Abstract
The authors examined the association between estimated glomerular filtration rate (eGFR), calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine‐cystatin C equation, and hemodynamics in 556 normotensive or never‐treated hypertensive patients without kidney disease (mean age, 46 years). Hemodynamic variables were recorded using pulse wave analysis and whole‐body impedance cardiography. The mean eGFR was 98 mL/min/1.73 m2 (range, 64–145 mL/min/1.73 m2 and one third of the patients had values below 92, while none had proteinuria. In linear regression analyses adjusted for differences in age, weight:height ratio, low‐density lipoprotein cholesterol, and sex, significant associations were found between lower eGFR and higher systolic (P=.001) and diastolic blood pressure (P<.001) and higher systemic vascular resistance (P=.001). There was no association between eGFR and cardiac output or extracellular volume. In the absence of clinical kidney disease, lower eGFR was associated with higher blood pressure and systemic vascular resistance. Therefore, early impairment in kidney function may be involved in the pathogenesis of essential hypertension.
The kidney plays a central role in the regulation of blood pressure (BP) through control of extracellular volume and renal perfusion pressure. Putative renal mechanisms in the pathogenesis of hypertension include reduced nephron mass and glomerular filtration rate (GFR), impaired tubular sodium handling, activation of the renin‐angiotensin‐aldosterone system, increased sympathetic tone, and increased endothelin release.1 However, the association between mild impairment of renal function, which could precede overt clinical chronic kidney disease (CKD), and hypertension is not well established.
Early impairment in kidney function may predict incident hypertension in patients without CKD or cardiovascular disease, independent of traditional risk factors.2, 3 In participants of the US National Health and Nutrition Examination Survey (NHANES) 1999–2002, mild reduction in kidney function in the absence of clinically recognized CKD was associated with hypertension in women but not in men.4 An association between mild reduction in GFR and elevated systolic BP has also been reported in elderly people.5 In patients with coronary artery disease, early impairment in kidney function, as judged by elevated serum cystatin C levels, was associated with higher systolic BP, even when creatinine‐based measures indicated normal kidney function.6 Several studies have shown an association between decreased renal function and increased arterial stiffness, an effect partially independent of the level of BP.7, 8
Creatinine‐based measures are rather insensitive markers of mild reduction in GFR (values >60 mL/min),6 whereas cystatin C may more precisely detect early impairment of kidney function.9 In addition, increased cystatin C concentration is a predictor of cardiovascular risk.6, 10 However, an equation including both serum cystatin C and creatinine concentrations may provide the most accurate value for estimated GFR (eGFR).11, 12, 13, 14
The association of kidney function with hemodynamics has not been studied in patients without prevalent cardiovascular diseases other than hypertension and not using antihypertensive medication. We tested the hypothesis of whether a mild decrease in eGFR, evaluated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) creatinine‐cystatin C formula,11 is associated with the hemodynamic determinants of BP. Normotensive and never‐treated hypertensive patients in this cross‐sectional study were without previously diagnosed diabetes, coronary artery disease, renal insufficiency, and peripheral or cerebrovascular disease.
Patients and Methods
Study Population
This study is part of an ongoing investigation on hemodynamics (the Haemodynamics in Hypertension [DYNAMIC] study, clinical trial registration number NCT01742702). Volunteers were recruited from announcements in Tampere University Hospital, University of Tampere, Varala Sports Institute, and organizations that provide occupational healthcare in the surrounding area. The participants were screened from a group of 830 individuals. All patients underwent interview, clinical examination, and anthropometric measurements by a medical doctor. Lifestyle habits and medical history were recorded. Smoking was calculated as pack‐years. The use of alcohol was evaluated as consumption of standard drinks (~12 g of absolute alcohol) per week. Seated office BP was measured using a manual sphygmomanometer after at least 5 minutes of rest.15
Exclusion criteria were use of medications with any influence on hemodynamics (such as β2‐adrenoreceptor agonists for asthma), presence of diabetes mellitus or clinical CKD (eGFR <60 mL/min/1.73 m2 or proteinuria), history of cardiovascular disease, and laboratory findings indicating secondary hypertension. Excluding elevated BP, the majority of patients did not have chronic medical conditions or require regular medications. Seventy of 275 women used hormones (contraception or hormone replacement therapy) and 96 were postmenopausal. Only 14 patients used statins (four women), one patient used ezetimib, 15 had hypothyroidism treated to euthyroidism with stable thyroxin treatment (one man), 17 were taking inhaled corticosteroids for asthma, and 21 used antidepressants. The final study group consisted of 556 patients (aged 20–72 years, 282 men).
