Abstract
Pulse pressure (PP) is an independent risk factor for cardiovascular (CV) disease and death but few studies have investigated the effect of antihypertensive treatments in relation to PP levels before treatment. The Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) trial showed that the combination of benazepril+amlodipine (B+A) is superior to benazepril+hydrochlorothiazide (B+H) in reducing CV events. We aimed to investigate whether the treatment effects in the ACCOMPLISH trial were dependent on baseline PP. High‐risk hypertensive patients (n=11,499) were randomized to double‐blinded treatment with single‐pill combinations of either B+A or B+H and followed for 36 months. Patients were divided into tertiles according to their baseline PP and events (CV mortality/myocardial infarction or stroke) were compared. Hazard ratios (HRs) for the treatment effect (B+A over B+H) were calculated in a Cox regression model with age, coronary artery disease, and diabetes mellitus as covariates and were compared across the tertiles. The event rate was increased in the high tertile of PP compared with the low tertile (7.2% vs 4.4% P<.01). In the high and medium PP tertiles, HRs were 0.75 (95% confidence interval [CI], 0.60–0.95; P=.018) and 0.74 (CI, 0.56–0.98, P=.034), respectively, in favor of B+A. There was no significant difference between the treatments in the low tertile and no significant differences in treatment effect when comparing the HRs between tertiles of PP. B+A has superior CV protection over B+H in high‐risk hypertensive patients independent of baseline PP although the absolute treatment effect is enhanced in the higher tertiles of PP where event rates are higher.
Hypertension has been identified as the most important global risk factor for premature death. It causes 45% of deaths caused by heart disease and 51% of deaths caused by stroke.1 Rapsomaniki and colleagues recently highlighted the importance of blood pressure (BP) for various manifestations of cardiovascular (CV) disease in 1.25 million patients. Diastolic and systolic pressure associations were not concordant, and pulse pressure (PP), rather than systolic BP (SBP), was associated with some CV diseases.2 The Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) trial investigated antihypertensive combination treatment with benazepril+amlodipine (B+A) or benazepril+hydrochlorothiazide (B+H) on CV outcomes in patients with systolic hypertension and with widely varying PPs.3, 4 The overall study result was significantly lower for CV outcomes in the patients randomized to B+A compared with B+H despite no differences in the achieved BP between groups.
PP is an indicator of arterial stiffness and is related to an increased risk for CV disease.5, 6, 7, 8, 9, 10, 11 Results from the Framingham Heart Study show that for a given SBP, coronary heart disease rates increase with lower diastolic BP (DBP) values (ie, increasing PP). Although this has been known for a long time, very little is addressed in guidelines and treatment recommendations about how to handle PP information in risk assessment. PP may be better for risk prediction than SBP in high‐risk populations such as the elderly. Different treatments for hypertension may affect PPs differently, which might have therapeutic implications. Despite this, very few studies have investigated the effect of different antihypertensive treatments in relation to PP12 and current guidelines lack recommendations on this subject.13, 14 The aim of this retrospective analysis of the ACCOMPLISH trial was to investigate whether the superiority of the combination treatment B+A over B+H on a combined primary endpoint derived from the ACCOMPLISH trial was dependent on baseline PP. Secondary aims were to study whether the superiority of the combination treatment B+A over B+H on total stroke and total myocardial infarction (MI) was separately dependent on baseline PP.
Material and Methods
The complete design of the ACCOMPLISH study has been published previously.4 In brief, the ACCOMPLISH trial was a randomized, double‐blinded, multicenter trial (a total of 548 centers in the United States, Sweden, Norway, Denmark, and Finland) that compared the effect of B+A and B+H in preventing a composite of fatal and nonfatal CV outcomes. Participants included in the trial were 55 years and older with either SBP ≥160 mm Hg or currently receiving antihypertensive therapy. Included patients had evidence of CV and or renal disease or other target organ damage or diabetes.
