Abstract
The aim of the study was to evaluate the role of conventional and new markers of early cardiac organ damage (OD) on 12‐lead electrocardiography (ECG) in 25 outpatients with newly diagnosed untreated essential hypertension compared with 15 normotensive, otherwise healthy individuals. Each participant underwent ECG, echocardiographic, and blood pressure (BP) measurements. Conventional and new ECG indexes for cardiac OD (Tp‐Te interval, ventricular activation time, and P‐wave analysis) were also measured. Clinic and 24‐hour ambulatory BP levels as well as left ventricular mass indexes were significantly higher in hypertensive than in normotensive patients. No significant differences were found between the two groups for ECG and echocardiographic markers of OD. Only Tp‐Te interval was higher in hypertensive than in normotensive individuals (3.06 mm vs 2.24 mm; P<.0001), even after adjustment for anthropometric and clinical parameters. Preliminary results of this study demonstrated prolonged Tp‐Te interval in newly diagnosed, untreated hypertensive outpatients compared with normotensive individuals.
Global cardiovascular risk assessment represents a fundamental step in the clinical management of hypertension.1, 2 Beyond proper measurement of clinic blood pressure (BP) levels, current European guidelines strongly recommend including a thorough assessment of markers of hypertension‐related organ damage (OD) at cardiac, renal, and vascular levels.3 Systematic search of OD has been demonstrated to be useful for the daily clinical management of hypertensive patients by: (1) ameliorating individual global cardiovascular risk stratification; (2) improving patients' own awareness of an asymptomatic disease; and (3) helping physicians choose the best diagnostic and therapeutic options.1, 2 Presence of OD, in fact, may suggest the use of select antihypertensive drug classes or molecules, which have been demonstrated to confer proven benefits in favoring prevention and promoting regression of markers of OD, beyond their BP‐lowering efficacy.4, 5
At the cardiac level, hypertension‐related OD is characterized by an increased left ventricular mass (LVM), leading to the development of left ventricular hypertrophy (LVH) and increased risk of major cardiovascular events.6, 7, 8, 9, 10 LVH can be detected on conventional 12‐lead electrocardiography (ECG) using Sokolow‐Lyon and Cornell indexes.11, 12 Even in the presence of high sensitivity; however, the diagnostic ability of ECG is blunted by its relatively low specificity, which may induce false‐negative results. To overcome this intrinsic limitation and to improve early detection of LVH in a setting of clinical practice, even in the asymptomatic stages of hypertension, a larger use for echocardiography has been proposed over the years.13, 14 This method has the advantage of providing more accurate estimation of LVM and LV geometry with both high sensitivity and specificity for LVH detection. Even in this case, however, the relatively high cost of the procedure as well as the need for adequate user expertise have limited the applicability of echocardiographic estimation of LVH to the general population of hypertensive patients.3 Other advanced diagnostic procedures, eg, computed tomography or magnetic resonance for LVM assessment, have limited applicability in the daily clinical practice of hypertension because of high cost and reduced availability in some hospital divisions and hypertension excellence centers.
The primary role of conventional 12‐lead ECG has recently been reaffirmed in the first‐line diagnostic workup of hypertension to estimate presence of cardiac OD.15 In the past few years, several new ECG parameters have been proposed for improving detection of LV dysfunction and hypertrophy. These parameters, which include the time interval between the peak and the end of the T wave (Tp‐Te interval),16 ventricular activation time (VAT),17 and the P‐wave analysis,18 have been demonstrated to be related to increased LVM, LV diastolic dysfunction, and risk of cardiac arrhythmias in several clinical settings other than hypertension. In particular, Tp‐Te interval, defined as the time interval between complete epicardial and myocardial repolarization, has been viewed as a powerful and independent index of transmural dispersion of LV repolarization.19, 20 From a pathophysiological point of view, increased LVM is considered to be the main determinant of prolonged Tp‐Te interval.21 This has been related to increased risk of cardiac arrhythmias in several clinical conditions, including long‐QT and Brugada syndromes,22 as well as in the early stages of acute myocardial infarction23 and ventricular tachyarrhythmias.24 More recently, prolonged Tp‐Te interval has been associated with impaired LV relaxation and diastolic dysfunction in unselected outpatients with or without hypertension.25 The potential implication of this ECG marker in essential hypertension, however, remains to be defined.
On the basis of these considerations, the primary aim of this study is to evaluate the role of these new ECG indexes as markers of early cardiac abnormalities in outpatients with newly diagnosed, untreated essential hypertension compared with normotensive individuals.
Methods
Study Population
Adult outpatients were consecutively recruited among those admitted to the adult outpatient service of the hypertension unit at Sant'Andrea Hospital in Rome, Italy, for hypertension assessment (including home, clinic, and 24‐hour ambulatory BP measurements).
To be included in the study protocol, participants had to meet the following inclusion criteria: (1) adult individuals younger than 55 years; (2) recently diagnosed (naive), untreated hypertension; and (3) signature of informed consent for study participation. Exclusion criteria were at least one of the following: (1) history of treated hypertension; (2) any history of supraventricular or ventricular arrhythmia, including atrial fibrillation; (3) history of any previous cardiovascular disease, including coronary artery disease, congestive heart failure, severe valvular heart disease; (4) hyperthyroidism, electrolyte imbalance, chronic kidney dysfunction (estimated glomerular filtration rate <60 mL/min by the Cockroft‐Gault formula); and (5) any neurological or psychiatric disease that may at least, in part, affect the signature of informed consent.