Laboratory Analyses
Blood samples were collected after fasting overnight. Urine protein excretion was assessed using dipstick screening and measurement of overnight urine albumin excretion (nephelometric BN Prospec System; Siemens AG, Erlangen, Germany). Blood cell count and hematocrit level were determined using ADVIA 120 or 2120 (Bayer Health Care, Tarrytown, NY) and other laboratory values using Cobas Integra 700/800 (F. Hoffmann‐LaRoche Ltd, Basel, Switzerland).
Tonometric Radial Artery Recordings
Radial BP and pulse wave form were determined by the use of an automatic tonometric sensor (Colin BP‐508T; Colin Medical Instruments Corp, San Antonio, TX) and recorded using the SphygmoCor PWMx system (AtCor Medical, West Ryde, Australia). Radial BP was calibrated by brachial measurements at the onset of each recording.
Whole‐Body Impedance Cardiography
The CircMon device (JR Medical Ltd, Tallinn, Estonia) was used to determine heart rate (HR), stroke index (stroke volume/body surface area), cardiac index (cardiac output/body surface area), systemic vascular resistance index (SVRI), aortic to popliteal pulse wave velocity (PWV), and extracellular fluid volume.16, 17, 18, 19 The description and repeatability of the method have been published elsewhere.16, 17, 18, 19 SVRI was calculated from radial tonometric BP and cardiac index measured by the CircMon device. With CircMon, cardiac output shows good agreement with the thermodilution method,17 while stroke volume shows good correlation with values obtained using 3‐dimensional ultrasound.18
Hemodynamic Recordings
Before the recordings, patients were advised to abstain from caffeine‐containing products, smoking, and heavy meals for at least 4 hours and from alcohol for at least 24 hours. While the patients were resting in a temperature‐controlled laboratory for 10 minutes, a tonometric sensor for pulse wave analysis was fixed on the left radial pulse, a brachial cuff for BP calibration on the right upper arm, and electrodes for whole‐body impedance cardiography on the body surface.16, 18, 19, 20, 21 Beat‐to‐beat hemodynamic data were captured for 5 minutes with the patient in the supine position, and mean values of the last 3 minutes were used in the analysis when the signal was most stable. The repeatability and reproducibility of the method is good.19
Statistical Analyses
GFR was estimated using the CKD‐EPI creatinine‐cystatin C equation.11 Stepwise linear regression analyses were used to investigate factors independently associated with hemodynamic variables and sex, age, waist:height ratio (WHtR), body mass index (BMI), hemoglobin, plasma uric acid, C‐reactive protein, total cholesterol, triglyceride, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, fasting glucose levels, smoking, and alcohol consumption were tested. Total and normalized extracellular volumes, given as extracellular volume balance by the CircMon software, were used in the analyses. In the final analyses, age, sex, WHtR, and LDL cholesterol level were included as independent variables in the multivariate models with eGFR. WHtR was chosen instead of BMI, since WHtR is considered a better determinant of cardiovascular risk factors.22 Analyses were also performed separately for sexes.
The patients were subsequently divided into tertiles according to eGFR values (ranges, 64–91, 92–104, and 105–145 mL/min/1.73 m2). Data were analyzed using two‐way analysis of variance, and the Bonferroni correction was applied in the post‐hoc analyses. SPSS 21.0 for Windows software (IBM SPSS, Armonk, NY) was used. The results were presented as means and standard deviations (SDs) or 95% confidence intervals (CIs), and P<.05 was considered statistically significant.
