Abstract
Cytokines play an important role in the pathogenesis of hypertension. The authors hypothesized that interleukin 17 (IL‐17) might contribute to the prehypertensive state. This study evaluated the relationship between serum levels of IL‐17 and prehypertension. A total of 394 participants were enrolled, after excluding for hypertension or treated hypertension, and divided into two groups (optimal blood pressure [BP] and prehypertension) based on the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure classification of BP. Optimal BP was defined as systolic BP <120 mm Hg and diastolic BP <80 mm Hg. Prehypertension was defined as systolic BP of 120 to 139 mm Hg or diastolic BP of 80 to 89 mm Hg. IL‐17A levels were determined by enzyme‐linked immunosorbent assay. The mean serum IL‐17 concentration in the prehypertension group was significantly higher than in the optimal BP group. The cohort was divided into quartiles Q1 (≤3.5 ng/L), Q2 (3.60 to 6.10 ng/L), Q3 (6.20 to 10.00 ng/L), and Q4 (≥10.10 ng/L) based on IL‐17 levels. The Q2 to Q4 groups had increasing odds ratios for having prehypertension compared with the Q1 group. Elevated serum IL‐17 was accompanied by a rise in systolic BP. Thus, increased serum IL‐17 levels are associated with prehypertension.
Studies have shown that both innate and adaptive immune responses contribute to vascular dysfunction and hypertension.1 Immune responses play a key role in the vascular remodeling that leads to blood pressure (BP) elevation.2, 3 Early studies suggested that the thymus is important in experimental hypertension in rodents.4, 5 Moreover, T lymphocytes infiltrate the kidney in hypertensive animals, and reduction of this infiltration decreases BP.6 Further evidence showed that T cells are essential for the development of hypertension. Mice without T and B cells have little or none of the associated vascular dysfunction. Adoptive transfer of T cells, but not B cells, restored both the hypertensive response and vascular abnormalities in these mice.7
The development of autoimmune and inflammatory disorders has been shown to be influenced by a recently described subset of CD4+ T cells that are characterized by the secretion of interleukin 17 (IL‐17) (T‐helper [Th] 17 cells).8 IL‐17 has been implicated in the pathogenesis of many autoimmune and inflammatory diseases, such as rheumatoid arthritis, psoriasis, multiple sclerosis, asthma, inflammatory bowel disease, and periodontal disease.9 Recent evidence also suggests that IL‐17 plays an important role in cardiovascular disease including coronary atherosclerosis and hypertension.10, 11
In 2003, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) proposed a new BP category, designated “prehypertension,” of 120 mm Hg to 139 mm Hg systolic blood pressure (SBP) or 80 mm Hg to 89 mm Hg diastolic blood pressure (DBP).12 Cardiovascular risk and end‐organ damage are already elevated in individuals with prehypertension when compared with those with BP <120/80 mm Hg.13 Some studies have found that a prehypertensive state is present in autoimmune and inflammatory disorders.1, 2, 3 Various inflammatory makers and cytokines, including high‐sensitivity C‐reactive protein (hs‐CRP), IL‐6, and tumor necrosis factor α14, 15, 16, 17 are associated with prehypertension.
IL‐17 plays a critical role in hypertension, and prehypertension is an important stage of progression to hypertension. We hypothesized that IL‐17 might also contribute to the prehypertensive state. To test this hypothesis, we examined serum levels of IL‐17 to evaluate the relationship between IL‐17 and prehypertension in a middle‐aged population.
Methods
Study Population
Study procedures were in accordance with the ethical standards of the responsible ethics committee on human experimentation of Tianjin Medical University General Hospital. All participants gave informed written consent prior to inclusion in the study.