Endpoints
The primary endpoint in the current subanalysis of the ACCOMPLISH trial was a combined endpoint of CV morbidity and/or mortality. CV morbidity was defined as nonfatal acute MI or nonfatal stroke. CV mortality was defined as death caused by sudden cardiac death, fatal MI, fatal stroke, death caused by coronary intervention, or death caused by congestive heart failure or other CV causes. This is the same as the overall primary endpoint in ACCOMPLISH (time to first event for CV death or CV event) except for the removal of the following (in CV events): hospitalization for unstable angina, coronary revascularization, or resuscitation after sudden cardiac arrest.
Secondary endpoints in the current subanalysis consisted of MI (nonfatal and fatal) and stroke (nonfatal and fatal). An endpoint committee adjudicated all endpoints according to standard criteria. The members of the endpoint committee were unaware of the study group assignments and were not active investigators or staff of the sponsor, Novartis Pharmaceuticals.
BP Measurement
BP was measured according to the 1988 American Heart Association committee report on BP determination15 using a calibrated standard sphygmomanometer or a calibrated digital device and an appropriately sized cuff. BP was measured three times at each study visit at 1‐ to 2‐minute intervals after the patient had remained in a seated position for 5 minutes and was recorded as the average of the three measurements.
There was no formal washout period for patients with ongoing antihypertensive treatment. Patients already taking treatment for hypertension were to discontinue ongoing treatment after visit 1, resulting in a 2‐week period of no antihypertensive treatment until switching to the blinded study drugs after randomization.
Statistical Analysis
In our current subanalysis, patients were divided into PP tertiles (high, medium, and low) based on their baseline PP. Normally distributed data were presented as mean±standard deviation in the three tertiles. First, hazard ratios (HRs) with 95% confidence intervals (CIs) for the primary and secondary endpoints for each of the tertiles (high vs low, high vs medium, and medium vs low) were calculated pooling the two treatment groups using a Cox regression model that included age, coronary artery disease (yes/no), and diabetes mellitus (yes/no) as covariates. Second, HRs with 95% CIs for the primary and secondary endpoints for treatment effect (B+A over B+H) were calculated in all PP tertiles using the same Cox regression model. Finally, HRs for treatment effects were compared among all PP tertiles. SAS version 9.3 (SAS Institute Inc, Cary, NC) was used for statistical analysis. A P value <.05 was considered significant.
Results
The ACCOMPLISH trial was terminated early when the limit of the prespecified stopping criterion was reached after a mean study duration of 35.7 months. There was a highly significant treatment effect in favor of the B+A combination, which has been described elsewhere.3 Of the 13,782 screened patients, 11,499 underwent randomization (5741 to B+A and 5758 to B+H) in ACCOMPLISH and were included in this subanalysis. Baseline characteristics between randomly assigned study patients in the two treatment arms were similar and are presented in Table 1 in relation to tertiles of PP. The mean age of patients in the ACCOMPLISH trial was 68.4 years, and 39.5% were women. Laboratory data of the study patients are presented in Table 2.
Table 1.