Results
Study Population
The mean age of patients was 46 years, and 282 (51%) of the 556 participants were men (Table 1). The average levels of creatinine (74 μmol/L), cystatin C (0.84 mg/L), and eGFR (99 mL/min/1.73 m2) were within the normal range. Because of sex‐related differences in the associations between hemodynamic variables and eGFR (see below), the values were also calculated separately for men and women. Most variables were not significantly different between men and women, but men had higher BMI, WHtR, plasma creatinine, and triglyceride levels and lower HDL cholesterol and consumed more alcohol than women (Table 1).
Table 1.
Clinical Characteristics and Laboratory Test Results
| All (N=556) | Men (n=282) | Women (n=274) | |
|---|---|---|---|
| Age, y | 46 (12) | 46 (12) | 45 (12) |
| BMI, kg/m2 | 26.7 (4.3) | 27.4 (4.0) | 25.9 a (4.5) |
| Waist:height ratio | 0.53 (0.07) | 0.55 (0.07) | 0.52 a (0.08) |
| Alcohol, standard drinks per wk | 5 (6) | 7 (7) | 3 a (4) |
| Current smokers, % | 12 | 13 | 11 |
| Office blood pressure, mm Hg | |||
| Systolic | 140 (21) | 146 (21) | 135 (20) |
| Diastolic | 89 (12) | 92 (12) | 85 (12) |
| Creatinine, μmol/L | 74 (13) | 82 (11) | 65 a (9) |
| Cystatin C, mg/L | 0.84 (0.15) | 0.88 (0.14) | 0.7 (0.14) |
| Estimated GFR, mL/min/1.73 m2 | 99 (15) | 98 (15) | 99 (14) |
| Hemoglobin, g/L | 144 (12) | 152 (8) | 136 (9) |
| C‐reactive protein, mg/L | 0.8 (0.5–1.9) | 0.8 (0.5–1.5) | 1.0 (0.5–2.2) |
| Sodium, mmol/L | 140 (2) | 141 (2) | 140 (2) |
| Potassium, mmol/L | 3.8 (0.3) | 3.9 (0.3) | 3.8 (0.3) |
| Uric acid, μmol/L | 305 (76) | 348 (66) | 259 (57) |
| Fasting plasma | |||
| Total cholesterol, mmol/L | 5.2 (1.0) | 5.3 (1.1) | 5.0 (1.0) |
| Triglycerides, mmol/L | 1.3 (1.0) | 1.5 (1.2) | 1.0 a (0.6) |
| HDL cholesterol, mmol/L | 1.6 (0.4) | 1.4 (0.3) | 1.8 a (0.4) |
| LDL cholesterol, mmol/L | 3.0 (1.0) | 3.3 (1.0) | 2.8 (0.9) |
| Glucose, mmol/L | 5.4 (0.6) | 5.6 (0.6) | 5.3 (0.6) |
Abbreviations: BMI, body mass index; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein. Values are expressed as mean (standard deviation). a P<.05 women vs men.
Hemodynamics at Rest
As observed previously,21 BP values in the laboratory were lower than those in the office (P<.001 for both systolic and diastolic BP) (Table 2). Based on the laboratory measurements, 166 of 556 participants (30%) were either hypertensive (BP ≥140/90 mm Hg) or had high‐normal BP (BP ≥130/85 mm Hg).15 Systolic and diastolic BP, cardiac index, SVRI, PWV, and extracellular volume balance were not significantly different between men and women, but men had slightly lower HR (Table 2).
Table 2.