The study patients were participants of an ongoing community‐based study that aimed to estimate the rate of progression of prehypertension to hypertension in Tianjin, China. From May 2011 to May 2012, residents aged 40 to 70 years living in the center of Tianjin City were enrolled after exclusion of participants with hypertension or treated hypertension. A total of 394 participants, including 174 men and 220 women, were enrolled. All participants were free of self‐reported stroke, transient ischemic attack, myocardial infarction, angina, renal failure, rheumatoid arthritis, psoriasis, multiple sclerosis, asthma, inflammatory bowel disease, periodontal disease, surgery, and trauma within the month prior. The prevalence of self‐reported diabetes mellitus was 8.9% (35 of 394) and 53.8% (19 of 35) diabetic patients received treatment with oral antidiabetic agents and/or insulin. The prevalence of self‐reported dyslipidemia was 31.4% (124 of 394), with 24.9% (31 of 124) receiving antidyslipidemic therapy.
Anthropometry and BP
An interview‐based survey was performed using a questionnaire by trained staff. Demographic data, disease history, family history of hypertension, current smoking (at least one cigarette per day), alcohol drinking (≥2 times per week), and medical and medication histories were recorded.
Three BP measurements were performed by two trained doctors while the study participants were seated, using a standard mercury sphygmomanometer according to a standard protocol, after the patients had been resting for 30 minutes. Standard cuff bladders (12–13 cm wide and 35 cm long) were used to measure BP, with larger and smaller bladders available for large (arm circumference >32 cm) and thin arms, respectively. The 1st and 5th Korotkoff sounds were recorded as SBP and DBP, respectively, and the mean of three measurements was used in the analysis. The classification of normotensive, prehypertensive, and hypertensive was based on the classification of BP from JNC 7.12 Optimal BP was defined as not being on antihypertensive medication, and having an SBP <120 mm Hg and a DBP <80 mm Hg. Prehypertension was defined as not being on antihypertensive medication and having an SBP of 120 mm Hg to 139 mm Hg or DBP of 80 mm Hg to 89 mm Hg.
Body weight and height were measured by trained staff with the patients wearing light clothing and without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. Waist circumference (WC) was measured at the level of 1 cm above the umbilicus.
Biochemical and Plasma IL‐17 Measurements
Blood samples were drawn from the antecubital vein in the morning after a 10‐hour fast. Fasting plasma glucose (FPG), uric acid (UA), total cholesterol (TC), triglycerides (TG), high‐density lipoprotein cholesterol (HDL‐C), and low‐density lipoprotein cholesterol (LDL‐C) were measured using a Hitachi 7180 Automatic Analyzer (Hitachi, Tokyo, Japan). hs‐CRP was assayed by a particle‐enhanced immunoturbidimetric method (Roche Diagnostics, Roche/Hitachi Analyzer, Tokyo, Japan).
Fasting serum and plasma were separated from blood cells in the field within 30 minutes and kept frozen at −80°C. Serum IL‐17A was measured by an enzyme‐linked immunosorbent assay using commercial kits (Bio‐Rad, Hercules, CA) on a microplate reader (Model 680; Bio‐Rad). All analyses were performed in accordance with the manufacturer's recommendations.
Statistical Analysis
SPSS version 17.0 software (IBM, Somers, NY) was used to perform all statistical analyses. Data are expressed as the mean±standard deviation after normality tested, or as the median with interquartile range for variables with a skewed distribution or percentage as appropriate. Differences between groups were analyzed by t test or Mann‐Whitney U test for measurement data and chi‐square test for enumeration data. Multiple logistic regression analysis was used to examine relationships between prehypertension and IL‐17 after adjustment for covariates: age, sex, BMI, WC, smoking status, drinking status, TC, TG, HDL‐C, LDL‐C, FPG, UA, diabetes, and dyslipidemia. Multiple linear regression analysis was used to establish the relationship between SBP, DBP, and IL‐17 after adjustment for age, sex, BMI, WC, smoking status, drinking status, TC, TG, HDL‐C, LDL‐C, FPG, UA, diabetes, and dyslipidemia. Odds ratios (ORs) and 95% confidence intervals were calculated. All statistical tests were two‐tailed, and P values of <.05 were considered statistically significant.
