Abstract
High aldosterone levels are considered to play a key role in arterial hypertension. Data on the relationship between the aldosterone to active renin ratio (AARR), a quantity of aldosterone excess, and ambulatory blood pressure (BP) monitoring (ABPM) during the night are, however, sparse. Hypertensive patients were recruited from local outpatient clinics who underwent 24‐hour urine collection and in parallel ABPM. Plasma aldosterone and renin concentrations were measured by radioimmunoassay. A total of 211 patients (age, 60.2±10.2 years; 51.9% female) with a mean systolic/diastolic ABPM value of 128.7±12.8/77.1±9.2 mm Hg were evaluated. In backwards linear regression analyses adjusted for age, sex, body mass index, smoking, glomerular filtration rate, hemoglobin A1c, N‐terminal prohormone of brain natriuretic peptide, urinary sodium/potassium ratio, and ongoing antihypertensive medication, AARR was significantly associated with nocturnal systolic (ß‐coefficient: 0.177; P=.017) and diastolic BP (ß‐coefficient: 0.162; P=.027). In patients with arterial hypertension, a significant association between AARR and nighttime BP even after adjustment for a broad panel of confounders was found.
Accumulating evidence suggests that aldosterone is a central hormone in the development and progression of arterial hypertension and plays a pivotal role in the cardiovascular disease continuum.1, 2 This is most evident in patients with primary aldosteronism (PA).3
A growing body of literature indicates that even in the absence of PA, a relative aldosterone excess, reflected by the aldosterone to active renin ratio (AARR), may also contribute to high blood pressure (BP).4 This is in line with the observation that in normotensive individuals the future incidence of hypertension is significantly increased as a function of elevated aldosterone levels at baseline.5 Underlying pathophysiological mechanisms seem to be changes in vascular smooth muscle cells and fluid homoeostasis6 and increased profibrotic and proinflammatory activity,1 as well as sympathetic drive,7, 8 which translates into a rise in BP.
The hypothesis of a direct link between aldosterone and arterial hypertension is further supported by evidence from clinical trials showing that mineralocorticoid receptor antagonists (MRAs)9, 10, 11, 12, 13, 14, 15 and aldosterone synthase inhibitors16 can significantly lower BP. It is, however, largely unclear whether and to what extent BP control with MRAs reduces hard clinical endpoints in hypertensive patients.17
Observational studies consistently demonstrate a positive association between circulating aldosterone and AARR levels with both clinic and ambulatory BP readings.18, 19, 20, 21 In a study including 3056 Caucasian patients referred for coronary angiography, AARR was significantly associated with office as well as central BP.18 In line with this, El‐Gharbawy and colleagues19 showed in a cohort of 182 Afro‐Americans and Caucasians that plasma active renin concentrations (PRCs) and plasma aldosterone concentrations (PACs) were significantly associated with nighttime systolic and diastolic ambulatory BP monitoring (ABPM) measurements.
Quantifying the net‐effect of inappropriately elevated circulating aldosterone levels on BP is, however, limited in most previous studies because of the use of office BP measurements instead of ABPM.22 Particularly, dietary salt intake and sex‐specific differences should be considered as potential confounders.23, 24 Furthermore, studies on aldosterone should ideally be performed under highly standardized sampling and laboratory conditions.25, 26, 27
We therefore aimed to investigate the association between relative and absolute aldosterone excess—measured by a standardized assessment of the AARR—and ABPM by adjusting for potential confounders, such as sex, dietary salt intake,28, 29 and antihypertensive medication.30, 31, 32 This cross‐sectional investigation was performed in a cohort of hypertensive patients derived from a tertiary care center. Particular attention was paid to nighttime ABPM in view of their strong relationship with cardiovascular mortality.33
Methods
Study Population
The Styrian Hypertension Study is an ongoing prospective cohort study with the main objective to study biomarkers in relation to arterial hypertension and cardiovascular risk. Patients with a history of arterial hypertension, ie, either arterial hypertension according to medical records or according to patient interview, were eligible for inclusion into the Styrian Hypertension Study. Study participants (18 years and older) were prospectively recruited from the Departments of Cardiology and Internal Medicine, Division of Endocrinology & Metabolism at the Medical University of Graz, Austria. Main exclusion criteria were stroke or myocardial infarction in the past 4 weeks, pregnancy and lactating women, and estimated life expectancy of less than a year. Patients with a positive screening result (AARR ≥3.7 ng/dL/μU/mL)3 for PA were referred for further diagnostic workup. Twenty‐four–hour urinary creatinine levels (g/24 h), where used to validate completeness of urine collection.