Baseline Characteristics of the Study Patients According to Tertiles of Pulse Pressure and Treatment
| Low Tertile (Mean PP=50.3 mm Hg) (<58 mm Hg) | Medium Tertile (Mean PP=63.9 mm Hg) (58–70.7 mm Hg) | High Tertile (Mean PP=82.2 mm Hg) (≥70.7 mm Hg) | ||||
|---|---|---|---|---|---|---|
| B+A (n=1888) | B+H (n=1881) | B+A (n=1924) | B+H (n=1887) | B+A (n=1929) | B+H (n=1990) | |
| Sex (male/female) | 1213/675 | 1247/634 | 1175/749 | 1157/730 | 1059/870 | 1109/881 |
| Mean age (y) | 66.9 (6.49) | 66.4 (6.36) | 68.4 (6.70) | 68.4 (6.74) | 70.0 (7.02) | 70.0 (6.97) |
| BMI (kg/m2) | 31.34 (6.09) | 31.56 (6.31) | 31.13 (6.30) | 30.89 (6.20) | 30.39 (6.24) | 30.36 (6.11) |
| Antihypertensive treatment at start (yes) | 1886 | 1874 | 1896 | 1857 | 1834 | 1914 |
| SBP (mm Hg) | 129.7 (11.9) | 129.7 (11.4) | 144.0 (11.2) | 144.1 (11.2) | 161.9 (15.1) | 161.4 (14.7) |
| DBP (mm Hg) | 80.3 (10.2) | 80.6 (9.8) | 80.1 (10.6) | 80.3 (10.6) | 79.9 (11.6) | 79.0 (14.7) |
| Heart rate (bpm) | 71.4 (10.8) | 71.4 (10.7) | 71.0 (10.9) | 70.5 (10.9) | 69.0 (10.8) | 69.1 (11.5) |
| History of CV disease | ||||||
| MI (yes) | 452 (23.9) | 487 (25.9) | 459 (23.9) | 450 (23.8) | 426 (22.1) | 435 (21.9) |
| Unstable angina (yes) | 241 (12.8) | 235 (12.5) | 201 (10.4) | 221 (11.7) | 210 (10.9) | 215 (10.8) |
| CABG (yes) | 394 (20.9) | 374 (19.9) | 441 (22.9) | 393 (20.8) | 412 (21.4) | 430 (21.6) |
| PCI (yes) | 424 (22.5) | 436 (23.2) | 333 (17.3) | 340 (18.0) | 296 (15.3) | 346 (17.4) |
| History of stroke (yes) | 254 (13.5) | 257 (13.7) | 249 (12.9) | 243 (12.9) | 258 (13.6) | 236 (11.9) |
| Diabetes mellitus (yes) | 1099 (58.2) | 1083 (57.6) | 1198 (62.3) | 1121 (59.4) | 1180 (61.2) | 1262 (63.4) |
| Other risk factors | ||||||
| Current smoking (yes) | 216 (11.4) | 230 (12.2) | 228 (11.9) | 226 (12.0) | 197 (10.2) | 202 (10.2) |
| Atrial fibrillation (yes) | 137 (7.3) | 128 (6.8) | 110 (5.7) | 135 (7.2) | 129 (6.7) | 139 (7.0) |
| LVH by ECG (yes) | 180 (9.5) | 152 (8.1) | 228 (11.9) | 231 (12.2) | 354 (18.4) | 374 (18.8) |
Abbreviations: B+A, benazepril+amlodipine; B+H, benazepril+hydrochlorothiazide; BMI, body mass index; CABG, coronary angioplastic bypass surgery; CV, cardiovascular; DBP, diastolic blood pressure; ECG, electrocardiogram; LVH, left ventricular hypertrophy; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; Unstable angina, hospitalization for unstable angina. Data are number of patients, (%) or mean (SD) were appropriate if nothing else is stated. Missing data in each subgroup varied from 0 to 4.
Table 2.
Laboratory Data in Relation to Tertiles of PP and Treatment
| Low Tertile (Mean PP=50.3 mm Hg) (<58 mm Hg) | Medium Tertile (Mean PP=63.9 mm Hg) (58–70.7 mm Hg) | High Tertile (Mean PP=82.2 mm Hg) (≥70.7 mm Hg) | ||||
|---|---|---|---|---|---|---|
| B+A (n=1888) | B+H (n=1881) | B+A (n=1924) | B+H (n=1887) | B+A (n=1929) | B+H (n=1990) | |
| Serum glucose, mg/dL | 125.3 (48.2) | 123.9 (44.0) | 128.2 (45.0) | 126.7 (44.4) | 130.0 (48.8) | 130.1 (48.6) |
| Total cholesterol, mg/dL | 182.9 (41.2) | 180.3 (37.4) | 184.4 (39.7) | 183.9 (39.2) | 187.5 (40.5) | 187.9 (40.8) |
| HDL cholesterol, mg/dL | 48.9 (14.0) | 48.1 (13.3) | 49.5 (13.8) | 49.4 (14.2) | 50.4 (14.5) | 51.0 (14.6) |
| hsCRP, mg/L | 0.459 (1.0) | 0.447 (0.7) | 0.466 (1.0) | 0.452 (0.8) | 0.450 (0.9) | 0.457 (0.8) |
| eGFR, mL/min/1.73 m2 | 79.5 (20.8) | 81.1 (21.6) | 79.7 (21.5) | 79.1 (21.3) | 77.5 (21.1) | 77.2 (21.5) |
| GFR (MDRD), % | ||||||
| <60 | 314 (16.6) | 292 (15.5) | 350 (18.2) | 338 (17.9) | 381 (19.8) | 398 (20.0) |
| 60–90 | 1046 (55.4) | 1009 (53.6) | 1031 (53.6) | 1042 (55.2) | 1074 (55.7) | 1136 (57.1) |
| >90 | 524 (27.8) | 576 (30.6) | 538 (28.0) | 503 (26.7) | 471 (24.4) | 453 (22.8) |
Abbreviations: B+A, benazepril+amlodipine; B+H, benazepril+hydrochlorothiazide; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; GFR (MDRD), estimated glomerular filtration rate calculated according to Modification of Diet in Renal Disease; PP, pulse pressure. Data are expressed as number of patients (percentage) or mean (standard deviation) unless otherwise indicated. Missing data in each subgroup varied from 0 to 4.