Results
Table 1 shows the general characteristics of the study population according to baseline BP. The two groups (optimal BP and prehypertension) differed significantly in many characteristics. The prehypertensive group showed a significantly higher percentage of male sex, smoking, alcohol drinking, diabetes, and dyslipidemia compared with the optimal BP group. The prehypertensive group also had higher mean age, BMI, WC, TG, FPG, and UA compared with the optimal BP group.
Table 1.
Baseline Characteristics of Study Participants (N=394)
| Characteristic | Optimal BP (n=198) | Prehypertension (n=196) | P Valuea |
|---|---|---|---|
| Age, y | 53.71±6.30 | 57.20±6.63 | <.01 |
| Men, No. (%) | 74 (37.37) | 100 (51.02) | <.01 |
| Diabetes, No. (%) | 14 (7.07) | 21 (10.71) | <.05 |
| Dyslipidemia, No. (%) | 55 (27.78) | 68 (34.80) | <.05 |
| Alcohol drinking, No. (%) | 63 (31.82) | 86 (43.88) | <.01 |
| Smoking, No. (%) | 34 (17.17) | 49 (25.00) | <.01 |
| BMI, kg/m2 | 23.32±2.88 | 25.16±2.98 | <.01 |
| WC, cm | 83.28±9.19 | 87.57±9.57 | <.01 |
| TC, mmol/L | 5.45±1.09 | 5.64±1.00 | .069 |
| TG, median, mmol/L | 1.21 (0.87–1.69) | 1.43 (1.05–2.10) | <.01b |
| HDL‐C, mmol/L | 1.54±0.40 | 1.48±0.38 | .132 |
| LDL‐C, mmol/L | 3.31±0.99 | 3.44±0.85 | .152 |
| FPG, median, mmol/L | 4.90 (4.60–5.43) | 5.20 (4.80–5.70) | <.01b |
| UA, median, μmol/L | 261.50 (221.75–311.25) | 281.50 (243.50–335.75) | <.01b |
Abbreviations: BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; UA, uric acid; WC, waist circumference. aComparisons between groups were analyzed by t test for measurement data and chi‐square test for enumeration data. bComparisons between groups were analyzed by Mann‐Whitney U test for TG, FPG, and UA.
As evident in Table 2, the mean serum levels of IL‐17 were 5.10 ng/L in the optimal BP group and 7.0 ng/L in the prehypertension group, with the mean serum concentration of IL‐17 being significantly higher in the prehypertension group than in the optimal BP group (P<.01). The level of hs‐CRP was also higher in the prehypertension group (P<.01).
Table 2.
Difference in Serum Level of IL‐17 Between Patients With Prehypertension and Optimal BP (N=394)
| Characteristic | Optimal BP (n=198) | Prehypertension (n=196) | P Valuea |
|---|---|---|---|
| IL‐17, median, ng/L | 5.10 (2.70–8.40) | 7.00 (4.33–11.23) | <.01 |
| hs‐CRP, median, mg/L | 0.90 (0.40–2.03) | 1.30 (0.70–3.30) | <.01 |
Abbreviations: BP, blood pressure; hs‐CRP, high‐sensitivity C‐reactive protein; IL‐17, interleukin 17. aComparisons between groups were analyzed by Mann‐Whitney U test.
The cohort was divided into four quartiles based on serum IL‐17 concentration: Q1 (≤3.5 ng/L), Q2 (3.60–6.10 ng/L), Q3 (6.20–10.00 ng/L), and Q4 (≥10.10 ng/L). The relationship between IL‐17 and prehypertension for the crude and adjusted covariate values are shown in Table 3. In the final multivariable analytic models, the Q2, Q3, and Q4 groups had a significantly higher OR for having prehypertension than the Q1 group. In order to further confirm the relationship between IL‐17 and prehypertension values, sensitivity analysis was performed where participants with diabetes and dyslipidemia were excluded. In the final multivariable analytic models, the significantly positive association was unchanged: the ORs for prehypertension of the Q2, Q3, and Q4 groups were 1.68 (1.16–3.73), 3.07 (1.37–6.08), and 3.33 (1.47–7.57), respectively.
Table 3.