Written informed consent was obtained from all study participants. The Styrian Hypertension Study was approved by the ethics committee at the Medical University Graz, Austria. The study is compatible with the Declaration of Helsinki.
Circumference of the upper arm was measured in all patients to select the appropriate cuff for BP measurements. ABPM were performed with a SPACELABS 90207 device (Spacelabs Healthcare, Inc, Issaquah, WA) every 15 minutes during the day (6 am–10 pm) and every 30 minutes during the night (10 pm–6 am). In parallel, 24‐hour urine samples were obtained from the study participants.
Laboratory Measurements
Blood samplings were performed in the morning (7 am–10 am) after an overnight fast and after 10 minutes in the sitting position. All blood samples were either measured at least 4 hours after blood collection or immediately stored at −20°C until analysis. Before analysis or freezing, all samples were kept at room temperature, except for the samples for determination of PAC, which were kept at 4°C. PRC were measured in EDTA plasma by a Renin III Generation radioimmunometric assay (RIA; Renin IRMA RIA‐4541; DRG Instruments GmbH, Marburg, Germany). Intra‐assay and interassay coefficients of variation (CV) of this assay are 0.6% to 4.5% and 2.7% to 14.5%, respectively. PAC values were also determined by means of an RIA (Active Aldosterone RIA DSL‐8600; Diagnostic Systems Laboratories, Inc, Webster, TX) with an intra‐assay and interassay CV of 3.3% to 4.5% and 5.9% to 9.8%, respectively. AARR was calculated as PAC divided by PRC (ng/dL divided by μU/mL).
Estimated glomerular filtration rate (eGFR) was calculated according to the Modification of Diet in Renal Disease formula as published, with no adjustment for race as only Caucasian patients were included in the present study.34 Quantitative determination of sodium in urine was performed by the Ion‐Selective Electrode Potentiometry module of a Roche/Hitachi Cobas 8000 analyzer (Mannheim, Germany). CV of within‐run was 0.6% at maximum and CV of total precision was 1.6% at maximum, depending on the sodium concentration. Quantitative determination of creatinine was performed by means of the rate‐blanked Jaffé method with compensation in human serum, plasma, and urine by a Cobas 8000 modular analyzer (Roche, Inc). The intra‐run and inter‐run CV for human serum was 0.7% and 2.3%, respectively. The intra‐run and inter‐run CV for human urine was 2.1% and 2.2%, respectively. All other measurements were performed by routine laboratory procedures.
Statistical Analyses
Under critical appraisal, normal distribution was evaluated visually (with histograms, Q‐Q plots, mean–median difference) and tested with Kolmogorov‐Smirnov and Shapiro‐Wilk tests, respectively. Where appropriate, skewed variables were log(10)‐transformed and indicated in the text with the prefix “log.” We formed quartiles according to the AARR values of the entire study cohort. Group comparisons were performed by Kruskal‐Wallis or chi‐square tests or analysis of variance (ANOVA), where appropriate.