The mean PP in the whole study group was 65.2 mm Hg. The mean PPs in the different tertiles of PP were 50.3 mm Hg, 63.9 mm Hg, and 82.2 mm Hg, respectively. Of randomized patients, most (97.2%) were taking antihypertensive treatment before the trial, although only 37.3% had a normal BP level at baseline. After 6 months in the trial, when titration of antihypertensive treatment was complete, mean doses in the B+A group were 36.3 mg benazepril and 7.7 mg amlodipine and 1662 (29%) patients received additional agents. In the B+H group, mean doses were 36.1 mg of benazepril and 19.3 mg of hydrochlorothiazide and 1636 (29%) patients received additional antihypertensive agents. At the end of the trial, outcome data were unavailable in 143 participants: 5 withdrew their consent, 21 were from sites affected by natural disaster that forced them to end their activity, and 117 (1.0%) were lost to follow‐up. The results are based on an intention‐to‐treat design.
Comparisons between tertiles of PP, pooling the two treatment groups, showed an increased incidence of the primary endpoint (CV mortality/nonfatal MI/nonfatal stroke) in the high tertile compared with the low tertile and in the high tertile compared with the medium tertile of PP (P<.01) (Table 3). For the secondary endpoint (all MI), a similar association was observed. No significant association was observed between PP and the incidence of stroke.
Table 3.
Number of Events According to Tertiles of Pulse Pressure and Between‐Tertile Hazard Ratios
| High vs Low | Medium vs Low | High vs Medium | |
|---|---|---|---|
| CV mortality/nonfatal MI/nonfatal stroke |
284 (7.2) vs 164 (4.4) 1.48 (1.22–1.80)a |
204 (5.4) vs 164 (4.4) 1.16 (0.94–1.42) |
284 (7.2) vs 204 (5.4) 1.28 (1.07–1.54)a |
| All MI |
136 (3.5) vs 64 (1.7) 2.01 (1.48–2.73)a |
84 (2.2) vs 64 (1.7) 1.29 (0.93–1.79) |
136 (3.5) vs 84 (2.2) 1.56 (1.19–2.05)a |
| All stroke |
104 (2.7) vs 66 (1.8) 1.22 (0.89–1.78) |
75 (2.0) vs 66 (1.8) 1.00 (0.72–1.40) |
104 (2.7) vs 75 (2.0) 1.22 (0.91–1.65) |
Abbreviations: CV, cardiovascular; MI, myocardial infarction. Data are expressed as number of patients with events (percentage) and hazard ratio (95% confidence interval). a P<.01.
Secondly, HRs for B+A over B+H were calculated in the three tertiles of PP in a Cox regression model adjusted for age, diabetes mellitus, and previous MI. The HRs for the primary endpoint for B+A over B+H were significant in the high and medium tertiles of PP (Table 4). There were, however, no significant differences between tertiles of PP when comparing HRs: high PP vs low PP (P=.34), medium PP vs low PP (P=.33), high PP vs medium PP (P=.93), and overall among tertiles (P=.56) (Table 4).