Multifactor Logistic Regression Analysis of IL‐17 Influence on Prehypertension (N=394)
| Serum IL‐17, ng/L | ||||
|---|---|---|---|---|
| Quartile 1 (n=100) | Quartile 2 (n=97) | Quartile 3 (n=98) | Quartile 4 (n=99) | |
| Patients with prehypertension, No. | 33 | 47 | 53 | 58 |
| Crude | Reference | 2.25 (1.27–3.99) | 2.45 (1.37–4.36) | 2.94 (1.65–5.26) |
| Adjusteda | Reference | 2.44 (1.21–4.93) | 2.59 (1.34–4.92) | 2.75 (1.41–5.34) |
Abbreviation: IL‐17, interleukin 17. Values are given as odds ratios (95% confidence intervals). aAdjusted for age, sex, body mass index, waist circumference, total cholesterol, triglycerides, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, uric acid, fasting plasma glucose, smoking status, drinking status, diabetes, and dyslipidemia.
To confirm the relationship between IL‐17 and SBP and DBP values, we performed linear regression and multiple regression analysis. The linear regression models showed a positive and significant relationship between IL‐17 and SBP and DBP (Figure 1 and Figure 2). In the multiple regression model, the IL‐17 distinctly showed a significant relationship with SBP (P<.01) after adjustment for potential confounding factors (Table 4).
Figure 1.
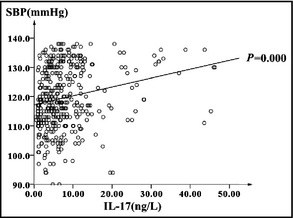
Linear regression analysis between systolic blood pressure (SBP) and interleukin (IL) 17 (SBP=0.304×IL‐17+117.23).
Figure 2.
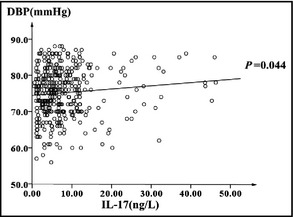
Linear regression analysis between diastolic blood pressure (DBP) and interleukin (IL) 17 (DBP=0.089×IL‐17+74.441).
Table 4.
Results of Multivariate Modelling for IL‐17 (N=394)
| SBP | DBP | |||
|---|---|---|---|---|
| Standard β Coefficient (SEM) | P Value | Standard β Coefficient (SEM) | P Value | |
| IL‐17 | 0.191 (0.046) | <.01 | 0.061 (0.050) | .23 |
| Age | 0.241 (0.048) | <.01 | 0.053 (0.052) | .31 |
| Sex | −0.131 (0.063) | <.05 | −0.116 (0.069) | <.05 |
| Diabetes | 0.025 (0.052) | .63 | 0.017 (0.057) | .76 |
| Dyslipidemia | 0.020 (0.047) | .67 | 0.007 (0.052) | .89 |
| Alcohol drinking | −0.030 (0.049) | .55 | 0.011 (0.055) | .84 |
| Smoking | 0.074 (0.054) | .17 | 0.111 (0.059) | .06 |
| BMI | 0.214 (0.061) | <.01 | 0.255 (0.067) | <.01 |
| WC | 0.073 (0.062) | .24 | 0.021 (0.068) | .76 |
| TC | 0.485 (0.180) | <.01 | 0.451 (0.198) | <.05 |
| TG | 0.100 (0.060) | .09 | 0.060 (0.066) | .37 |
| HDL‐c | −0.079 (0.073) | .28 | −0.072 (0.080) | .37 |
| LDL‐c | 0.372 (0.168) | <.05 | 0.301 (0.184) | .10 |
| FPG | 0.149 (0.054) | <.01 | 0.038 (0.059) | .52 |
| UA | 0.030 (0.053) | .57 | 0.029 (0.058) | .62 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; IL‐17, interleukin 17; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; UA, uric acid; WC, waist circumference.