Diagnosis of hypertension was made in the presence of clinic BP levels above the normal values (average of three BP measurements), performed according to the recommendations of the latest set of European guidelines.26 On the other hand, normotension was defined in the presence of clinic BP levels below the normal values (140/90 mm Hg).26
Once included in the study protocol, participants were classified into two groups, including hypertensive patients (cases) and otherwise healthy normotensive individuals (controls) on the basis of the presence or absence of hypertension, as defined by current European guidelines.3
The study conformed to the Declaration of Helsinki and its subsequent modifications. The confidentiality of the data of each patient included in the present study was carefully and strictly protected. Informed consent was obtained in all patients and the study was approved by the local ethical committee.
BP Measurements
Clinic BP measurements were performed in the hypertension clinic during the morning section (from 8 am to 10 am). Sequential BP measurements were performed in a quiet room, after 10 minutes of rest, on the same arm and with the participant in the sitting position, by using an automated oscillometric device (Omron 705 IT, Lake Forest, IL). The average of three consecutive BP measurements and heart rate was considered as clinic systolic/diastolic BP levels.
Ambulatory BP monitoring was performed by an oscillometric Spacelabs 90207 (Spacelabs Inc, Redmond, WA) device. The device was set in the hypertension clinic after completion of the clinic BP measurements, and the monitoring was started at about 9 am. Automatic BP readings were obtained every 15 minutes during the daytime period (from 6 am to 10 pm) and every 30 minutes during the nighttime period (from 10 pm to 6 am) over 24 hours. Each patient was instructed not to alter her/his usual 24‐hour schedule during the monitoring period, but was asked to avoid unusual physical activities and to maintain the arm still during BP measurements. Average values for the 24‐hour, daytime, and nighttime systolic and diastolic BP levels were reported. In addition, standard deviation from the average values, as well as number of BP measurements above the normal BP thresholds were reported for each time period (24‐hour, daytime, and nighttime) in each participant. Ambulatory BP monitoring examinations were included in the calculation of the 24‐hour, daytime, and nighttime average values, if there were at least two valid readings per hour for at least 21 hours.
ECG Analysis
All study patients had to be in sinus rhythm on the day of examination. A 12‐lead surface ECG was performed for all patients in the supine position using a Mortara Eli 350 ECG device (Milwaukee, WI). The 12‐lead ECG was recorded at a paper speed of 25 mm/s and 1 mV/cm standardization. All ECGs were scanned at 600 dpi and conventional and new ECG parameters were measured on a high‐resolution computer screen.
Conventional ECG parameters for LVH were defined according to standard criteria by using Sokolow‐Lyon, Cornell voltage, and Cornell product indexes, as recommended by current hypertension guidelines.3
In addition, the following novel ECG parameters were calculated for each patient included in the study: (1) Tpeak‐Tend (Tp‐Te) interval, defined as the distance between the peak and the end of the T wave and expressed as millimeters (mm);16 (2) VAT, defined as the time interval between the onset of the Q wave and the peak of the R wave (QR interval) and expressed as mm;17 (3) P‐wave analysis,18 including average P‐wave duration in each ECG lead, maximum P‐wave duration in any measurable leads (P maximum), minimum P‐wave duration in any measurable leads (P minimum), P‐wave dispersion (PWD), defined as the difference between the maximum P‐wave duration and the minimum P‐wave duration, and P‐wave area (PWA), defined as the product of the P‐wave amplitude per half of the duration in DII.
The onset of the P and T waves was defined as the point of the first visible upward departure of the trace from the bottom of the baseline for the positive waves and as the point of first downward departure from the top of the baseline for negative waves. The return to the baseline of the bottom of the trace in positive waves and of the top of the trace in negative waves were considered the end of the P and T waves, respectively. Duration of P and T waves was assessed by two investigators blinded to patient clinical information. All these parameters were calculated using Adobe Photoshop CS6 (average of three measurements; San Jose, CA). ECG with measurable P and T waves in fewer than nine of 12 ECG leads was excluded from the study.
Echocardiography
All participants underwent Doppler echocardiographic examination performed by an Acuson Sequoia C512 (Siemens Medical Solution, Mountain View, CA) with a multi‐frequency transducer (2.5–4 MHz). Images were implemented using standardized acquisition methods. LV dimensions were measured at end diastole and end systole, just below the mitral leaflets, through the standard left parasternal window. LV ejection fraction was calculated according to the Simpson method. Left atrial size was calculated as the anteroposterior diameter and measured as the distance from the leading edge of the posterior aortic wall to the leading edge of the posterior left atrial wall at end systole. LV mass (LVM) was calculated and then normalized by body surface area and height2.7. Echocardiographic LVH was defined according to standard criteria.
