Abstract
The accuracy of the spot urine analyte/creatinine ratio in estimating 24‐hour excretion of the analyte is compromised because it is not adjusted for 24‐hour creatinine excretion. The authors developed a model for conveniently estimating 24‐hour creatinine excretion. The model was derived from 24‐hour urine collections using multiple linear regression, including sex, weight, race, and age. The model was then evaluated in a validation cohort, assessing the correlation between estimated and measured 24‐hour creatinine excretion and by comparing their correlation with muscle mass. Estimated creatinine excretion correlated strongly with measured creatinine excretion (r=0.80 in the entire cohort and 0.93 after eliminating patients with incomplete collections), and correlated at least as strongly as measured creatinine excretion with lean muscle mass (r=0.94 vs r=0.82, respectively). Adjusting spot urine analyte/creatinine ratios using the estimated 24‐hour creatinine excretion by this convenient method can improve the accuracy of estimating 24‐hour excretion of albumin, sodium, and other analytes.
The traditional method for assessing the level of excretion of an analyte of clinical interest is the time‐honored 24‐hour urine collection. However, this method is fraught with problems, such as inconvenience and incomplete collections. In addition, particularly in assessing an analyte whose excretion varies considerably from day to day, the result of a single 24‐hour collection might not be representative of usual excretion on other days, and multiple 24‐hour collections are usually not feasible.
To circumvent the problems of inconvenience and incomplete collection, analysis of a spot urine analyte/creatinine ratio has been adopted to provide an estimate of 24‐hour excretion of analytes such as albumin, catecholamines, and others. Creatinine concentration serves as a surrogate for the state of concentration or dilution of the urine, varying inversely with urine volume. It is widely used in estimating albumin excretion, and, as recently reported, could potentially be very useful in more conveniently estimating sodium excretion.1, 2
A widely overlooked but major problem with the analyte/creatinine ratio, however, is that it ignores the considerable interindividual differences in daily creatinine excretion, which can range from <600 mg/d to >2800 mg/d. These interindividual differences are mostly related to differences in muscle mass, as creatinine is derived from muscle catabolism. This can result in as much as a 4‐fold variance in estimating 24‐hour excretion. Thus, failure to adjust for an individual's 24‐hour creatinine excretion unacceptably compromises the accuracy of the analyte/creatinine ratio.
A convenient and reasonably accurate method to estimate 24‐hour creatinine excretion is an overlooked necessity in improving estimation of 24‐hour excretion of any analyte from the spot urine analyte/creatinine ratio. The Cockcroft‐Gault formula3 and a more recent equation reported by Ix and colleagues4 have been put forward to do so, but the Cockcroft‐Gault formula considerably underestimates 24‐hour creatinine excretion and the Ix equations were limited to patients with chronic kidney disease.4 Neither of the two takes into account the substantial problem of incomplete collections in developing the equations.
In developing a method for estimating 24‐hour creatinine excretion, another challenge lies in how to assess its accuracy. The usual gold standard to which it is compared, 24‐hour urine creatinine excretion, is problematic because it is often inaccurate. Incomplete collection may affect 30% of collections.5 Overcollection can also occur. Further, day‐to‐day variation in protein intake and exercise cause significant variation in 24‐hour creatinine excretion.6, 7 The inaccuracy of the gold standard 24‐hour urine collection will thus result in an understatement of the accuracy of any method of estimation being tested if differences between estimate and gold standard are attributed solely to inaccuracy of the method of estimation being tested.
An alternative method of evaluating the accuracy of a method for estimation of 24‐hour creatinine excretion would be to determine its correlation with muscle mass. Since excreted creatinine is derived from catabolism of muscle, the amount of creatinine excreted in 24 hours should, and does, correlate strongly with muscle mass. Thus, the accuracy of a method of estimating 24‐hour creatinine excretion could be assessed by comparing the strength of its correlation with muscle mass in comparison to that of the actual measurement of 24‐hour creatinine excretion.
The purpose of this study was to construct an equation for estimating 24‐hour creatinine excretion based on easily obtained demographic variables and to assess its accuracy both by determining its correlation with measured 24‐hour creatinine excretion, and by comparing the correlation of both estimated and measured 24‐hour creatinine excretion with muscle mass. The latter analysis solves the problem of the frequent inaccuracy of the 24‐hour collection, as discussed above.