Discussion
Over the past few years, it has become increasingly recognized that immunopathological and inflammatory mechanisms play an important role in cardiovascular disease. Indeed, vascular inflammation characterized by infiltration of immune cells is an important mechanism in the development of cardiovascular disease and hypertension.18 Many studies have identified a subset of T cells characterized by IL‐17 production (Th17 cells) in cardiovascular disease.19 There are six known members of the IL‐17 family. IL‐17A is the most widely studied and has been implicated in the pathogenesis of multiple autoimmune and inflammatory diseases.9 Recent evidence also supports a role for IL‐17 in cardiovascular disease and hypertension.10 Notably, the serum IL‐17 level in hypertension patients was significantly higher than in normotensive individuals.11 Prehypertension is a transition stage from normotension to hypertension. A growing body of evidence indicates that chronic low‐grade inflammation is a common pathological process and an important contributing factor to prehypertension.2, 3 However, few studies have investigated the relationship between prehypertension and the immune response.
In our study, we examined serum levels of IL‐17 in prehypertensive patients and those with optimal BP. The serum level of IL‐17 was significantly higher in the prehypertension group compared with the optimal BP group. The level of hs‐CRP was also higher in the prehypertension group. This suggests that immune dysfunction and vascular inflammation may already exist in prehypertensive individuals. In our study, the serum level of IL‐17 in the prehypertension group was very close to those reported in the hypertension group, and the serum level of IL‐17 in the optimal BP group was much higher than that seen in the study by Madhur and colleagues.11 A possible reason was that the IL‐17 level might have been underestimated in the study by Madhur and colleagues. The participants in that study all had type 2 diabetes, 66% had hypercholesterolemia (3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors could inhibit IL‐17 gene expression and secretion from human T cells),20 88% of hypertensive patients were taking angiotensin‐converting enzyme inhibitors, which can suppress Th17 cells.21 To observe the relationship between IL‐17 and prehypertension, we divided the cohort into four groups according to the serum level of IL‐17 by the quartile method. Compared with those in the Q1 group, participants in the Q2 to Q4 groups had an increasing risk of having prehypertension. We subsequently adjusted for age, sex, BMI, FPG, UA, and other risk factors. However, adjustment for these factors did not significantly affect the relationship between IL‐17 and prehypertension, leading us to consider that the direct relationship between IL‐17 and prehypertension was independent of age, sex, BMI, TG, FPG, and UA. Since diabetes and dyslipidemia might affect the serum level of IL‐17, sensitivity analysis was performed. Furthermore, there was also a strong relationship between IL‐17 and prehypertension among participants without diabetes and dyslipidemia. To further investigate the relationship between IL‐17 and BP, linear regression and multiple regression analyses were performed and showed a strong relationship between IL‐17 and SBP values. These findings demonstrated that IL‐17 may dependently or independently contribute to prehypertension. The possible mechanism responsible for the elevation of the serum levels of IL‐17 in prehypertension is currently not clear. It may possibly be associated with angiotensin II–induced hypertension and vascular dysfunction; however, further research is required to elucidate the exact mechanism. IL‐17 and Th17 cells may represent potential therapeutic targets for this widespread disease.
Study Limitations
There were some limitations to our study. First, it was refined to prehypertensive patients aged 40 to 70 years; therefore, the results were not suitable to massive prehypertensive people. Second, excluded diseases such as stroke, transient ischemic attack, myocardial infarction, angina, renal failure, rheumatoid arthritis, psoriasis, multiple sclerosis, asthma, inflammatory bowel disease, and periodontal disease were self‐reported. If the participants had these diseases but without diagnosis, it would have affected our results. Third, the present study used a cross‐sectional design and the sample was not large; therefore, the present findings were inherently limited in their ability to eliminate causal relationships between prehypertension and IL‐17. In addition, the study was part of a larger continuing study, and hence could not estimate the risk of progression to hypertension in prehypertensive individuals with elevated IL‐17, thus further studies of the cohort are required. However, given these limitations, we believe that our results provide insight on the role of IL‐17 in prehypertension.
Conclusions
The present study showed that IL‐17 is associated with prehypertension. This might suggest that proinflammatory immune responses begin to increase with elevation of BP, even when BP is below the hypertensive range. Thus, examining the serum level of IL‐17 in prehypertensive individuals may provide improved predictive or prognostic values. The results of our study encourage further investigation in this field.