The following echocardiographic indexes of LV systolic function were considered: (A) Conventional Doppler analysis: (1) LV ejection fraction and (2) LV fractional shortening; (B) TDI analysis: (1) systolic myocardial peak flow velocity (Sm) wave amplitude; (2) isovolumetric contraction time; and (3) myocardial performance index. At the same time, the following indexes of diastolic function were considered: (A) Conventional Doppler analysis: (1) early diastolic peak flow velocity (E); (2) late diastolic peak flow velocity (A); and (3) ratio of early to late peak (E/A ratio); (B) TDI analysis of the lateral wall of the left ventricle: (1) early diastolic myocardial peak flow velocity (Em); (2) late diastolic myocardial peak flow velocity (Am); (3) ratio of early to late myocardial peak (Em/Am ratio); and (4) isovolumetric relaxation time. Finally, the ratio of early diastolic peak flow velocity (E) at conventional Doppler and early diastolic myocardial peak flow velocity (Em) at TDI was also considered a marker of diastolic dysfunction.
Statistical Analysis
All data were entered into Microsoft Access for Windows (Microsoft Office, Microsoft Corp, Redmond, WA). Baseline characteristics of patients are presented as number and percentage for dichotomous variables and mean±standard deviation of the mean for continuous variables. Normal distribution of data was assessed using histograms and Kolmogorov‐Smirnov test. All variables were normally distributed, with the exceptions of BP levels and LVM, which were log‐transformed. Differences between continuous variables were assessed using Student t test. Categorical variables were compared among groups by chi‐square test. To evaluate the association among clinical variables, hazard ratios (HRs) and 95% confidence intervals (CIs) were derived from logistic regression analysis. A multivariable model was fitted with baseline covariates associated with the primary endpoint at the <.05 significance level. All tests were two‐sided, and a P value of <.05 was considered statistically significant. All calculations were generated using SPSS version 15.0 (SPSS Inc, Chicago, IL).
Results
Study Population
From March to May 2014 we consecutively enrolled 50 young individuals who were referred to our hypertension unit for home, clinic, and 24‐hour ambulatory BP evaluation. On the basis of the presence or absence of hypertension, and according to inclusion and exclusion criteria, patients were classified into two groups, including 32 (64%) hypertensive patients and 18 (36%) otherwise normotensive individuals.
General characteristics of the study population are reported in Table 1. There were no differences between the groups with regard to anthropometric characteristics, lipid and glucose profile, and renal parameters, with the only exception of BMI, which was higher in hypertensive than in normotensive outpatients (27.0±2.6 vs 24.7±5.2; P=.043).
Table 1.
General Characteristics of the Study Population and Conventional ECG Parameters for Cardiac Organ Damage
| Parameters | Overall (N=50) | Normotensive (n=18) | Hypertensive (n=32) | P Value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 42.8±9.1 | 41.2±9.6 | 43.8±8.8 | .458 |
| Height, cm | 172.0±9.1 | 171.9±9.8 | 172.1±8.9 | .934 |
| Weight, kg | 78.0±16.6 | 73.8±21.0 | 80.3±13.3 | .072 |
| BMI, kg/m2 | 26.1±4.0 | 24.7±5.3 | 27.0±2.6 | .043 |
| TC, mg/dL | 220.3±56.8 | 224.5±53.9 | 220.3±56.8 | .562 |
| HDL‐C, mg/dL | 50.0±13.0 | 51.6±13.5 | 50.0±13.0 | .618 |
| LDL‐C, mg/dL | 138.6±28.5 | 136.9±28.4 | 138.6±28.5 | .745 |
| TG, mg/dL | 122.9±69.9 | 121.7±70.1 | 122.9±69.9 | .663 |
| Fasting glucose, mg/dL | 88.1±6.8 | 89.0±6.3 | 88.1±6.8 | .379 |
| BUN, mg/dL | 31.8±10.7 | 31.3±10.5 | 31.8±10.7 | .187 |
| Serum creatinine, mg/dL | 0.90±0.16 | 0.92±0.17 | 0.90±0.16 | .692 |
| BP profile | ||||
| Clinic systolic BP, mm Hg | 144.0±18.5 | 125.1±13.0 | 153.8±12.2 | <.001 |
| Clinic diastolic BP, mm Hg | 95.2±14.8 | 84.1±10.3 | 101.0±13.6 | <.001 |
| Heart rate, beats per min | 80.3±14.1 | 78.2±15.4 | 81.3±13.7 | .511 |
| 24‐h systolic BP, mm Hg | 133.7±14.7 | 120.0±14.9 | 140.8±8.4 | <.001 |
| 24‐h diastolic BP, mm Hg | 84.2±9.7 | 74.4±8.6 | 89.2±5.5 | <.001 |
| 24‐h heart rate, beats per min | 74.9±8.0 | 73.9±8.8 | 75.4±7.7 | .568 |
| Daytime systolic BP, mm Hg | 138.0±15.3 | 122.4±13.1 | 146.1±8.8 | <.001 |
| Daytime diastolic BP, mm Hg | 88.7±10.8 | 77.9±8.8 | 94.3±6.8 | <.001 |
| Daytime heart rate, beats per min | 78.0±8.4 | 76.1±8.2 | 78.9±8.5 | .306 |
| Nighttime systolic BP, mm Hg | 122.4±15.1 | 110.5±14.4 | 128.6±11.5 | <.001 |
| Nighttime diastolic BP, mm Hg | 74.0±9.5 | 66.4±8.8 | 77.9±7.3 | <.001 |
| Nighttime heart rate, beats per min | 67.3±9.2 | 67.0±9.0 | 67.4±9.5 | .890 |
| Conventional ECG parameters | ||||
| PR interval, ms | 152.6±20.1 | 149.1±20.6 | 154.4±19.9 | .389 |
| QRS duration, ms | 99.4±18.5 | 97.5±18.2 | 100.4±18.8 | .606 |
| QT interval, ms | 373.3±32.1 | 375.0±32.0 | 372.4±32.6 | .791 |
| QTc interval, ms | 397.7±24.7 | 396.9±21.9 | 398.2±26.4 | .866 |
| Sokolow‐Lyon index, mV | 24.8±6.9 | 26.6±7.6 | 23.8±6.3 | .178 |
| Cornell voltage index, mV | 15.3±6.4 | 13.9±5.6 | 16.1±6.7 | .248 |
| Cornell product index, mV × ms | 1553.6±755.9 | 1365.1±630.9 | 1657.0±807.3 | .204 |
| Positive Sokolow‐Lyon index, No. | 4 (8.3) | 3 (17.6) | 1 (3.2) | .084 |
| Positive Cornell voltage index, No. | 11 (22.9) | 4 (23.5) | 7 (22.6) | .940 |
| Positive Cornell product index, No. | 6 (12.5) | 1 (5.9) | 5 (16.1) | .305 |
Abbreviations: BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; ECG, electrocardiographic; HDL‐C, high‐density lipoprotein cholesterol; TC, total cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TG, triglycerides.