Table 4.
Between‐Treatment HRs Across PP Tertiles for the Primary Endpoint Cardiovascular Mortality/Nonfatal Myocardial Infarction/Nonfatal Stroke
| Baseline PP Tertiles | CV Events/No. (B+A) | CV Events/No. (B+H) | HR | 95% CI | P Value |
|---|---|---|---|---|---|
| High | 120/1929 (6.2) | 164/1990 (8.2) | 0.75 | 0.60–0.95 | .018 |
| Medium | 89/1929 (4.6) | 115/1887 (6.1) | 0.74 | 0.56–0.98 | .034 |
| Low | 79/1888 (4.2) | 85/1881 (4.5) | 0.91 | 0.67–1.23 | .54 |
Abbreviations: B+A, benazepril+amlodipine; B+H, benazepril+hydrochlorothiazide; CI, confidence interval; CV, cardiovascular; PP, pulse pressure. Comparing treatment hazard ratios (HRs) between tertiles (high vs low P=.34, medium vs low P=.33, high vs medium P=.93, overall among tertiles P=.56). Values are expressed as numbers (percentages) in each tertile and treatment group, respectively.
The difference in event rates of the secondary endpoints MI and stroke between the two treatment groups across the PP tertiles is shown in the Figure. The HRs favored B+A in both endpoints and tertiles except for all MI in the low PP tertile. However, none of these HRs were significant. Differences in HRs between tertiles were not significant: high PP vs low PP (P=.27), medium PP vs low PP (P=.18), high PP vs medium PP (P=.70), and overall among tertiles (P=.39) for all MI and high PP vs low PP (P=.54), medium PP vs low PP (P=.99), high PP vs medium PP (P=.53), and overall among tertiles (P=.76) for all stroke.
Figure 1.
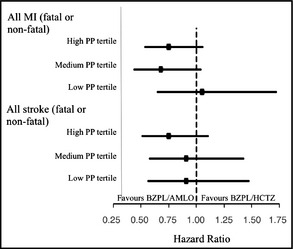
Between‐treatment hazard ratios across pulse pressure (PP) tertiles by baseline PP for the indicated endpoint. AMLO, amlodipine; BZPL, benazepril; HCTZ, hydrochlorothiazide; MI, myocardial infarction; PP, pulse pressure; CAD, coronary artery disease. Bars express the 95% confidence interval. Hazard ratio for BZPL/AMLO over BZPL/HCTZ is based on a Cox regression model with treatment, baseline PP tertile, and treatment‐by‐PP tertile interaction as factors and baseline age, CAD (yes/no), and Diabetes Mellitus (yes/no) as covariates. Hazard ratios comparing treatments between tertiles were not significant at P<.05 (High vs Low, Medium vs Low, High vs Medium, and overall among tertiles P=.39 for all MI and P=.76 for all stroke) by baseline PP.
Discussion
In this subanalysis of the ACCOMPLISH trial, PP was a strong predictor of future CV events, thus confirming findings from previous studies.6, 7, 8, 9, 10, 11 The novel finding of this study was that the superiority of B+A treatment compared with B+H was independent of the baseline office PP.