Backwards linear regression analyses (P in <.05 and P out >.10) were used to evaluate the association between nocturnal systolic and diastolic BP (dependent variable) and the AARR (independent variable). Cumulative adjustments were performed for various confounders that were carefully selected based on existing literature and pathophysiological considerations regarding their interaction with the renin‐angiotensin‐aldosterone‐system (RAAS). These included log age (years), sex, log body mass index (kg/m²), smoking status (active smoker, yes/no), eGFR (mL/min/1.73 m²), hemoglobin A1c (mmol/mol), log N‐terminal prohormone of brain natriuretic peptide (pg/mL), urinary sodium/potassium (Na+/K+) ratio, and treatment with angiotensin‐converting enzyme inhibitors or angiotensin II type 1 receptor blockers. After the analysis of the basic model, we additionally adjusted for intake of β‐blockers and serum potassium. Patients under treatment with MRAs or with a renin level <5 μg/mL (functional sensitivity threshold of the RIA) were excluded from the analysis. Stability of the model was assessed by repeating the analysis as a forward linear regression model.
In a second‐step AARR, quartiles were built and further stratified into subgroups based on sex (male vs female) and 24‐hour urinary sodium concentration (below and above the median, ie, 95.5 mmol/L). ANOVA was used to test for BP changes across these AARR quartiles.
To exclude the possibility that our results simply reflected elevated BP in patients with PA, we repeated the analysis in patients with a negative screening result for PA (AARR <3.7 ng/dL/μU/mL).3
Collinearity was assessed for all included parameters (criteria were variance inflation factor <1.96 equivalent to tolerance >0.51). All statistical analyses were performed with SPSS 20 (SPSS, Inc, Chicago, IL) and a P value <.05 was considered statistically significant.
Results
We examined 211 hypertensive patients (age, 60.2±10.2 years; 51.9% females) with a mean systolic/diastolic 24‐hour ABPM value of 128.7±12.8/77.1±9.2 mm Hg and a median of 2 antihypertensive drugs (Table 1).
Table 1.
Baseline Characteristics According to AARR Quartiles
| Patients, No. (N=211) | 53 | 53 | 53 | 52 | P Value |
|---|---|---|---|---|---|
| AARR, ng/dL/μU/mL | 0.17±0.07 | 0.55±0.17 | 1.24±0.28 | 3.43±2.44 | |
| AARR range | 0.03–0.3 | 0.32–0.83 | 0.85–1.72 | 1.73–13.26 | |
| Age, y | 62.0±11.65 | 61.3±8.1 | 57.0±10.0 | 60.0±11.0 | .04 |
| Women, % | 50.9 | 43.4 | 54.7 | 56.6 | .49 |
| BMI, kg/m² | 30.9±4.6 | 30.0±4.7 | 29.8±4.6 | 28.6±5.1 | .03 |
| BP | |||||
| Daytime systolic BP, mm Hg | 129.9±11.4 | 127.5±13.9 | 134.4±14.2 | 132.6±12.9 | .04 |
| Nighttime systolic BP, mm Hg | 115.9±13.5 | 116.0±15.0 | 119.2±16.0 | 119.0±13.6 | <.01 |
| Daytime diastolic BP, mm Hg | 75.7±8.5 | 78.8±8.4 | 81.5±8.1 | 81.4±11.0 | .49 |
| Nighttime diastolic BP, mm Hg | 65.3±7.6 | 68.5±8.4 | 69.1±8.13 | 70.6±8.1 | .01 |
| Laboratory | |||||
| Plasma aldosterone concentration, ng/dL | 15.0 (10.5–19.3) | 12.3 (9.5–18.4) | 16.3 (12.6–21.7) | 18.4 (14.8–23.9) | <.01 |
| Plasma renin concentration, μU/mL | 86.1 (64.6–157.8) | 24.3 (15.0–42.8) | 14.0 (10.5–18.2) | 6.6 (3.9–9.8) | <.01 |
| 24‐H urinary sodium, mmol/24 h | 165.0 (116.0–217.5) | 139.0 (108.0–192.0) | 152.0 (107.0–193.0) | 136.0 (97.6–198.8) | .26 |
| 24‐H total creatinine excretion, g/24 h | 1.2 (1.0–1.55) | 1.3 (1.0–1.5) | 1.3 (0.8–1.65) | 1.1 (0.8–1.5) | .52 |