BP Profile
As expected, systolic and diastolic BP levels were significantly higher in hypertensive outpatients and normotensive individuals, both at clinic (153.8±12.2/101.0±13.6 mm Hg vs 125.1±13.0/84.1±10.3 mm Hg; P<.001) and 24‐hour ambulatory (140.8±8.4/89.2±5.5 mm Hg vs 120.0±14.9/74.4±8.6 mm Hg; P<.001) BP measurements. Also, daytime (146.1±8.8/94.3±6.8 mm Hg vs 122.4±13.1/77.9±8.8 mm Hg; P<.001) and nighttime (128.6±11.5/77.9±7.3 vs 110.5±14.4/66.4±8.8 mm Hg; P<.001) BP levels were significantly higher in the former than in the latter group. No significant differences were found with regard to clinic and 24‐hour ambulatory heart rate between the two study groups.
Conventional ECG Parameters
Conventional ECG parameters for cardiac OD are reported in Table 1. No significant differences were found between the two study groups with regard to general ECG parameters, including PR, QT, and QTc intervals, as well as QRS duration. At the same time, no significant differences were found with regard to conventional ECG indexes of cardiac OD in hypertensive outpatients compared with normotensive individuals, including Sokolow‐Lyon (26.6±7.6 mV vs 23.8±6.3; P=.178), Cornell voltage (16.1±6.7 vs 13.9±5.6 mV; P=.248), and Cornell product (1657.0±807.3 vs 1365.1±630.9 mV × ms; P=.204) indexes.
ECG criteria for LVH were met by three (15.8%) normotensive individuals and one (3.2%) hypertensive outpatient according to Sokolow‐Lyon criterion (P=.084), by four (21.0%) normotensive individuals and seven (22.6%) hypertensive outpatients according to Cornell voltage criterion (P=.940), and by one (5.2%) normotensive individual and five (16.1%) hypertensive outpatients according to Cornell product criterion (P=.305).
Novel ECG Parameters
New ECG parameters for cardiac OD are reported in Table 2. No significant differences were found between the two study groups with regard to VAT and P‐wave analysis (which includes P‐wave duration measured in all leads, maximum, minimum, and average duration, as well as dispersion, area, and amplitude). On the contrary, Tp‐Te was significantly higher in hypertensive outpatients compared with normotensive individuals, both as absolute values (2.9554±0.52002 mm vs 2.2234±0.32531 mm; P<.001) (Figure 1). In addition, receiver operating characteristic analysis revealed an area under the curve of 0.886 (range, 0.795–0.977) for log Tp‐Te interval, 0.528 (range, 0.357–0.698) for log VAT, and 0.494 (range, 0.318–0.670) for log P duration (Figure 2).
Table 2.