Methods
Study participants were recruited between October 2006 and December 2008. Informed consent was obtained as approved by the institutional review board of Weill Cornell Medical College. Participants older than 21 years were recruited irrespective of hypertensive status and renal function, as long as renal function and appetite were stable. Participants were considered to be hypertensive if their average clinic blood pressure was ≥140/90 mm Hg or if they were taking antihypertensive medication.
Height was measured by a technician. Weight and impedance were simultaneously measured using a bioelectric impedance instrument, and muscle mass was derived by the instrument's microprocessor based on equation formulas using dual‐energy x‐ray absorptiometry (DEXA) as a reference (Tanita, Model BC‐554 Ironman Body Composition Monitor; Tanita, Tokyo, Japan). This system uses 2 foot‐pad electrodes, with the participants' shoes and socks removed. Using a similar 2‐limb foot‐to‐foot device, previous researchers have found that this method provides accurate estimation of muscle mass with reported differences between DEXA of −1.4% to −2.5%8 and with 4 compartment bioelectrical impedance analysis of 2 to 3 kg.9
All study participants were given a 3‐L jug and instructed to collect a 24‐hour urine sample by discarding the first voided urine upon rising in the morning and then collecting all voided urine up to and including the first void the following morning. The volume of the 24‐hour collection was recorded and an aliquot was sent to The New York Presbyterian Hospital Laboratories for measurement of creatinine concentration.10 Collections were considered incomplete if 24‐hour creatinine excretion was <20 mg/kg in men and <15 mg/kg in women.11
In the development cohort, after exclusion of incomplete collectors, leaving n=50, multiple linear regression was used to develop an equation for estimation of 24‐hour creatinine excretion. The equation, which evaluated the influence of age, sex, race, and weight, was then evaluated in the second, independent cohort (n=83) in 3 ways: (1) repeating the regression analysis in complete collectors (n=41), (2) assessing the correlation between estimated and measured creatinine excretion in the entire cohort and in complete collectors, and (3) computing the correlation with muscle mass of estimated and of measured 24‐hour creatinine excretion in both the development and validation cohorts, as well as in subgroups characterized by complete or incomplete 24‐hour urine collections.
Subsequently, we derived a regression model that combined data from both the development and validation cohorts. This combined cohort is referred to as the merged dataset.
Statistical Analysis
Correlations between estimated 24‐hour creatinine excretion and measured 24‐hour excretion were assessed by Pearson's correlation coefficient, as was the correlation with muscle mass of both estimated and measured 24‐hour creatinine excretion. Two‐tailed probability levels for statistical significance tests are reported, with P<.05 considered statistically significant.
Multiple linear regression was used to predict 24‐hour creatinine excretion in both the development dataset and the merged dataset. We forced the inclusion of age, sex, weight, and race in the multivariable model, regardless of statistical significance, due to their known relationship with creatinine excretion. Adjusted beta (slope) estimates (with associated 95% confidence intervals) are reported from the multivariable model.
Results
Select characteristics of the development and validation cohorts are presented in Table 1.
Table 1.
Participant Characteristics in the Development and Validation Cohortsa
| Characteristic | Development | Validation | ||
|---|---|---|---|---|
| No. | Mean±SD | No. | Mean±SD | |
| Age, y | 50 | 54.2±15.2 | 83 | 59.1±12.1 |
| Body mass index, kg/m2 | 49 | 28.1±5.4 | 78 | 27.2±5.2 |
| Muscle mass | 26 | 116.1±20.8 | 79 | 116.4±25.7 |
| Serum creatinine, mg/dL | 41 | 1.10±0.37 | 78 | 1.22±0.63 |
| Male, % | 50.0 | 59.0 | ||
| Race/ethnicity, % | ||||
| White | 60.0 | 89.2 | ||
| Black | 40.0 | 10.8 | ||
| Hypertensive, % | 74.0 | 86.7 | ||
Abbreviation: SD, standard deviation. aThe development cohort included complete collectors only; the validation cohort included both complete and incomplete collectors.
Equation Development
An equation to estimate 24‐hour creatinine excretion was derived from the development dataset using linear regression, and included age, race/ethnicity, sex, and weight as predictors. Sex and weight were significant independent predictors (R 2=0.83). Applying that model (Table 2) to the validation dataset yielded an R 2 of 0.89.
Table 2.
Regression Analysis Estimating 24‐Hour Creatinine Excretion
| Predictor | Estimation Equation | ||
|---|---|---|---|
| Development Dataseta | |||
| B | SE | P Value | |
| Sex | 513.97 | 60.399 | <.001 |
| Weight | 7.43 | 0.860 | <.001 |
| Race | 71.56 | 60.621 | .24 |
| Age | 2.20 | 1.873 | .25 |
Abbreviations: B, unstandardized regression coefficient; SE, standard error.