Hemodynamic Measurements
| All | Men | Women | |
|---|---|---|---|
| Systolic blood pressure, mm Hg | 134 (104–171) | 139 (112–171) | 129 (101–165) |
| Diastolic blood pressure, mm Hg | 78 (57–101) | 81 (59–104) | 75 (55–98) |
| Heart rate, beats per min | 63 (62–64) | 62 (61–63) | 64 (63–65) |
| Cardiac index, L/min/m2 | 2.91 (2.19–3.79) | 2.94 (2.21–3.79) | 2.88 (2.18–3.76) |
| Systemic vascular resistance index, dyn*s/cm5*m2 | 2624 (1700–3665) | 2703 (1815–3685) | 2542 (1652–3655) |
| Pulse wave velocity, m/s | 8.47 (6.03–12.43) | 8.92 (6.27–12.97) | 8.00 (5.93–10.80) |
| Extracellular volume balance | 1.09 (0.93–1.26) | 1.06 (0.92–1.20) | 1.11 (0.96–1.27) |
Values are expressed as mean (95% confidence interval).
BP and Hemodynamic Variables in Linear Regression Analyses
In stepwise linear multivariate regression analysis including all study patients, the variables that showed a significant independent association with systolic and diastolic BP were eGFR, age, WHtR, plasma LDL cholesterol level, and sex (Table 3). The variables that were associated with SVRI were eGFR, age, and WHtR (Table 4). No relationship was observed between eGFR and HR, cardiac index, PWV, or extracellular volume balance (not shown). The other laboratory variables (hemoglobin, plasma sodium, potassium, C‐reactive protein, uric acid, fasting glucose, triglycerides, and HDL cholesterol), smoking, and alcohol intake were not significantly related with hemodynamic variables.
Table 3.
Significant Explanatory Variables for Systolic and Diastolic BP in Linear Regression Analysis
| Systolic BP | All (R 2=0.28) | Men (R 2=0.13) | Women (R 2=0.32) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | Beta | P Value | B | Beta | P Value | B | Beta | P Value | |
| Constant | 109 | 108 | 119 | ||||||
| eGFR | −0.23 | −0.17 | <.001 | −0.11 | −0.10 | .161 | −0.36 | −0.25 | <.001 |
| Age | 0.23 | 0.14 | <.001 | 0.21 | 0.14 | .050 | 0.23 | 0.13 | .055 |
| WHtR | 46 | 0.17 | .004 | 50 | 0.19 | .004 | 42 | 0.16 | .007 |
| LDL cholesterol | 2.9 | 0.14 | .001 | 1.5 | 0.08 | .18 | 4.6 | 0.20 | .001 |
| Male sex | 7.2 | 0.18 | <.001 | ||||||
| Diastolic BP | All (R 2=0.29) | Men (R 2=0.25) | Women (R 2=0.23) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | Beta | P Value | B | Beta | P Value | B | Beta | P Value | |
| Constant | 71 | 66 | 79 | ||||||
| eGFR | −0.21 | −0.23 | <.001 | −0.18 | −0.21 | .001 | −0.24 | −0.27 | <.001 |
| Age | 0.15 | 0.14 | .004 | 0.19 | 0.18 | .008 | 0.10 | 0.09 | .23 |
| WHtR | 23 | 0.13 | .003 | 34 | 0.18 | .003 | 14 | 0.09 | .16 |
| LDL cholesterol | 2.1 | 0.15 | <.001 | 1.6 | 0.13 | .025 | 2.7 | 0.18 | .006 |
| Male sex | 4.6 | 0.17 | .001 | ||||||
Abbreviations: BP, blood pressure; eGFR, estimated glomerular filtration rate; LDL, low‐density lipoprotein; WHtR, waist:height ratio. Bold values indicate significance.
Table 4.