When analyzing the different tertiles, the difference in PP between the tertiles is a consequence of an increase in SBP. This implies that our findings are caused by differences in SBP rather than PP per se. However, in hypertensive patients with healthy vascular morphology, a rise in SBP would be accompanied by a rise in DBP as well, resulting in almost the same PP between patients independently of SBP values. In our study population, elevated SBPs are obviously not appropriately accompanied by elevated DBPs, resulting in higher PPs. This is most likely the result of a more advanced vascular disease with arterial stiffness in the groups with higher PP.5, 6, 7, 8, 9, 10, 11
When comparing CV events in the pooled treatment groups, high and medium baseline PP was associated with CV morbidity and/or mortality and total MI. We also found a trend toward more strokes in the highest PP tertile, although not significant. This is consistent with another subanalysis of ACCOMPLISH that showed better CV outcomes in patients with achieved SBP <140 mm Hg and <130 mm Hg compared with SBP >140 mm Hg.16 A high PP may be a stronger predictor for MI than stroke. Two possible explanations for this effect on MI may be that an elevated SBP may promote cardiac hypertrophy while a decrease in DBP can cause a decrease in coronary perfusion.12 Stroke, on the other hand, appears to be primarily related to SBP levels.16 Similar findings to those found in our study have been seen in healthy patients,8 patients with high PP,6 and in untreated hypertensive men.9 Our findings are also compatible with the recently reported finding that the effect of high BP varies by CV disease endpoint.2
In our subanalysis of ACCOMPLISH, there was no significant difference between PP tertiles for the treatment effect. To our knowledge, the association between PP and treatment effect has been reported from only one large hypertension study. The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study demonstrated the superiority of treatment with losartan (an angiotensin II receptor antagonist) over atenolol (a β‐blocker) in reducing CV events in high‐risk hypertensive patients.17 In a post hoc analysis of that study, a higher PP was significantly related to an increased number of CV events in the atenolol‐treated group, whereas the same pattern, although not significant, was observed in the group treated with losartan.17, 18 Although the relationship of the reported numbers of events in categories of PP and treatment show similar patterns compared with our study, the differences in baseline characteristics and statistical methodology hamper the comparison to our study.
In absolute numbers, a larger treatment effect was observed in the two higher tertiles compared with the lowest tertile. The lack of significant differences between PP tertiles for the relative treatment effect in the current study could be the result of a type 2 error. Further, a categorization of PP based on standard BP measurements may be inferior in comparison to 24‐hour19 and central pressure measurements.20, 21 In the ACCOMPLISH trial, the difference in SBP between the two treatment groups was <1 mm Hg. This indicates that there are other mechanisms than the reduction of brachial BP that are responsible for the superiority of B+A over B+H in preventing CV disease events. Another trial that studied the effect of a calcium channel blocker (CCB) in patients with high PP is the Systolic Hypertension in Europe (Syst‐Eur) trial.22 In Syst‐Eur, the 24‐hour average PP, before the initiation of drug therapy, was the most important factor predicting CV disease risk.23 It was shown that the reduction of CV events correlated with the PP reduction on CCB treatment. Twenty‐four–hour BP monitoring is a more precise method for determining a patient's true BP over time and is therefore a better marker for CV risk and outcomes.24 It has also been suggested that office BP underestimates 24‐hour BP in patients with established CV disease.25 Ambulatory BP measurement was performed in only a subset of the ACCOMPLISH patients. Achieved BP after 2 years did not differ between the two treatment arms.26 However, the reduction in 24‐hour BP from baseline BP in relation to the two treatment arms in ACCOMPLISH have not been reported. Taking this into account, BP values at baseline in our current study (especially in patients with more advanced CV disease) may not be the best predictors of events and so might have affected the findings in our present study.
Since high PP is a risk factor for CV disease, identification of a treatment tailored to patients with high PP would have high clinical relevance. High PP is common among the elderly22 and clearly identifies patients at high risk. Further, PP is a powerful predictor for CV events27 and seems to play a particularly important prognostic role in older, hypertensive, and diseased populations than in healthy, middle‐aged populations.28, 29, 30, 31 Studies are needed to investigate whether the effects of treatments to prevent CV events are dependent on PP levels and also to investigate whether patients with stiff arteries benefit more from CCB‐based treatment (measuring central pressure), especially since PP is the most important predictor of CV disease risk in elderly patients.32 However, no studies have evaluated PP as a treatment goal and should now be considered. Such studies could be performed using available data from previous large outcomes trials.
Conclusions
This subanalysis of the ACCOMPLISH trial shows that high PP is related to higher incidence of CV death, nonfatal MI, and stroke in a group of high‐risk hypertensive patients. The superiority of the combination treatment B+A over B+H in hypertensive patients existed irrespective of baseline PP, but the absolute treatment effect may be enhanced in the higher tertiles of PP.