Novel ECG Parameters for Cardiac Organ Damage
| Parameters | Overall (N=50) | Normotensive (n=18) | Hypertensive (n=32) | P Value |
|---|---|---|---|---|
| Tp‐Te, mm | 2.7015±0.577 | 2.2234±0.32531 | 2.9554±0.52002 | <.001 |
| VAT, mm | 0.9930±0.1836 | 0.9786±.1973843 | 1.0008±0.178492 | .693 |
| P‐wave duration | ||||
| DI | 2.2123±0.5331 | 2.1368±0.6221 | 2.2525±0.4853 | .475 |
| DII | 2.6377±0.3987 | 2.5759±0.3839 | 2.6706±0.4085 | .435 |
| DIII | 2.1921±0.5995 | 2.3756±0.3977 | 2.0945±0.6683 | .119 |
| aVR | 2.4751±0.3936 | 2.3654±0.3652 | 2.5333±0.4012 | .157 |
| aVL | 1.7968±0.5116 | 1.9925±0.3987 | 1.6929±0.5395 | .050 |
| aVF | 2.4179±0.5493 | 2.3195±0.5618 | 2.4702±0.5442 | .336 |
| V1 | 2.0581±0.5057 | 2.0049±0.4399 | 2.0863±0.5419 | .597 |
| V2 | 1.8437±0.4073 | 1.7631±0.3924 | 1.8866±0.4147 | .317 |
| V3 | 2.1528±0.4752 | 2.0175±0.4226 | 2.2247±0.4921 | .148 |
| V4 | 2.4795±0.4540 | 2.3338±0.5178 | 2.5569±0.4036 | .102 |
| V5 | 2.5048±0.5067 | 2.4618±0.5272 | 2.5276±0.5026 | .670 |
| V6 | 2.5105±0.5149 | 2.4758±0.5549 | 2.5290±0.5006 | .734 |
| Maximum duration | 2.9699±0.4043 | 2.8653±0.3903 | 3.0254±0.4066 | .190 |
| Minimum duration | 1.3784±0.3249 | 1.4485±0.3374 | 1.3412±0.3172 | .276 |
| Average duration | 2.2285±0.2892 | 2.2332±0.2824 | 2.2260±0.2971 | .934 |
| P‐wave dispersion | 1.5027±0.4387 | 1.4126±0.4288 | 1.5505±0.4431 | .300 |
| P‐wave area | 2.0900±0.7746 | 2.1524±0.8706 | 2.0568±0.7311 | .685 |
| P‐wave amplitude (DIII) | 1.5652±0.5401 | 1.6455±0.6326 | 1.5226±0.4896 | .454 |
Abbreviations: ECG, electrocardographic; VAT, ventricular activation time.
Figure 1.
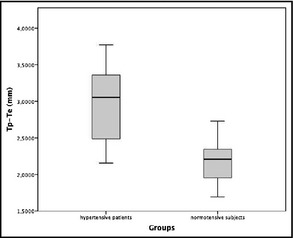
Box plot reporting Tp‐Te intervals in hypertensive patients (group A) and normotensive individuals (group B).
Figure 2.
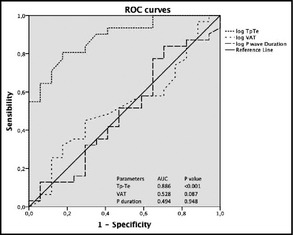
Receiver operating characteristics (ROC) curves illustrating ability of Tp‐Te interval, ventricular activation time (VAT), and average P duration to predict presence of hypertension.
Echocardiographic Parameters
Echocardiographic parameters for cardiac OD are reported in Table 3. Absolute LVM (158.7±41.3 vs 130.9±35.9 g; P=.009), as well as LVM indexed by body surface area (81.9±18.0 vs 69.5±12.8 g/m2; P=.003), by height (91.4±21.3 vs 75.5±18.3 g/cm; P=.005), and by height2.7 (36.6±7.8 vs 29.7±6.1 g/cm2.7; P=.001) were significantly higher in hypertensive outpatients compared with normotensive individuals. Of note, although these parameters were significantly different between the two groups, they were all within the normal thresholds. In particular, in the hypertensive group, seven (22.6%) patients showed concentric remodeling and 24 (77.4%) showed normal LV geometry.
Table 3.
Echocardiographic Parameters of the Study Population
| Parameters | Overall (N=50) | Normotensive (n=18) | Hypertensive (n=32) | P Value |
|---|---|---|---|---|
| LV mass, g | 148.2±41.3 | 130.9±35.9 | 158.7±41.3 | .009 |
| LV mass/BSA, g/m2 | 77.2±17.2 | 69.5±12.8 | 81.9±18.0 | .003 |
| LV mass/height, g/cm | 85.4±21.5 | 75.5±18.3 | 91.4±21.3 | .005 |
| LV mass/height2.7, g/cm2.7 | 34.0±7.9 | 29.7±6.1 | 36.6±7.8 | .001 |
| Systolic parameters | ||||
| LVEF, % | 69.5±7.1 | 70.7±5.6 | 68.8±7.8 | .220 |
| LVFS, % | 40.0±5.7 | 40.6±4.5 | 39.7±6.4 | .408 |
| LV Sm wave | 0.148±0.041 | 0.152±0.044 | 0.145±0.039 | .658 |
| Conventional diastolic parameters | ||||
| E wave | 74.4±16.7 | 71.9±13.3 | 76.2±19.0 | .430 |
| A wave | 63.9±13.7 | 58.4±9.4 | 67.9±15.1 | .026 |
| E/A ratio | 1.4±1.5 | 1.8±2.3 | 1.2±0.5 | .330 |
| DT, ms | 209.3±56.3 | 194.9±34.5 | 219.5±66.8 | .160 |
| Left atrium area, cm2 | 16.6±3.6 | 16.5±4.3 | 16.6±3.1 | .095 |
| TDI diastolic parameters | ||||
| LV Em wave | 0.185±0.049 | 0.195±0.058 | 0.177±0.041 | .311 |
| LV Am wave | 0.162±0.051 | 0.144±0.036 | 0.176±0.056 | .045 |
| LV Em/Am ratio | 1.223±0.390 | 1.420±0.406 | 1.082±0.317 | .013 |
| LV E/Em ratio | 4.17±0.96 | 3.81±1.06 | 4.40±0.84 | .149 |
Abbreviations: BSA, body surface area; DT, deceleration time; LV, left ventricular; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening.
No significant differences were found between the two study groups with regard to indexes of LV systolic function, including LV ejection fraction, fractional shortening, and Sm wave amplitude at TDI analysis.