Significant Explanatory Variables for SVRI and PWV in Linear Regression Analysis
| SVRI | All (R 2=0.21) | Men (R 2=0.16) | Women (R 2=0.25) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | Beta | P Value | B | Beta | P Value | B | Beta | P Value | |
| Constant | 2033 | 1756 | 2468 | ||||||
| eGFR | −8.5 | −0.20 | <.001 | −5.2 | −0.12 | .049 | −12.2 | −0.28 | <.001 |
| Age | 6.5 | 0.13 | .012 | 6.8 | 0.13 | .057 | 5.8 | 0.11 | .13 |
| WHtR | 2023 | 0.24 | <.001 | 2098 | 0.21 | <.001 | 1974 | 0.25 | .001 |
| Male sex | 90 | 0.07 | .057 | ||||||
| PWV | All (R 2=0.46) | Men (R 2=0.43) | Women (R 2=0.45) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | Beta | P Value | B | Beta | P Value | B | Beta | P Value | |
| Constant | 2.058 | 0.300 | 4.279 | ||||||
| eGFR | −0.009 | −0.07 | .100 | −0.002 | −0.03 | .812 | −0.017 | −0.14 | .015 |
| Age | 0.078 | 0.46 | <.001 | 0.086 | 0.48 | <.001 | 0.067 | 0.46 | <.001 |
| WHtR | 6.323 | 0.24 | <.001 | 8.882 | 0.28 | <.001 | 4.539 | 0.21 | <.001 |
| Male sex | 0.684 | 0.17 | <.001 | ||||||
Abbreviations: SVRI, systemic vascular resistance index; eGFR, estimated glomerular filtration rate; WHtR, waist:height ratio; PWV, pulse wave velocity. Bold values indicate significance.
In analyses performed separately for sexes, WHtR was related with systolic BP in men, while age had a borderline significant influence (Table 3). In men, eGFR, age, WHtR, LDL cholesterol, and alcohol consumption (P=.029) were associated with diastolic BP. In women, eGFR and LDL cholesterol were associated with systolic and diastolic BP, WHtR was significantly associated with systolic BP, and age had a borderline significant effect (Table 3). In both sexes, eGFR and WHtR were independently associated with SVRI (Table 4). In men, age and WHtR were associated with PWV, whereas in women, eGFR, age, and WHtR were associated with PWV.
BP and Hemodynamic Variables in Tertiles of eGFR
Systolic and diastolic BPs were different in subjects in all eGFR tertiles after unadjusted analyses. When the analyses were adjusted for differences in age, WHtR, LDL cholesterol, and sex, higher systolic and diastolic BPs were still observed in subjects in the tertiles with the lowest and intermediate eGFR when compared with those in the highest eGFR tertile (Figure 1).
Figure 1.
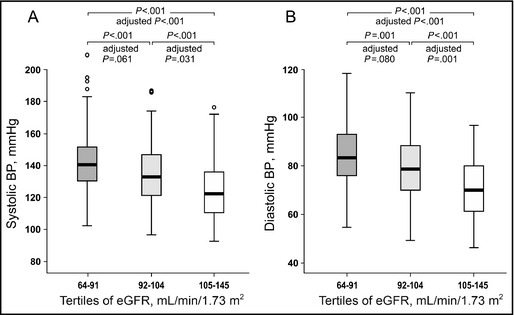
Systolic blood pressure (BP) (A) and diastolic BP (B) depicted in tertiles of estimated glomerular filtration rate (eGFR). Median (thick line inside box), 25th to 75th percentile (box), range (whiskers), and outliers (circles). P values for unadjusted analyses, and P values adjusted for differences in age, waist:height ratio, low‐density lipoprotein cholesterol, and sex.
No differences were found in cardiac index, PWV, and extracellular water volume between eGFR tertiles in analyses adjusted for differences in age, WHtR, LDL cholesterol, and sex (and also for body weight in analyses of extracellular water volume). However, the tertiles with the lowest and intermediate eGFR showed higher SVRI than the tertile with the highest eGFR (Figure 2).
Figure 2.