Equation Evaluation
Using the equation derived from the development cohort, the estimated 24‐hour creatinine excretion in the validation cohort correlated strongly (P<.0001) with the measured creatinine excretion, with r=0.80 (Figure a) in the entire cohort (N=83). In complete collectors (n=41), the correlation was particularly strong (r=0.93; P<.0001) (Figure b).
Figure 1.
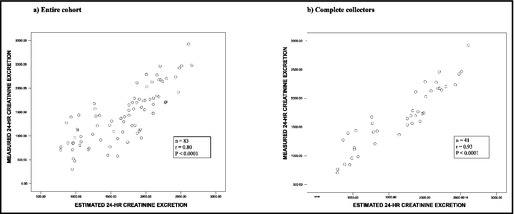
Relationship between estimated 24‐hour creatinine excretion and measured 24‐hour creatinine excretion in (a) entire cohort and (b) complete collectors.
Table 3 shows correlations with muscle mass of both estimated and measured 24‐hour creatinine excretion. In the validation cohort, which included participants having either complete or incomplete collections, the correlation with muscle mass was r=0.95 for estimated and r=0.82 for measured 24‐hour creatinine excretion. Among complete collectors, the correlations of muscle mass with estimated and measured 24‐hour creatinine excretion were similarly strong (r=0.97 and 0.91, respectively). Among the incomplete collectors, the correlations were r=0.93 and r=0.85, respectively.
Table 3.
Correlation With Muscle Mass of Estimated and Measured 24‐Hour Creatinine Excretion in the Validation Cohort by Completeness of Collection
| No. | R | P Value | |
|---|---|---|---|
| Validation cohort | |||
| Entire cohort | |||
| Estimated 24‐h creatinine excretion | 80 | 0.95 | <.0001 |
| Measured 24‐h creatinine excretion | 79 | 0.82 | <.0001 |
| Complete collectors | |||
| Estimated 24‐h creatinine excretion | 39 | 0.97 | <.0001 |
| Measured 24‐h creatinine excretion | 39 | 0.91 | <.0001 |
| Incomplete collectors | |||
| Estimated 24‐h creatinine excretion | 40 | 0.93 | <.0001 |
| Measured 24‐h creatinine excretion | 40 | 0.85 | <.0001 |
R=correlation coefficient.
Final Equation Model
After merging participants from the development cohort with the complete collectors in the validation cohort (n=91), we determined a final regression equation to estimate 24‐hour creatinine excretion from age, race/ethnicity, sex, and weight. The following equation was derived:
Table 4 reports the mean values for weight, muscle mass, and 24‐hour creatinine excretion for participants in the merged dataset according to the completeness of their urinary collection.
Table 4.
Mean Values for Weight, Muscle Mass, and Measured and Estimated 24‐Hour Creatinine Excretion in the Merged Dataset in Complete and Incomplete Collectors
| Characteristic | Complete Collectors | Incomplete Collectors | P Valuea | ||
|---|---|---|---|---|---|
| No. | Mean±SD | No. | Mean±SD | ||
| Weight, lb | 91 | 177.17±40.12 | 42 | 180.17±38.29 | .69 |
| Muscle mass, lb | 65 | 117.94±25.55 | 40 | 113.73±22.73 | .40 |
| Measured 24‐h creatinine excretion, mg/d | 91 | 1686.56±490.01 | 42 | 1190.36±472.67 | <.0001* |
| Estimated 24‐h creatinine excretion, mg/d | 91 | 1686.61±450.74 | 42 | 1690.20±428.61 | .97 |
Abbreviations: SD, standard deviation. a P value represents difference between (a) means of complete and incomplete collectors and (b) measured and estimated 24‐hour creatinine excretion among incomplete collectors.
Among complete and incomplete collectors, despite similar means for weight and muscle mass, the mean for measured 24‐hour creatinine excretion was significantly lower (P<.0001) among incomplete collectors (1190 mg) than among complete collectors (1687 mg). In contrast, the means for estimated 24‐hour creatinine excretion were very similar (1687 mg and 1690 mg, respectively).
Discussion
The 24‐hour urine collection for creatinine excretion has always been assumed to be the gold standard for assessment of urine creatinine excretion. In the real world, however, where, in contrast to the metabolic ward, 24‐hour collections are often undercollected or performed incorrectly, and where diet and activity vary, measurement of 24‐hour creatinine excretion is prone to considerable variation and error. For that reason, an accurate method to estimate 24‐hour creatinine excretion without the need for a 24‐hour urine collection could rival, or perhaps even exceed, that of the 24‐hour collection, while also obviating the need for the impractical 24‐hour collection.