| NT‐proBNP, pg/mL | 83.0 (42.5–153.0) | 88.0 (41.5–171.5) | 77.0 (38.0–132.5) | 95.0 (53.0–231.0) | .19 |
| Serum creatinine, μmol/L | 91±13 | 95±16 | 97±13 | 97±13 | .12 |
| eGFR, mL/min/1.73 m² | 71.2±19.8 | 72.4±13.6 | 78.6±18.7 | 79.2±17.5 | .04 |
| Diabetes mellitus, % | 34.0 | 22.6 | 17.0 | 15.4 | .09 |
| Hemoglobin A1c, mmol/mol | 43.0 (39.5–57.0) | 39.0 (36.0–44.5) | 37.0 (36.0–42.0) | 39.0 (37.0–41.0) | <.01 |
| Active smokers, % | 3.7 | 9.4 | 20.8 | 25.0 | <.01 |
| Medication | |||||
| Different antihypertensive drugs, No. | 2 (1–3) | 2 (1–3) | 1 (1–3) | 2 (1–3) | .08 |
| ACE inhibitors, % | 49.0 | 43.4 | 34.0 | 40.4 | .46 |
| AT1 blockers, % | 39.2 | 35.9 | 18.9 | 17.3 | .01 |
| Thiazide diuretics, % | 52.5 | 44.3 | 36.1 | 43.3 | .34 |
| Loop diuretics, % | 9.8 | 3.3 | 0.0 | 0.0 | <.01 |
| β‐Blockers, % | 47.5 | 54.1 | 47.5 | 63.3 | .25 |
| Calcium antagonists, % | 29.5 | 24.6 | 24.6 | 18.3 | .55 |
| Birth control in premenopausal women, % | 66.7 | 33.3 | 25.0 | 0.0 | .28 |
| Postmenopausal, % | 90.3 | 80.7 | 87.1 | 89.2 | .66 |
| Hormone replacement therapy, % | 3.5 | 3.7 | 0.0 | 0.0 | .51 |
Abbreviations: AARR, aldosterone to active renin ratio; ACE, angiotensin‐converting enzyme; AT1, angiotensin II type 1 receptor; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide. Continuous data are presented as mean±standard deviation and as medians with interquartile range, and categorical data are shown as percentages. Analysis of variance with P for trend, Kruskal‐Wallis, and chi‐square tests were used.
In backwards linear regression analysis, nocturnal systolic BP was significantly associated with the AARR, in the final model adjusted for sex, hemoglobin A1c, and urinary Na+/K+ ratio (ß‐coefficient for AARR as an independent variable: 0.177; P=.017) (Table 2). Nighttime diastolic BP was also significantly associated with AARR, including log age, sex, and urinary Na+/K+ ratio in the last model of the backwards linear regression (ß‐coefficient for AARR as an independent variable: 0.162; P=.067). Further adjustments for β‐blockers and serum potassium did not materially change the results (data not shown). Repeating the analysis as a forward linear regression model showed virtually no different results, indicating a good stability of the statistical model. The association between AARR and nocturnal BP remained materially unchanged in the subgroup analysis of participants with a negative screening for PA (AARR <3.7 ng/dL/μU/mL; n=184; ß‐coefficient for AARR as an independent variable: 0.157, P=.035 and ß‐coefficient: 0.169, P=.023 for systolic and diastolic nocturnal BP, respectively), suggesting that our findings are not driven by undiagnosed PA patients. There was no significant collinearity in any of our statistical analyses.
Table 2.
Results of the Final Step of the Backwards Linear Regression Analyses for Nighttime Systolic and Diastolic Blood Pressure, Respectively
| Model (Final Step R²=0.209) | ß‐Coefficient | Significance | Tolerance |
|---|---|---|---|
| Sex | .162 | .02 | .987 |
| Hemoglobin A1c (mmol/mol) | .393 | <.01 | .943 |
| Urinary_Na_K_ratio | .126 | .07 | .995 |
| Aldosterone to active renin ratio | .177 | .01 | .947 |
| Model (Final Step R²=0.191) | ß‐Coefficient | Significance | Tolerance |
| Log age | −.194 | .01 | .935 |
| Sex | .248 | <.01 | .971 |
| Urinary_Na_K_ratio | .177 | .01 | .971 |
| Aldosterone to active renin ratio | .162 | .02 | .975 |
Dependent variable: mean nighttime systolic blood pressure (mm Hg).