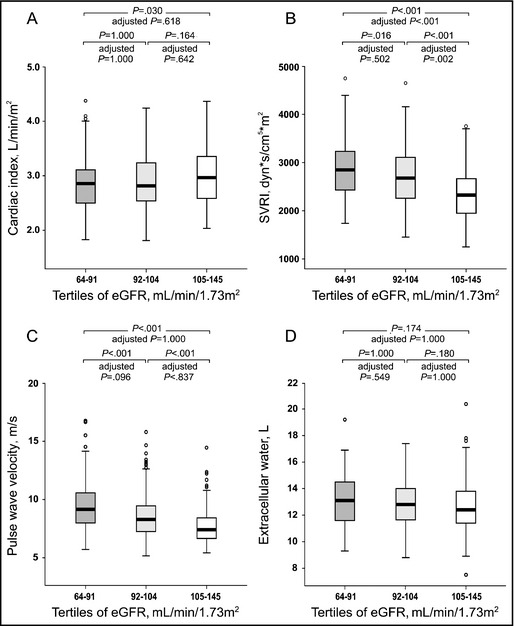
Cardiac index (A), systemic vascular resistance index (SVRI) (B), pulse wave velocity (C), and volume of extracellular water (D) depicted in tertiles of estimated glomerular filtration rate (eGFR). Median (thick line inside box), 25th to 75th percentile (box), range (whiskers), and outliers (circles). P values for unadjusted analyses, and P values adjusted for differences in age, waist:height ratio, low‐density lipoprotein cholesterol, and sex (and weight in analyses of extracellular water).
Discussion
We investigated the relationship of eGFR with the level of BP and hemodynamic profile in normotensive and hypertensive subjects without clinically recognized CKD who did not use medications with direct cardiovascular actions. The combined CKD‐EPI creatinine and cystatin C formula was applied to evaluate renal function, since several recent studies support the view that this equation provides the most accurate value for eGFR.11, 12, 13, 14, 23, 24 The present results suggest an independent association between lower eGFR and higher BP, and increased systemic vascular resistance was the hemodynamic alteration that was related to elevated BP.
The prevalence of hypertension is known to be inversely associated with the level of kidney function.25 In NHANES III, an almost linear relationship was found between the prevalence of arterial hypertension and the level of eGFR, as assessed using the creatinine‐based Modification of Diet in Renal Disease equation.25 Already mild reduction in renal function has been implicated in the pathogenesis of primary hypertension, as significant associations have been found between higher serum cystatin C concentrations and the incidence2, 3 and prevalence4 of hypertension among the general population. However, several previous studies examining the relationship between BP and kidney function have included patients medicated with BP‐lowering drugs and other agents with direct cardiovascular actions.4, 26, 27, 28
Renal mechanisms may predispose to the development of hypertension via several pathways.1 Structural and hemodynamic changes in the kidney may lead to defective sodium excretion and increased volume load.1, 29 In line with this view, extracellular fluid excess has been found in patients who are in the early stages of manifest CKD.26 In the present study, eGFR was inversely associated with BP, but BP was not associated with cardiac output or extracellular fluid volume, suggesting that hyperkinetic hemodynamics or volume retention did not explain the relationship between higher BP and lower eGFR. Endothelium‐mediated vasodilatation has been reported to be impaired in patients with mild renal insufficiency and uncomplicated essential hypertension.30 Such a mechanism can be expected to lead to a rise in peripheral arterial resistance. In the present study, eGFR was inversely associated with systemic vascular resistance, implying that altered regulation of the arterial tree may have been the pathophysiological process that elevated BP.