With regard to indexes of LV diastolic dysfunction, no significant differences were found between the two groups, with the only exception of A‐wave amplitude, which was slightly but significantly higher in hypertensive outpatients compared with normotensive individuals both at conventional Doppler (68±15 vs 58±9; P=.026) and TDI (0.176±0.056 vs 0.144±0.036; P=.045) analyses.
Correlations and Multivariate Analysis
Increased Tp‐Te interval was significantly related to both clinic (r=0.380; P=.012) and 24‐hour (r=0.448; P=.03) BP levels. In addition, significant positive correlations were found between Tp‐Te interval and LVM (r=0.313; P=.29) and LVM indexed by height2.7 (r=0.345; P=.015). However, Tp‐Te interval did not show any significant correlation with BMI (r=0.251; P=.082), as well as with different parameters of LV diastolic dysfunction, including E/A ratio (r=0.068; P=.694), Em/Am ratio (r=−0.265; P=.119), and E/Em ratio (r=0.136; P=.428). Finally, when the Cox regression model was fitted with all covariates predictive of hypertension at the 0.1 significance level at univariate analysis, several parameters, including Tp‐Te and BMI, LVM indexed by height2.7 and Em/Am ratio independently predicted the presence of hypertension, with Tp‐Te interval emerging as the only predictor of hypertension at multivariate analysis (Table 4).
Table 4.
Univariate and Multivariate Analyses
| Variable | Univariate Analysis (95% CI) | P Value | Multivariate Analysis (95% CI) | P Value |
|---|---|---|---|---|
| Male sex (categorical) | 0.529 (0.164–1.711) | .288 | – | |
| Age | 1.034 (0.970–1.101) | .308 | – | |
| BMI | 1.204 (1.001–1.447) | .049 | 2.059 (0.867–4.889) | .102 |
| Heart rate | 1.021 (0.972–1.071) | .407 | – | |
| QRS | 1.015 (0.979–1.053) | .412 | – | |
| QT | 1.003 (0.985–1.021) | .748 | – | |
| QTc | 1.003 (0.980–1.027) | .773 | – | |
| Tp‐Tea | 1.497 (1.176–1.905) | .001 | 1.835 (1.064–3.167) | .029 |
| VATb | 1.070 (0.770–1.488) | .686 | – | |
| Average P duration | 1.185 (0.156–8.979) | .869 | – | |
| LV mass indexed 2.7 | 1.160 (1.042–1.292) | .007 | 1.366 (0.951–1.964) | .092 |
| E/A ratio | 0.490 (0.111–2.156) | .345 | – | |
| Em/Am ratio | 0.052 (0.004–0.628) | .020 | 0.064 (0.000–11–942) | .303 |
Abbreviations: BMI, body mass index; LV, left ventricular. aHazard ratio is expressed as relative risk for each 0.1‐mm increase of Tp‐Te interval. bHazard ratio is expressed as relative risk for each 0.1‐mm increase of ventricular activation time (VAT).
Discussion
First, our study demonstrated that recently diagnosed, untreated hypertensive outpatients had higher values of Tp‐Te interval than those observed in normotensive individuals. This was associated with significantly higher, although normal, values of LVM and diastolic function in untreated hypertensive outpatients compared with normotensive individuals. These findings may be of potential clinical relevance on the basis of the following considerations.
ECG assessment of cardiac OD has been recently reaffirmed by the most recent sets of European guidelines26 as a fundamental step in both diagnostic and therapeutic processes during the clinical course of the very complex, although asymptomatic disease, that is hypertension. Several characteristics of this technique has prompted its first‐line application in order to identify those hypertensive patients with LV remodeling or dysfunction. Among these, the large diffusion in almost all clinical settings, the relatively low cost of the procedure in various countries, the objective (semiautomatic) interpretation of the data needed for the diagnostic criteria, and the high sensitivity, even in the presence of its relatively low specificity, have substantially contributed to its predominant positioning compared with echocardiographic assessment of cardiac OD. ECG detection of LVH has, in fact, been demonstrated to be independently correlated to an increased risk of major cardiovascular events in hypertensive patients with different cardiovascular risk profiles.27 Nonetheless, ECG regression of LVH under pharmacologic treatment has been demonstrated to confer a significant reduction of such increased risk of major cardiovascular complications.28 In fact, evidence from large, randomized clinical trials have, indeed, has demonstrated the beneficial effects of LVH regression in terms of reduced incidence of major cardiovascular complications in hypertension, mostly stroke.29, 30, 31, 32 As such, ECG detection, as well as regression of cardiac OD, namely LVH, can be viewed as an intermediate endpoint that may help physicians during the long‐term clinical management of hypertension.33, 34
Over the past years, several novel ECG criteria for LVH have been tested for the diagnostic workup of hypertensive outpatients in order to try to overcome some intrinsic limitations of this approach and to ameliorate its relatively low specificity.35 The applicability of these new diagnostic criteria, however, was at least, in part, limited to frankly hypertensive populations (ie, patients with established diagnosis of hypertension under pharmacologic treatment), and it has not been tested in untreated, recently diagnosed hypertensive patients.
