics involving 573 veterans evaluated BP control at the end of a 6‐month intervention.26 Systolic and diastolic BP decreased significantly (11.2 mm Hg and 4.6 mm Hg, respectively; P<.001) for both comparisons.
Unlike the previously mentioned study, the present study also evaluated patients' perceptions of pharmacist‐managed and team‐based care of chronic disease utilizing a summative evaluation. A number of themes were identified, including many patients' desire for co‐managed care and preference for a more aggressive approach to the management of their hypertension. Patients also expressed the perception that they had increased access to the pharmacist for education and questions relating to their chronic disease, and that they believed their BP was under better control when the pharmacist managed their hypertension compared with usual care.
Patients who do not take their medication as prescribed are more likely to experience a major cardiovascular event or even death, magnifying the importance of adhering to their medication regimen.27 Medication adherence can be difficult to assess in patients. No single method has been shown to be reliable and accurate.28 The use of multiple tools may be preferred for assessment. We utilized two tools to measure adherence, the Morisky and Hill‐Bone questionnaires, in order to reduce bias from self‐report.
Study Limitations
There are several limitations to the present study. First is the unknown generalizability of our results. The patient population examined was largely male, Midwestern, and Caucasian. Second, 19 patients had missing BP data at 6 months. To address this issue and any potential bias introduced by differences between study completers and noncompleters, we performed a sensitivity analysis using a multiple imputation procedure to replace missing values and compared those results to the LOCF method. Another limitation is a relatively small sample size. Many patients who were originally identified as having high BP from the medical record had controlled BP at their enrollment visit when BP was measured using the proper technique. Therefore, these patients were excluded from further participation. Finally, because all patients received the intensive BP intervention for the first phase of the trial reported here, firm conclusions cannot be made regarding the effectiveness of the approach based on these initial 6‐month findings because of the lack of a control group. Long‐term outcomes comparing those assigned to continue vs not continue the intervention during the second phase of the trial will allow more definitive conclusions regarding the impact of the intervention on BP. Those results will be available in late 2014.
Conclusions
This study found significantly lower mean BP levels after a 6‐month intensive physician‐pharmacist collaborative intervention. Our findings suggest that pharmacist‐managed hypertension reduces BP, therefore decreasing the risk of cardiovascular disease. The summative evaluation suggests the majority of patients responded favorably to the intervention. Patients expressed a desire to have a pharmacist and their primary care provider co‐manage their hypertension, as well as other chronic diseases. The physician‐pharmacist collaborative model could therefore be used as a long‐term strategy for controlling hypertension, as well as other chronic diseases.
Acknowledgments and disclosures
This project was funded by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development Service (grant IIR 07‐145), and Comprehensive Access and Delivery Research and Development (CADRE), Iowa City VA Health Care System, Iowa City, IA. None of these sponsors had a role in the study design, methods, analyses, and interpretation or in the preparation of the manuscript or the decision to submit it for publication. The views expressed are those of the authors and do not necessarily reflect the position or policy of the VA. The authors have no conflicts of interest with the research presented in this paper.
J Clin Hypertens (Greenwich). 2014;16:133–140. DOI: 10.1111/jch.12250. ©2014 Wiley Periodicals, Inc.