Several studies have found evidence of increased large arterial stiffness in patients with CKD, usually documented by the measurement of PWV.6, 7, 8, 31 In the present study, eGFR was associated with PWV and systolic BP in women but not in men even in the absence of kidney disease. However, men showed higher levels of triglycerides and adiposity and lower HDL cholesterol than women. Obesity, dyslipidemia, and atherosclerosis contribute to both the development of hypertension and arterial aging,15 and these factors may have overshadowed the influence of the prevailing renal function on PWV in men. It is noteworthy that the Framingham Heart Study did not find a correlation between arterial stiffness and mild to moderate CKD.32
Study Strengths and Limitations
The strengths of this study are that detailed hemodynamic information was captured in a relatively large number of participants (n=556) and that patients taking medications with direct cardiovascular influences were excluded. However, our study has limitations, and the observational design does not allow conclusions about causal relationship. As GFR was estimated, all associations of kidney function with the hemodynamic profile must be interpreted with caution. Hemodynamic recordings were performed on a single occasion, and white‐coat hypertension and masked hypertension may have influenced the results.15 PWV was recorded using the whole‐body impedance cardiography method, and hard end‐point data on the significance of arterial stiffness measured using this approach are lacking. Finally, the measurement of extracellular water volume was based on a single‐frequency 30‐kHz alternating current, and this method may not as accurately evaluate body water content as multifrequency impedance systems.33 However, the single‐frequency method has been found to be valid in extracellular fluid volume analyses in healthy patients in whom the extracellular to intracellular water ratio remains in the normal range.33
Conclusions
We examined healthy normotensive subjects and patients with prehypertension or newly discovered primary hypertension who were free of other cardiovascular or kidney diseases. Lower eGFR was associated with higher BP, and the related hemodynamic pattern was increased peripheral vascular resistance without changes of cardiac index, corresponding to the “classical” picture of primary hypertension.15, 34 It seems feasible to calculate creatinine and cystatin C–based eGFR in all patients with hypertension, as early impairment in kidney function may be involved in the pathogenesis of primary hypertension.
Acknowledgments and disclosure
The authors thank Reeta Kulmala, RN, Paula Erkkilä, RN, Marika Päällysaho, RN, Pirjo Järventausta, RN, and Mirja Ikonen, RN, for their invaluable technical assistance and Heini Huhtala, MSc, for statistical support. The authors declare no conflicts of interest.
Funding
This work was supported by Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital, Finnish Foundation for Cardiovascular Research, Sigrid Jusélius Foundation, Paavo Nurmi Foundation, Pirkanmaa Regional Fund of the Finnish Cultural Foundation, Tampere Tuberculosis Foundation, Aarne Koskelo Foundation, the Finnish Medical Foundation, and Orion‐Farmos Research Foundation.
J Clin Hypertens. 2014;16:722–728. DOI: 10.1111/jch.12405. © 2014 Wiley Periodicals, Inc.
References
- 1. Wadei HM, Textor SC. The role of the kidney in regulating arterial blood pressure. Nat Rev Nephrol. 2012;8:602–609. [DOI] [PubMed] [Google Scholar]
- 2. Kestenbaum B, Rudser KD, de Boer IH, et al. Differences in kidney function and incident hypertension: the multi‐ethnic study of atherosclerosis. Ann Intern Med. 2008;148:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otsuka T, Kato K, Kachi Y, et al. Serum cystatin C, creatinine‐based estimated glomerular filtration rate, and the risk of incident hypertension in middle‐aged men. Am J Hypertens. 2013;27:596–602. [DOI] [PubMed] [Google Scholar]