In ANOVA, nocturnal systolic and diastolic BP increased significantly from the first to the fourth AARR quartile (Figure 1). Similar results were observed for subgroup analyses stratified by sex and salt intake, suggesting that the association between AARR and BP is not significantly modified by sex or salt intake (Figures 2 and 3, respectively).
Figure 1.
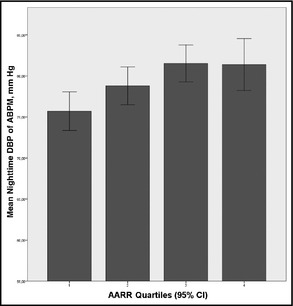
Association between increasing aldosterone to active renin ratio (AARR) expressed as quartiles (the error bars indicate 95% confidence interval [CI]) and nocturnal diastolic blood pressure (DBP) (analysis of variance P for trend=.002). ABPM indicates ambulatory blood pressure monitoring.
Figure 2.
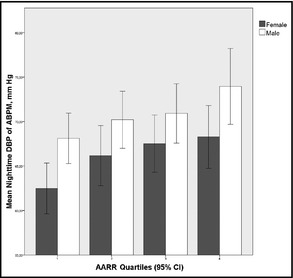
Association between increasing aldosterone to active renin ratio (AARR) subgrouped by sex (the error bars indicate 95% CI) and nocturnal diastolic blood pressure (DBP). Analysis of variance with P for trend was similar for both subgroups (males: P=.02 and females: P=.008). ABPM indicates ambulatory blood pressure monitoring.
Figure 3.
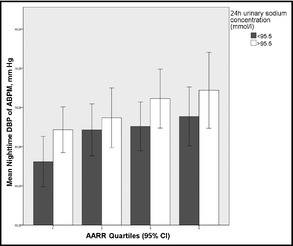
Analysis of variance across the different aldosterone to active renin ratio (AARR) quartiles with P for trend was similar for both subgroups according to 24 hour urinary sodium concentration (<95.5 mmol/L: P=.021 and >95.5 mmol/L: P=.031). DBP indicates diastolic blood pressure; ABPM, ambulatory blood pressure monitoring.
Discussion
In patients with arterial hypertension, we found a significant association between AARR and nocturnal BP, as well as 24‐hour diastolic ABPM. The results remained significant even after multivariate adjustments. These findings underline the potential role of a relative aldosterone excess in the pathophysiology of arterial hypertension.
Our findings extend previous observations indicating a strong relationship between relative aldosterone excess and arterial BP. El‐Gharbawy and colleagues19 described an association of plasma renin and aldosterone concentration with nocturnal BP and left ventricular mass. Notably, a large number of the patients studied in this cohort were of Afro‐American ancestry and the results may thus not necessarily be applicable to Caucasians.21 In addition, dietary salt intake reflected by 24‐hour urinary Na+/K+ ratio was not considered in these previous studies. Scott and colleagues35 demonstrated that an increased AARR modifies a substantial proportion of the relationship between urinary Na+/K+ and office BP at a community level, although no 24‐hour BP readings were reported. This is further underlined by adverse changes in left ventricular (LV) geometry and increased LV mass index in hypertensive patients with high aldosterone and high urinary sodium concentrations.36, 37 Interestingly, these latter associations were independent of office BP, supporting the notion that aldosterone might also exert BP‐independent adverse cardiovascular effects. We extended the above‐mentioned studies by evaluating the relationship between relative aldosterone excess and 24‐hour BP readings under consideration of salt excretion. We believe that our findings, if confirmed in further studies, may suggest that aldosterone excess is particularly related to nocturnal BP. While our findings underline the clinical relevance of AARR determinations, it should also be mentioned that apart from diagnosing PA and or relative aldosterone excess, the determination of the AARR is also of clinical value for the diagnosis of other RAAS‐related disorders such as Liddle's syndrome.38
There is a growing body of evidence that aldosterone through various mechanisms1, 5, 8, 9, 24, 39 may directly contribute to elevated nocturnal BP and may thus be the underlying pathophysiological explanation for our findings. Alternatively, there is also evidence supporting the hypothesis that the association between AARR and nighttime ABPM could be related to sleep‐disordered breathing symptoms.40
From a clinical point of view, it should also be underlined that MRAs9, 10, 11, 12, 13, 14, 15 are potent antihypertensive agents and are considered in current guidelines as 3rd‐ or 4th‐line treatment for arterial hypertension.17 Furthermore, it has recently been recommended that these drugs should be more widely introduced in the treatment of arterial hypertension, in particular resistant hypertension.41, 42
Study Limitations and Strengths
Based on our findings and the existing literature on aldosterone and arterial hypertension, further randomized controlled trials are warranted to evaluate the effect of aldosterone blockade on BP control and hard clinical endpoints in hypertensive patients.