Disclosures
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This study was supported by the Science and Technology Fund of Tianjin Health Bureau (11KG133).
J Clin Hypertens (Greenwich). 2015:770–774. DOI: 10.1111/jch.12612. © 2015 Wiley Periodicals, Inc.
References
- 1. Harrison DG, Guzik TJ, Goronzy J, Weyand C. Is hypertension an immunologic disease? Curr Cardiol Rep. 2008;10:464–469. [DOI] [PubMed] [Google Scholar]
- 2. Virdis A, Schiffrin EL. Vascular inflammation: a role in vascular disease in hypertension? Curr Opin Nephrol Hypertens. 2003;12:181–187. [DOI] [PubMed] [Google Scholar]
- 3. Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci. 2007;112:375–384. [DOI] [PubMed] [Google Scholar]
- 4. Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand A. 1976;84:523–528. [DOI] [PubMed] [Google Scholar]
- 5. Bataillard A, Freiche JC, Vincent M, et al. Antihypertensive effect of neonatal thymectomy in the genetically hypertensive LH rat. Thymus. 1986;8:321–330. [PubMed] [Google Scholar]
- 6. Rodriguez‐Iturbe B, Quiroz Y, Nava M, et al. Reduction of renal immune cell infiltration result in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–F201. [DOI] [PubMed] [Google Scholar]
- 7. Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong C. Diversification of T‐helper‐cell lineages: finding the family root of IL‐17‐producing cells. Nat Rev Immunol. 2006;6:329–333. [DOI] [PubMed] [Google Scholar]
- 9. Tesmer LA, Lundy SK, Sarkar S, et al. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eid RE, Rao DA, Zhou J, et al. Interleukin‐17 and interferon‐gamma are produced concomitantly by human coronary artery infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes angiotensin II‐induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chobanian AV, Bakris GL, Black HR, et al.; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure , National High Blood Pressure Education Program Coordinating Committee . The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 13. Natali A, Muscelli E, Casolaro A, et al. Metabolic characteristics of prehypertension: role of classification criteria and gender. J Hypertens. 2009;27:2394–2402. [DOI] [PubMed] [Google Scholar]
- 14. Fung MM, Rao F, Poddar S, et al. Early inflammatory and metabolic changes in association with AGTR1 polymorphisms in prehypertensive subjects. Am J Hypertens. 2011;24:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chrysohoou C, Panagiotakos DB, Pitsavos C, et al. The association between prehypertension status and oxidative stress markers related to atherosclerosis disease: the ATTICA study. Atherosclerosis. 2007;192:169–176. [DOI] [PubMed] [Google Scholar]
- 16. Wang G, Wang A, Tong W, et al. Association of elevated inflammatory and endothelial biomarkers with prehypertension among Mongolians in China. Hypertens Res. 2011;34:516–520. [DOI] [PubMed] [Google Scholar]
- 17. Navarro‐Gonzalez JF, Mora C, Muros M, et al. Relationship between inflammation and microalbuminuria in prehypertension. J Hum Hypertens. 2013;27:119–125. [DOI] [PubMed] [Google Scholar]
- 18. Eriksson EE, Xie X, Werr J, et al. Importance of primary capture and L‐selectindependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J Exp Med. 2001;194:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yao Z, Painter SL, Fanslow WC, et al. Human IL‐17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 20. Zhang X, Jin J, Peng X, et al. Simvastatin inhibits IL‐17 secretion by targeting multiple IL‐17‐regulatory cytokines and by inhibiting the expression of IL‐17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180:6988–6996. [DOI] [PubMed] [Google Scholar]
- 21. Platten M, Youssef S, Hur EM, et al. Blocking angiotensin‐converting enzyme induces potent regulatory T cells and modulates TH1‐ and TH17‐mediated autoimmunity. Proc Natl Acad Sci USA. 2009;106:14948–14953. [DOI] [PMC free article] [PubMed] [Google Scholar]