- 4. Shankar A, Teppala S. Relationship between serum cystatin C and hypertension among US adults without clinically recognized chronic kidney disease. J Am Soc Hypertens. 2011;5:378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rifkin DE, Katz R, Chonchol M, et al. Blood pressure components and decline in kidney function in community‐living older adults: the Cardiovascular Health Study. Am J Hypertens. 2013;26:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peralta CA, Whooley MA, Ix JH, et al. Kidney function and systolic blood pressure new insights from cystatin C: data from the Heart and Soul Study. Am J Hypertens. 2006;19:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schillaci G, Pirro M, Mannarino MR, et al. Relation between renal function within the normal range and central and peripheral arterial stiffness in hypertension. Hypertension. 2006;48:616–621. [DOI] [PubMed] [Google Scholar]
- 8. Tomiyama H, Tanaka H, Hashimoto H, et al. Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis. 2010;212:345–350. [DOI] [PubMed] [Google Scholar]
- 9. Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. [DOI] [PubMed] [Google Scholar]
- 10. Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin‐to‐creatinine ratio and association with progression to end‐stage renal disease and mortality. JAMA. 2011;305:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fan L, Inker LA, Rossert J, et al. Glomerular filtration rate estimation using cystatin C alone or combined with creatinine as a confirmatory test. Nephrol Dial Transplant. 2014;29:1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 16. Kööbi T, Kähönen M, Iivainen T, et al. Simultaneous non‐invasive assessment of arterial stiffness and haemodynamics – a validation study. Clin Physiol Funct Imaging. 2003;23:31–36. [DOI] [PubMed] [Google Scholar]
- 17. Kööbi T, Kaukinen S, Turjanmaa VM, et al. Whole‐body impedance cardiography in the measurement of cardiac output. Crit Care Med. 1997;25:779–785. [DOI] [PubMed] [Google Scholar]
- 18. Koskela JK, Tahvanainen A, Haring A, et al. Association of resting heart rate with cardiovascular function: a cross‐sectional study in 522 Finnish subjects. BMC Cardiovasc Disord. 2013;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tahvanainen A, Koskela J, Tikkakoski A, et al. Analysis of cardiovascular responses to passive head‐up tilt using continuous pulse wave analysis and impedance cardiography. Scand J Clin Lab Invest. 2009;69:128–137. [DOI] [PubMed] [Google Scholar]
- 20. Tahvanainen A, Leskinen M, Koskela J, et al. Ageing and cardiovascular responses to head‐up tilt in healthy subjects. Atherosclerosis. 2009;207:445–451. [DOI] [PubMed] [Google Scholar]
- 21. Tikkakoski AJ, Tahvanainen AM, Leskinen MH, et al. Hemodynamic alterations in hypertensive patients at rest and during passive head‐up tilt. J Hypertens. 2013;31:906–915. [DOI] [PubMed] [Google Scholar]
- 22. Lee CM, Huxley RR, Wildman RP, et al. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta‐analysis. J Clin Epidemiol. 2008;61:646–653. [DOI] [PubMed] [Google Scholar]
- 23. Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157:471–481. [DOI] [PubMed] [Google Scholar]
- 24. Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. [DOI] [PubMed] [Google Scholar]
- 25. K/DOQI . Clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 26. Essig M, Escoubet B, de Zuttere D, et al. Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol Dial Transplant. 2008;23:239–248. [DOI] [PubMed] [Google Scholar]
- 27. Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2001;161:1207–1216. [DOI] [PubMed] [Google Scholar]
- 28. Mena C, Robles NR, de Prado JM, et al. Cystatin C and blood pressure: results of 24 h ambulatory blood pressure monitoring. Eur J Intern Med. 2010;21:185–190. [DOI] [PubMed] [Google Scholar]
- 29. Johnson RJ, Herrera‐Acosta J, Schreiner GF, et al. Subtle acquired renal injury as a mechanism of salt‐sensitive hypertension. N Engl J Med. 2002;346:913–923. [DOI] [PubMed] [Google Scholar]
- 30. Perticone F, Maio R, Tripepi G, et al. Endothelial dysfunction and mild renal insufficiency in essential hypertension. Circulation. 2004;110:821–825. [DOI] [PubMed] [Google Scholar]
- 31. Gosse P, Safar ME. Arterial stiffness and plasma creatinine in untreated hypertensive patients. Am J Hypertens. 2005;18:1140–1145. [DOI] [PubMed] [Google Scholar]
- 32. Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild‐to‐moderate CKD. J Am Soc Nephrol. 2009;20:2044–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gudivaka R, Schoeller DA, Kushner RF, et al. Single‐ and multifrequency models for bioelectrical impedance analysis of body water compartments. J Appl Physiol. 1999;87:1087–1096. [DOI] [PubMed] [Google Scholar]
- 34. Staessen JA, Wang J, Bianchi G, et al. Essential hypertension. Lancet. 2003;361:1629–1641. [DOI] [PubMed] [Google Scholar]