Our study may be limited by the fact that participants were recruited from a tertiary care teaching hospital and our findings may thus not be applicable to a random community sample. As our data are cross‐sectional, no conclusions with regard to causality can be drawn. Furthermore, hard clinical endpoints are lacking, so we cannot describe any association along the cardiovascular continuum. Another possible limitation may be the use of the direct renin measurement by means of RIA instead of determinations of renin activity. Missing AARR values at night are another drawback of our work, because some regulators of the RAAS such as adrenocorticotropic hormone may have a greater (different) impact during the night. Although it should be noted that the difference between overall daytime/nighttime aldosterone secretion is only modest and much less pronounced than those differences induced by salt intake.43
The strengths of the present investigation are the thorough biochemical and anthropometrical characterization including ABPM, availability of 24‐hour urine specimen, and careful standardized laboratory measurements. The exclusion of patients with very low renin levels (n=27) helped us to avoid a denominator phenomenon, as those participants may have had plasma renin concentrations below the detection threshold of the assay and may have thus skewed the AARR.
Conclusions
We have shown that in a cohort of hypertensive patients of Caucasian origin the AARR is significantly associated with nocturnal BP, despite multivariate adjustments. Further studies are needed to evaluate cardioprotective effects of MRAs in dependence of varying dietary salt intake and sex in hypertensive patients and especially its effects on nocturnal BP control.
Disclosures
The authors have no conflicts of interest.
Acknowledgments
We would like to especially thank the Laboratory of the Division of Endocrinology and Metabolism for its work and support to the present research. Katharina Kienreich and Martin Gaksch are supported by funding of the Austrian National Bank (Jubilaeumsfond: project numbers: 13878 and 13905). Andreas Tomaschitz is partially supported by the project EU‐MASCARA (“Markers for Sub‐Clinical Cardiovascular Risk Assessment”; FP7‐HEALTH [HEALTH.2011.2.4.2‐2]; grant agreement number: 278249); and by the project EU‐HOMAGE (“Validation of ‐omics‐based biomarkers for diseases affecting the elderly” [HEALTH.2012.2.1.1‐2]; grant agreement number: 305507). Nicolas Verheyen is supported by funding of the Austrian National Bank (Jubilaeumsfond: project number: 14621).
J Clin Hypertens(Greenwich).2014; 289–294 DOI: 10.1111/jch.12274. © 2014 Wiley Periodicals, Inc.