In this study, the estimated 24‐hour creatinine excretion correlated very strongly with the measured 24‐hour creatinine excretion (r=0.80). When data of likely undercollectors were excluded, the correlation between the two was even stronger (r=0.93). This strong correlation is particularly notable, given the inaccuracies and variability of the measured 24‐hour collection, as described above.
The accuracy of the estimate of 24‐hour creatinine excretion is further supported by its correlation with muscle mass, which was as strong as, or possibly stronger than, the correlation between the measured 24‐hour creatinine excretion and muscle mass. Importantly, the strong correlation of estimated creatinine excretion and muscle mass is unaffected by the completeness or incompleteness of a 24‐hour urine collection. Our finding that the estimated excretion correlated at least as strongly as the measured 24‐hour collections with the more consistent measure of muscle mass argues that the accuracy of the estimated creatinine excretion is comparable to that of the actual 24‐hour collection.
Thus, the equation‐derived estimation of 24‐hour creatinine excretion appears to stand strongly as a potential substitute for measurement of 24‐hour creatinine excretion. This is of considerable clinical importance since 24‐hour urine collections are very inconvenient, are often incorrectly performed, and often are not even requested as a result. Further, adjustment of the spot urine analyte/creatinine ratio, whether for estimation of 24‐hour excretion of creatinine, or of any clinically important analyte, such as albumin or sodium, greatly improves the accuracy of the ratio while maintaining the convenience of a spot urine sample. Finally, utilizing the estimated 24‐hour creatinine excretion, the analyte/creatinine ratio can be converted into a much more easily understood estimate of the 24‐hour excretion of the analyte being assessed by simply multiplying the: [analyte]/[creatinine] ratio by the estimated 24‐hour creatinine excretion/10.1, 2 This substantial improvement in accuracy and ease of interpretation offers considerable clinical relevance.
The inaccuracy of the analyte/creatinine ratio, if it is not adjusted for the 24‐hour creatinine excretion, is evident in studies that employ it to estimate 24‐hour albumin excretion. The ratio, on average, is higher in women than in men because of the lower 24‐hour creatinine excretion in women, a function of both weight and sex. Consistent with this, investigators found a multivariate adjusted odds ratio for microalbuminuria of 1.62 for women as compared with men, but when sex‐specific albumin/creatinine cutpoints were used, no association between sex and microalbuminuria was found.9 Although providing a normal range based on sex would be helpful on average, considerable inaccuracy would remain when looking at individual patients unless the ratio was also adjusted for weight, which is the major correlate of muscle mass and creatinine excretion. The substantial impact on the ratio of the lower creatinine excretion of a smaller/lighter individual cannot be ignored. The 24‐hour creatinine excretion accounts for the effects of both weight and sex. In the absence of a 24‐hour urine collection, this method of estimating 24‐hour creatinine excretion can greatly improve the accuracy of the ratio.
Similarly, estimation of 24‐hour sodium excretion from a spot urine sodium/creatinine ratio is subject to as much as a 4‐fold error unless adjusted for 24‐hour creatinine excretion. As we previously reported, the accuracy of the spot urine sodium/creatinine ratio in estimating 24‐hour sodium excretion is greatly improved after adjusting for creatinine excretion measured by 24‐hour urine collection.1, 2 The correlation of the unadjusted sodium/creatinine ratio with 24‐hour sodium excretion increased from 0.67 to 0.86 when the ratio was adjusted for 24‐hour creatinine excretion.1 Given the accuracy of the estimate of 24‐hour creatinine excretion observed in this study, its use appears to provide a convenient and reliable alternative to the 24‐hour urine collection. Use of this method could similarly improve the accuracy of estimation of the 24‐hour excretion of any analyte from spot urine by enabling easy‐to‐perform adjustment for interindividual differences in 24‐hour creatinine excretion.
In this study, using an equation derived from the variables of sex, age, race, and weight, the correlation of estimated 24‐hour creatinine excretion was extremely strong with both measured 24‐hour creatinine excretion and with muscle mass. Adding additional variables would add little to the strong correlation observed, while increasing complexity and impracticality. The use of 4 variables seems to be most parsimonious, providing excellent correlation with measured 24‐hour excretion and with muscle mass, yet strikingly simple and therefore clinically useful. It should be noted, in this study, that race was a binary variable, and results are limited to values of white or black.