More recently, additional ECG indexes, including VAT, P‐wave analysis, and Tp‐Te interval, have been proposed for improving ECG detection of LVH and LV dysfunction. In particular, available evidence has demonstrated significant, positive, and independent correlations between increased LVM and prolonged Tp‐Te interval, which has been viewed as an index of impaired transmural dispersion of LV repolarization.19, 20, 21 These findings, however, have been obtained in various clinical conditions other than hypertension,19, 20, 21 a condition in which both LVH and LV dysfunction are extremely frequent and independently related to worse prognosis.
At the same time, clinical studies specifically designed for hypertensive populations have reported significant correlations between increased LVM and prolonged LV repolarization, mostly defined as QTc interval,36, 37, 38 without addressing the potential role of new ECG indexes of cardiac OD. The results of these studies, however, are similar to those reported in our analysis. Indeed, they demonstrated that prolonged LV repolarization throughout the 24‐hour period was significantly related to nondipping status and increased LVM, which may lead to prolongation of QTc, potentially facilitating ventricular arrhythmias in nondipper hypertensive patients compared with dipper hypertensive or normotensive individuals.
On the basis of these considerations, the main findings of our analysis demonstrated for the first time that prolonged Tp‐Te interval was associated with both increased LVM and BP levels in a relatively small sample of newly diagnosed, untreated hypertensive outpatients compared with normotensive individuals. Although preliminary, these results may have potential clinical relevance, since they suggest the use of a new, easy and low‐cost diagnostic tool that may improve global cardiovascular risk stratification and proper assessment of cardiac impairment in the early, asymptomatic stages of hypertension, in which conventional markers of cardiac OD may not help physicians in the clinical decision process.
Potential Limitations
Our study has some limitations that should be acknowledged. First of all, the relatively small sample size may limit the applicability of Tp‐Te measurement in a setting of clinical practice. The design of the study did not allow us to speculate on potential prognostic and therapeutic implications of Tp‐Te in the long‐term clinical management of hypertensive outpatients. In addition, the data on the reproducibility of these ECG parameters over time are still lacking and should be tested before considering them for routine ECG testing. The cutoff age has been arbitrarily chosen according to the median age of the outpatient population referred to our hypertension unit. Finally, the need for additional software to calculate these novel ECG parameters may also limit the applicability of these indexes in a setting of daily clinical practice. Larger and more extended studies in hypertensive outpatients are needed to better clarify the potential clinical usefulness and prognostic value of these new indexes parameters.
Conclusions
Preliminary results of our study demonstrated that Tp‐Te interval may be considered an early marker of cardiac abnormalities at 12‐lead ECG. Prolonged Tp‐Te interval was also related to an increased independent risk of having hypertension, even after adjusting for anthropometric and clinical parameters. Further studies are required to confirm our findings in patients with different degrees of hypertension and LV geometries, and to better clarify the potential role of this parameter in the diagnostic workup of hypertension and global cardiovascular risk stratification.
Disclosure
The authors report no specific funding in relation to this research and have no conflicts of interest to disclose.
J Clin Hypertens (Greenwich). 2015;17:441–449. DOI: 10.1111/jch.12522. © 2015 Wiley Periodicals, Inc.
References
- 1. Volpe M, Battistoni A, Tocci G, et al. Cardiovascular risk assessment beyond systemic coronary risk estimation: a role for organ damage markers. J Hypertens. 2012;30:1056–1064. [DOI] [PubMed] [Google Scholar]
- 2. Volpe M, Tocci G. 2007 ESH/ESC Guidelines for the management of hypertension, from theory to practice: global cardiovascular risk concept. J Hypertens. 2009;27(suppl 3):S3–S11. [DOI] [PubMed] [Google Scholar]
- 3. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 4. Volpe M, Alderman MH, Furberg CD, et al. Beyond hypertension toward guidelines for cardiovascular risk reduction. Am J Hypertens. 2004;17:1068–1074. [DOI] [PubMed] [Google Scholar]
- 5. Alderman MH, Furberg CD, Kostis JB, et al. Hypertension guidelines: criteria that might make them more clinically useful. Am J Hypertens. 2002;15:917–923. [DOI] [PubMed] [Google Scholar]
- 6. Lonn E, Mathew J, Pogue J, et al. Relationship of electrocardiographic left ventricular hypertrophy to mortality and cardiovascular morbidity in high‐risk patients. Eur J Cardiovasc Prev Rehabil. 2003;10:420–428. [DOI] [PubMed] [Google Scholar]
- 7. Bots ML, Nikitin Y, Salonen JT, et al. Left ventricular hypertrophy and risk of fatal and non‐fatal stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health. 2002;56(suppl 1):i8–i13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verdecchia P, Porcellati C, Reboldi G, et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation. 2001;104:2039–2044. [DOI] [PubMed] [Google Scholar]
- 9. Moser M, Hebert PR. Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J Am Coll Cardiol. 1996;27:1214–1218. [DOI] [PubMed] [Google Scholar]