References
- 1. Tomaschitz A, Pilz S, Ritz E, et al. Aldosterone and arterial hypertension. Nat Rev Endocrinol. 2010;6:83–93. [DOI] [PubMed] [Google Scholar]
- 2. Tomaschitz A, Pilz S, Ritz E, et al. Association of plasma aldosterone with cardiovascular mortality in patients with low estimated GFR: the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Am J Kidney Dis. 2011;57:403–414. [DOI] [PubMed] [Google Scholar]
- 3. Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. [DOI] [PubMed] [Google Scholar]
- 4. Gekle M, Grossmann C. Actions of aldosterone in the cardiovascular system: the good, the bad, and the ugly? Pflüg Arch Eur J Physiol. 2009;458:231–246. [DOI] [PubMed] [Google Scholar]
- 5. Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. [DOI] [PubMed] [Google Scholar]
- 6. Funder JW. The nongenomic actions of aldosterone. Endocr Rev. 2005;26:313–321. [DOI] [PubMed] [Google Scholar]
- 7. Geerling JC, Loewy AD. Aldosterone in the brain. Am J Physiol Ren Physiol. 2009;297:559–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang BS, Wang H, Leenen FHH. Chronic central infusion of aldosterone leads to sympathetic hyperreactivity and hypertension in Dahl S but not Dahl R rats. Am J Physiol Heart Circ Physiol. 2005;288:517–524. [DOI] [PubMed] [Google Scholar]
- 9. Batterink J, Stabler SN, Tejani AM, Fowkes CT. Spironolactone for hypertension. Cochrane Database Syst Rev. 2010;8:CD008169. [DOI] [PubMed] [Google Scholar]
- 10. Chapman N, Dobson J, Wilson S, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845. [DOI] [PubMed] [Google Scholar]
- 11. Václavík J, Sedlák R, Plachy M, et al. Addition of Spironolactone in Patients with Resistant Arterial Hypertension (ASPIRANT) a randomized, double‐blind, placebo‐controlled trial. Hypertension. 2011;57:1069–1075. [DOI] [PubMed] [Google Scholar]
- 12. Calhoun DA, White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens. 2008;2:462–468. [DOI] [PubMed] [Google Scholar]
- 13. Weber MA. Clinical implications of aldosterone blockade. Am Heart J. 2002;144:12–18. [DOI] [PubMed] [Google Scholar]
- 14. White WB, Carr AA, Krause S, et al. Assessment of the novel selective aldosterone blocker eplerenone using ambulatory and clinical blood pressure in patients with systemic hypertension. Am J Cardiol. 2003;92:38–42. [DOI] [PubMed] [Google Scholar]
- 15. Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild‐to‐moderate hypertension. Am J Hypertens. 2002;15:709–716. [DOI] [PubMed] [Google Scholar]
- 16. White WB, Calhoun DA, Krum H, et al. Blockade of aldosterone production as a novel approach to the management of high blood pressure: efficacy and tolerability of the aldosterone synthase inhibitor LCI699 in patients with stage 1–2 hypertension. J Am Coll Cardiol. 2010;55:582. [Google Scholar]
- 17. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 18. Tomaschitz A, Maerz W, Pilz S, et al. Aldosterone/renin ratio determines peripheral and central blood pressure values over a broad range. J Am Coll Cardiol. 2010;55:2171–2180. [DOI] [PubMed] [Google Scholar]
- 19. El‐Gharbawy AH, Nadig VS, Kotchen JM, et al. Arterial pressure, left ventricular mass, and aldosterone in essential hypertension. Hypertension. 2001;37:845–850. [DOI] [PubMed] [Google Scholar]
- 20. Kidambi S, Kotchen JM, Grim CE, et al. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension. 2007;49:704–711. [DOI] [PubMed] [Google Scholar]
- 21. Grim CE, Cowley AW Jr, Hamet P, et al. Hyperaldosteronism and hypertension: ethnic differences. Hypertension. 2005;45:766–772. [DOI] [PubMed] [Google Scholar]
- 22. Pickering TG, Shimbo D, Haas D. Ambulatory blood‐pressure monitoring. N Engl J Med. 2006;354:2368–2374. [DOI] [PubMed] [Google Scholar]
- 23. Satoh M, Kikuya M, Hara A, et al. Aldosterone‐to‐renin ratio and home blood pressure in subjects with higher and lower sodium intake: the Ohasama Study. Hypertens Res. 2011;34:361–366. [DOI] [PubMed] [Google Scholar]