Limitations
An obvious concern in estimating creatinine excretion is the expected difference in creatinine excretion between two individuals of the same weight, one of whom is obese and the other more muscular. Here, the estimated 24‐hour creatinine excretion would be expected to underestimate creatinine excretion in the muscular individual and overestimate it in the obese individual. This error is partly mitigated by the increase in muscle mass associated with obesity, as a 30 kg increase in fat mass is accompanied by a 12 kg increase in lean muscle mass.12 Regardless, the strong correlations observed assure a reasonable estimate, one that is certainly superior to reliance on the analyte/creatinine ratio assessed by itself, without adjustment for 24‐hour creatinine excretion.
Conclusions
The accuracy of the widely used spot urine analyte/creatinine ratio in estimating 24‐hour excretion of any analyte is compromised by failure to take into account the 24‐hour creatinine excretion of the individual being assessed. Refinement of the ratio by adjusting for estimated 24‐hour creatinine excretion, as described in this study, can greatly enhance the accuracy of spot urine estimates of 24‐hour excretion of any analyte, while avoiding the need for 24‐hour urine collections. Further, it also eliminates the widespread problem of inaccuracy due to undercollection of the 24‐hour urine. Adjusting the analyte/creatinine ratio using the estimated 24‐hour creatinine excretion could considerably enhance its accuracy in estimating 24‐hour excretion of analytes. This method merits attention for its ability to greatly improve the accuracy of the widely used albumin/creatinine ratio, and for its potential role in enabling assessment of sodium excretion without the requirement for the cumbersome, often inaccurate, and widely avoided 24‐hour urine collection. It also enables frequent assessments of albumin excretion while following patients with renal disease, as well as monitoring for albuminuria in pregnant patients.
Disclosure
Study funding was provided by the Julia and Seymour Gross Foundation and by Allied Minds, Inc. The authors have applied for intellectual property rights concerning this and other related research directed toward development of a method for estimation of sodium excretion from spot urine samples. Portions of this work were presented at the American Society of Hypertension Annual Meeting, New York, NY, May 3, 2010.
J Clin Hypertens (Greenwich). 2014;16:367–371. DOI: 10.1111/jch.12294. ©2014 Wiley Periodicals, Inc.
References
- 1. Mann SJ, Gerber LM. Estimation of 24‐hour sodium excretion from spot urine samples. J Clin Hypertens (Greenwich). 2010;12:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mann SJ, Gerber LM. Estimation of 24‐hour sodium excretion from spot urine sample using chloride and creatinine dipsticks. Am J Hypertens. 2010;23:743–748. [DOI] [PubMed] [Google Scholar]
- 3. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 4. Ix JH, Wassel CL, Stevens LA, et al. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol. 2011;6:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christopher‐Stine L, Petri M, Astor BC, et al. Urine protein‐to‐creatinine ratio is a reliable measure of proteinuria in lupus nephritis. J Rheumatol. 2004;31:1557–1559. [PubMed] [Google Scholar]
- 6. Calles‐Escandon J, Cunningham JJ, Snyder P, et al. Influence of exercise on urea, creatinine, and 3‐methylhistidine excretion in normal human subjects. Am J Physiol. 1984;246:E334–E338. [DOI] [PubMed] [Google Scholar]
- 7. Mayersohn M, Conrad KA, Achari R. The influence of a cooked meat meal on creatinine plasma concentration and creatinine clearance. Br J Clin Pharmacol. 1983;15:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang JG, Zhang Y, Chen HE, et al. Comparison of two bioelectrical impedance analysis devices with dual energy X‐ray absorptiometry and magnetic resonance imaging in the estimation of body composition. J Strength Cond Res. 2013;27:236–243. [DOI] [PubMed] [Google Scholar]
- 9. LaForgia J, Gunn S, Withers RT. Body composition: validity of segmental bioelectrical impedance analysis. Asia Pac J Clin Nutr. 2008;17:586–591. [PubMed] [Google Scholar]
- 10. Rose BD. Diagnostic approach to the patient with renal disease. In: Rose BD, ed. Pathophysiology of Renal Disease. New York: McGraw Hill;1981:35. [Google Scholar]
- 11. Mattix HJ, Hsu C‐Y, Shaykevich S, et al. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13:1034–1039. [DOI] [PubMed] [Google Scholar]
- 12. Shibao C, Gamboa A, Diedrich A, et al. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. [DOI] [PubMed] [Google Scholar]