Acknowledgments and disclosures
Preliminary results were presented as a poster at the European Society of Hypertension meeting in London 2012. The sponsor of the ACCOMPLISH study was Novartis Pharmaceuticals, which is the producer of Lotrel, a combination of amlodipine and benazepril. Per H. Skoglund, Joline Asp, and Per Svensson have nothing to declare. Björn Dahlöf was a member of the steering committee of ACCOMPLISH and has been on advisory boards for Novartis, MSD, Pfizer, and Boehringer Ingelheim and has been lecturing and received honoraria from Novartis, MSD, Pfizer, Boehringer Ingelheim, and Vicore Pharma. Björn Dahlöf is also a part owner in Mintage Scientific AB and Cereno Scientific AB. Sverre E. Kjeldsen has received lecture honoraria from AstraZeneca, Bayer, Medtronic, MSD, and Takeda; honoraria for consulting from Bayer, Medtronic, Serodus, and Takeda; and research support from AstraZeneca, Hemo Sapiens, and Pronova. Kenneth A. Jamerson reports receiving advisory board/consulting fees from Daiichi Sankyo Pharmaceuticals and Paradigm Medical Communications, LLC; lecture fees from Daiichi Sankyo and Merck Pharmaceuticals; and research support from the National Heart, Lung, and Blood Institute, National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Disease, and Novartis. Kenneth A. Jamerson serves as a board and/or committee member with the American Society of Hypertension, the International Society of Hypertension in Blacks, and Pfizer. Michael A. Weber was a member of the steering committee of ACCOMPLISH and has consulted for Novartis, Forest, and Takeda. Yan Jia and Dion Zappe are employees of Novartis Pharmaceuticals. Jan Östergren was a member of the steering committee of ACCOMPLISH and has also been involved in other studies supported by Novartis.
J Clin Hypertens (Greenwich). 2015;17:141–146. DOI: 10.1111/jch.12460. © 2014 Wiley Periodicals, Inc.
References
- 1. Ezzati M, Lopez AD, Rodgers A, et al. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. [DOI] [PubMed] [Google Scholar]
- 2. Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 4. Jamerson KA, Bakris GL, Wun CC, et al. Rationale and design of the avoiding cardiovascular events through combination therapy in patients living with systolic hypertension (ACCOMPLISH) trial: the first randomized controlled trial to compare the clinical outcome effects of first‐line combination therapies in hypertension. Am J Hypertens. 2004;17:793–801. [DOI] [PubMed] [Google Scholar]
- 5. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham heart study. Circulation. 2001;103:1245–1249. [DOI] [PubMed] [Google Scholar]
- 6. Madhavan S, Ooi WL, Cohen H, Alderman MH. Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infarction. Hypertension. 1994;23:395–401. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell GF, Moyé LA, Braunwald E, et al. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96:4254–4260. [DOI] [PubMed] [Google Scholar]
- 8. Benetos A, Safar M, Rudnichi A, et al. Pulse pressure: a predictor of long‐term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. [DOI] [PubMed] [Google Scholar]
- 9. Millar JA, Lever AF, Burke V. Pulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension Trial. J Hypertens. 1999;17:1065–1072. [DOI] [PubMed] [Google Scholar]
- 10. Avanzini F, Alli C, Boccanelli A, et al. High pulse pressure and low mean arterial pressure: two predictors of death after a myocardial infarction. J Hypertens. 2006;24:2377–2385. [DOI] [PubMed] [Google Scholar]
- 11. Benetos A, Zureik M, Morcet J, et al. A decrease in diastolic blood pressure combined with an increase in systolic blood pressure is associated with a higher cardiovascular mortality in men. J Am Coll Cardiol. 2000;35:673–680. [DOI] [PubMed] [Google Scholar]
- 12. Safar ME. Pulse pressure, heart rate, and drug treatment of hypertension. Curr Hypertens Rep. 2004;6:190–194. [DOI] [PubMed] [Google Scholar]