References
- 1. Stamler J, Rose G, Stamler R, et al. INTERSALT study findings. Public health and medical care implications. Hypertension. 1989;14:570–577. [DOI] [PubMed] [Google Scholar]
- 2. Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339:1957–1963. [DOI] [PubMed] [Google Scholar]
- 3. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988‐2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 4. Fletcher RD, Amdur RL, Kolodner R, et al. Blood pressure control among US veterans: a large multiyear analysis of blood pressure data from the Veterans Administration health data repository. Circulation. 2012;125:2462–2468. [DOI] [PubMed] [Google Scholar]
- 5. VA/DoD Clinical practice guideline for diagnosis and management of hypertension in the primary care setting. Department of Veterans Administration, Department of Defense. 2004, Revision 2005. [Google Scholar]
- 6. Bond CA, Monson R. Sustained improvement in drug documentation, compliance, and disease control. A four‐year analysis of an ambulatory care model. Arch Intern Med. 1984;144:1159–1162. [PubMed] [Google Scholar]
- 7. Carter BL, Zillich AJ, Elliott WJ. How pharmacists can assist physicians with controlling blood pressure. J Clin Hypertens (Greenwich). 2003;5:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Erickson SR, Slaughter R, Halapy H. Pharmacists' ability to influence outcomes of hypertension therapy. Pharmacotherapy. 1997;17:140–147. [PubMed] [Google Scholar]
- 9. Monson R, Bond CA, Schuna A. Role of the clinical pharmacist in improving drug therapy. Clinical pharmacists in outpatient therapy. Arch Intern Med. 1981;141:1441–1444. [PubMed] [Google Scholar]
- 10. Carter BL, Barnette DJ, Chrischilles E, et al. Evaluation of hypertensive patients after care provided by community pharmacists in a rural setting. Pharmacotherapy. 1997;17:1274–1285. [PubMed] [Google Scholar]
- 11. Park JJ, Kelly P, Carter BL, Burgess PP. Comprehensive pharmaceutical care in the chain setting. J Am Pharm Assoc (Wash). 1996;36:443–451. [DOI] [PubMed] [Google Scholar]
- 12. Mehos BM, Saseen JJ, MacLaughlin EJ. Effect of pharmacist intervention and initiation of home blood pressure monitoring in patients with uncontrolled hypertension. Pharmacotherapy. 2000;20:1384–1389. [DOI] [PubMed] [Google Scholar]
- 13. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. [DOI] [PubMed] [Google Scholar]
- 14. Wright JT Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 15. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 16. Kaboli P, Hoth A, Carter BL, et al. The VA Enhanced Pharmacy Outpatient Clinic (EPOC) Study: a randomized‐controlled pharmacist‐physician intervention trial. J Gen Intern Med. 2004;1:227. [Google Scholar]
- 17. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 18. Lowry KP, Dudley TK, Oddone EZ, Bosworth HB. Intentional and unintentional nonadherence to antihypertensive medication. Ann Pharmacother. 2005;8:1198–1203. [DOI] [PubMed] [Google Scholar]
- 19. Bandura A, Jeffery RW, Wright CL. Efficacy of participant modeling as a function of response induction aids. J Abnorm Psychol. 1974;83:56–64. [DOI] [PubMed] [Google Scholar]
- 20. Bandura A, Reese L, Adams NE. Microanalysis of action and fear arousal as a function of differential levels of perceived self‐efficacy. J Pers Soc Psychol. 1982;43:5–21. [DOI] [PubMed] [Google Scholar]
- 21. Bandura A. Self‐regulatory mechanisms governing the impact of social comparison on complex decision making. J Person Social Pyschol. 1991;60:941–951. [Google Scholar]
- 22. Bandura A. Self Efficacy in Changing Societies. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- 23. Cary NC. What's New in SAS® 9.3. USA: SAS Institute Inc; 2012. [Google Scholar]
- 24. Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich). 2008;10:260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bex SD, Boldt AS, Needham SB, et al. Effectiveness of a hypertension care management program provided by clinical pharmacists for veterans. Pharmacotherapy. 2011;31:31–38. [DOI] [PubMed] [Google Scholar]
- 27. Nelson MR, Ryan P, Willson K, Yelland L. Self‐reported adherence with medication and cardiovascular disease outcomes in the Second Australian National Blood Pressure Study (ANBP2). Med J Aust. 2006;185:487–489. [DOI] [PubMed] [Google Scholar]
- 28. MacLaughlin EJ, Raehl CL, Treadway AK, et al. Assessing medication adherence in the elderly: which tools to use in clinical practice? Drugs Aging. 2005;22:231–255. [DOI] [PubMed] [Google Scholar]