- 10. Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med. 1970;72:813–822. [DOI] [PubMed] [Google Scholar]
- 11. Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. [DOI] [PubMed] [Google Scholar]
- 12. Levy D, Labib SB, Anderson KM, et al. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–820. [DOI] [PubMed] [Google Scholar]
- 13. Devereux RB, Bella J, Boman K, et al. Echocardiographic left ventricular geometry in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE Study. Blood Press. 2001;10:74–82. [DOI] [PubMed] [Google Scholar]
- 14. Devereux RB, Casale PN, Hammond IW, et al. Echocardiographic detection of pressure‐overload left ventricular hypertrophy: effect of criteria and patient population. J Clin Hypertens (Greenwich). 1987;3:66–78. [PubMed] [Google Scholar]
- 15. Gosse P, Jan E, Coulon P, et al. ECG detection of left ventricular hypertrophy: the simpler, the better? J Hypertens. 2012;30:990–996. [DOI] [PubMed] [Google Scholar]
- 16. Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp‐Te interval and its diagnostic value. J Electrocardiol. 2008;41:575–580. [DOI] [PubMed] [Google Scholar]
- 17. Boles U, Almuntaser I, Brown A, et al. Ventricular activation time as a marker for diastolic dysfunction in early hypertension. Am J Hypertens. 2010;23:781–785. [DOI] [PubMed] [Google Scholar]
- 18. Tsai WC, Lee KT, Wu MT, et al. Significant correlation of P‐wave parameters with left atrial volume index and left ventricular diastolic function. Am J Med Sci. 2013;346:45–51. [DOI] [PubMed] [Google Scholar]
- 19. Antzelevitch C. T peak‐Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest. 2001;31:555–557. [DOI] [PubMed] [Google Scholar]
- 20. Antzelevitch C, Sicouri S, Di Diego JM, et al. Does Tpeak‐Tend provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4:1114–1116; author reply 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolk R, Mazurek T, Lusawa T, et al. Left ventricular hypertrophy increases transepicardial dispersion of repolarisation in hypertensive patients: a differential effect on QTpeak and QTend dispersion. Eur J Clin Invest. 2001;31:563–569. [DOI] [PubMed] [Google Scholar]
- 22. Castro Hevia J, Antzelevitch C, Tornes Barzaga F, et al. Tpeak‐Tend and Tpeak‐Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao X, Xie Z, Chu Y, et al. Association between Tp‐e/QT ratio and prognosis in patients undergoing primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction. Clin Cardiol. 2012;35:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolk R, Stec S, Kulakowski P. Extrasystolic beats affect transmural electrical dispersion during programmed electrical stimulation. Eur J Clin Invest. 2001;31:293–301. [DOI] [PubMed] [Google Scholar]
- 25. Sauer A, Wilcox JE, Andrei AC, et al. Diastolic electromechanical coupling: association of the ECG T‐peak to T‐end interval with echocardiographic markers of diastolic dysfunction. Circ Arrhythm Electrophysiol. 2012;5:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 27. Gosse P, Cremer A, Vircoulon M, et al. Prognostic value of the extent of left ventricular hypertrophy and its evolution in the hypertensive patient. J Hypertens. 2012;30:2403–2409. [DOI] [PubMed] [Google Scholar]
- 28. Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A meta‐analysis of randomized double‐blind studies. JAMA. 1996;275:1507–1513. [PubMed] [Google Scholar]
- 29. Verdecchia P, Sleight P, Mancia G, et al. Effects of telmisartan, ramipril, and their combination on left ventricular hypertrophy in individuals at high vascular risk in the Ongoing Telmisartan Alone and in Combination with Ramipril Global End Point Trial and the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease. Circulation. 2009;120:1380–1389. [DOI] [PubMed] [Google Scholar]
- 30. Wachtell K, Okin PM, Olsen MH, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation. 2007;116:700–705. [DOI] [PubMed] [Google Scholar]
- 31. Okin PM, Devereux RB, Harris KE, et al. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med. 2007;147:311–319. [DOI] [PubMed] [Google Scholar]
- 32. Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new‐onset atrial fibrillation in patients with hypertension. JAMA. 2006;296:1242–1248. [DOI] [PubMed] [Google Scholar]
- 33. Volpe M, Tocci G, Pagannone E. Fewer mega‐trials and more clinically oriented studies in hypertension research? The case of blocking the renin‐angiotensin‐aldosterone system. J Am Soc Nephrol. 2006;17:S36–S43. [DOI] [PubMed] [Google Scholar]
- 34. Okin PM, Devereux RB, Liu JE, et al. Regression of electrocardiographic left ventricular hypertrophy predicts regression of echocardiographic left ventricular mass: the LIFE study. J Hum Hypertens. 2004;18:403–409. [DOI] [PubMed] [Google Scholar]
- 35. Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy in essential hypertension. J Am Coll Cardiol. 1998;31:383–390. [DOI] [PubMed] [Google Scholar]
- 36. Passino C, Magagna A, Conforti F, et al. Ventricular repolarization is prolonged in nondipper hypertensive patients: role of left ventricular hypertrophy and autonomic dysfunction. J Hypertens. 2003;21:445–451. [DOI] [PubMed] [Google Scholar]
- 37. Angeli F, Angeli E, Cavallini C, et al. Electrocardiographic abnormalities of left ventricular repolarization: prognostic implications in hypertensive post‐menopausal women. Maturitas. 2010;67:159–165. [DOI] [PubMed] [Google Scholar]
- 38. Passino C, Franzoni F, Gabutti A, et al. Abnormal ventricular repolarization in hypertensive patients: role of sympatho‐vagal imbalance and left ventricular hypertrophy. Int J Cardiol. 2004;97:57–62. [DOI] [PubMed] [Google Scholar]