- 24. Edelmann F, Tomaschitz A, Wachter R, et al. Serum aldosterone and its relationship to left ventricular structure and geometry in patients with preserved left ventricular ejection fraction. Eur Heart J. 2012;33:203–212. [DOI] [PubMed] [Google Scholar]
- 25. Tomaschitz A, Pilz S. Aldosterone to renin ratio – a reliable screening tool for primary aldosteronism? Horm Metab Res. 2010;42:382–391. [DOI] [PubMed] [Google Scholar]
- 26. Pilz S, Tomaschitz A, Stepan V, et al. Graz Endocrine Causes of Hypertension (GECOH) study: a diagnostic accuracy study of aldosterone to active renin ratio in screening for primary aldosteronism. BMC Endocr Disord. 2009;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pilz S, Tomaschitz A, März W. Diagnostic procedures for primary aldosteronism/diagnostische methoden für den primären hyperaldosteronismus. LaboratoriumsMedizin. 2009;33:202–209. [Google Scholar]
- 28. Griffing GT, Wilson TE, Melby JC. Alterations in aldosterone secretion and metabolism in low renin hypertension. J Clin Endocrinol Metab. 1990;71:1454–1460. [DOI] [PubMed] [Google Scholar]
- 29. Makhanova N, Hagaman J, Kim H‐S, Smithies O. Salt‐sensitive blood pressure in mice with increased expression of aldosterone synthase. Hypertension. 2008;51:134–140. [DOI] [PubMed] [Google Scholar]
- 30. Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin‐angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2013;302:1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone theory and therapy. Hypertension. 2004;43:518–524. [DOI] [PubMed] [Google Scholar]
- 32. Bochud M, Nussberger J, Bovet P, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–245. [DOI] [PubMed] [Google Scholar]
- 33. Burr ML, Dolan E, O'Brien EW, et al. The value of ambulatory blood pressure in older adults: the Dublin outcome study. Age Ageing. 2008;37:201–206. [DOI] [PubMed] [Google Scholar]
- 34. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 35. Scott L, Woodiwiss AJ, Maseko MJ, et al. Aldosterone‐to‐renin ratio and the relationship between urinary salt excretion and blood pressure in a community of African ancestry. Am J Hypertens. 2011;24:951–957. [DOI] [PubMed] [Google Scholar]
- 36. Du Cailar G, Fesler P, Ribstein J, Mimran A. Dietary sodium, aldosterone, and left ventricular mass changes during long‐term inhibition of the renin‐angiotensin system. Hypertension. 2010;56:865–870. [DOI] [PubMed] [Google Scholar]
- 37. Jin Y, Kuznetsova T, Maillard M, et al. Independent relations of left ventricular structure with the 24‐hour urinary excretion of sodium and aldosterone. Hypertension. 2009;54:489–495. [DOI] [PubMed] [Google Scholar]
- 38. Tapolyai M, Uysal A, Dossabhoy NR, et al. High prevalence of Liddle syndrome phenotype among hypertensive US veterans in Northwest Louisiana. J Clin Hypertens. 2010;12:856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ritz E, Tomaschitz A. Aldosterone, a vasculotoxic agent–novel functions for an old hormone. Nephrol Dial Transplant. 2009;24:2302–2305. [DOI] [PubMed] [Google Scholar]
- 40. Fulop T, Hickson DA, Wyatt SB, et al. Sleep‐disordered breathing symptoms among African–Americans in the Jackson heart study. Sleep Med. 2012;13:1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Funder J. Mineralocorticoid receptor antagonists for heart failure. Expert Opin Pharmacother. 2011;12:2767–2769. [DOI] [PubMed] [Google Scholar]
- 42. Armanini D, Fiore C. Choice of diuretic therapy and reconsideration for aldosterone receptors blockers. Hypertension. 2010;55:5. [DOI] [PubMed] [Google Scholar]
- 43. Thomas JP, Oake RJ. An accurate method for measurement of aldosterone production. Biochem J. 1969;115:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]