- 13. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 14. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 15. Frohlich ED, Grim C, Labarthe DR, et al. Recommendations for human blood pressure determination by sphygmomanometers. Hypertension. 1988;11:210A–222A. [Google Scholar]
- 16. Weber MA, Bakris GL, Hester A, et al. Systolic blood pressure and cardiovascular outcomes during treatment of hypertension. Am J Med. 2013;126:501–508. [DOI] [PubMed] [Google Scholar]
- 17. Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 18. Fyhrquist F, Dahlöf B, Devereux RB, et al. Pulse pressure and effects of losartan or atenolol in patients with hypertension and left ventricular hypertrophy. Hypertension. 2005;45:580–585. [DOI] [PubMed] [Google Scholar]
- 19. Ernst ME. Ambulatory blood pressure monitoring: recent evidence and clinical pharmacy applications. Pharmacotherapy. 2013;33:69–83. [DOI] [PubMed] [Google Scholar]
- 20. Vlachopoulos C, Aznaouridis K, O'Rourke MF, et al. Prediction of cardiovascular events and all‐cause mortality with central haemodynamics: a systematic review and meta‐analysis. Eur Heart J. 2010;31:1865–1871. [DOI] [PubMed] [Google Scholar]
- 21. Protogerou AD, Stergiou GS, Vlachopoulos C, et al. The effect of antihypertensive drugs on central blood pressure beyond peripheral blood pressure. Part II: Evidence for specific class‐effects of antihypertensive drugs on pressure amplification. Curr Pharm Des. 2009;15:272–289. [DOI] [PubMed] [Google Scholar]
- 22. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 23. Staessen JA, Thijs L, O'Brien ET, et al. Ambulatory pulse pressure as predictor of outcome in older patients with systolic hypertension. Am J Hypertens. 2002;1(10 pt 1):835–843. [DOI] [PubMed] [Google Scholar]
- 24. Redon J. The importance of 24‐hour ambulatory blood pressure monitoring in patients at risk of cardiovascular events. High Blood Press Cardiovasc Prev. 2013;20:13–18. [DOI] [PubMed] [Google Scholar]
- 25. Svensson P, de Faire U , Niklasson U, Ostergren J. Office blood pressure underestimates ambulatory blood pressure in peripheral arterial disease in comparison to healthy controls. J Hum Hypertens. 2004;18:193–200. [DOI] [PubMed] [Google Scholar]
- 26. Jamerson KA, Devereux R, Bakris GL, et al. Efficacy and duration of benazepril plus amlodipine or hydrochlorothiazide on 24‐hour ambulatory systolic blood pressure control. Hypertension. 2011;57:174–179. [DOI] [PubMed] [Google Scholar]
- 27. Gasowski J, Fagard RH, Staessen JA, et al. Pulsatile blood pressure component as predictor of mortality in hypertension: a meta‐analysis of clinical trial control groups. J Hypertens. 2002;20:145–151. [DOI] [PubMed] [Google Scholar]
- 28. Glynn RJ, Chae CU, Guralnik JM, et al. Pulse pressure and mortality in older people. Arch Intern Med. 2000;160:2765–2772. [DOI] [PubMed] [Google Scholar]
- 29. Vaccarino V, Berger AK, Abramson J, et al. Pulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly program. Am J Cardiol. 2001;88:980–986. [DOI] [PubMed] [Google Scholar]
- 30. Hadaegh F, Shafiee G, Hatami M, Azizi F. Systolic and diastolic blood pressure, mean arterial pressure and pulse pressure for prediction of cardiovascular events and mortality in a Middle Eastern population. Blood Press. 2012;21:12–18. [DOI] [PubMed] [Google Scholar]
- 31. Miura K, Dyer AR, Greenland P, et al. Pulse pressure compared with other blood pressure indexes in the prediction of 25‐year cardiovascular and all‐cause mortality rates: the Chicago Heart Association Detection Project in Industry Study. Hypertension. 2001;38:232–237. [DOI] [PubMed] [Google Scholar]
- 32. Asmar R, Safar M, Queneau P. Pulse pressure: an important tool in cardiovascular pharmacology and therapeutics. Drugs. 2003;63:927–932. [DOI] [PubMed] [Google Scholar]


