Abstract
The current study examined the degree of blood pressure (BP) control and incidence of myocardial ischemia in hypertensive patients (n=2039) referred for cardiac stress test. Patients were categorized into well‐controlled (<140/90 mm Hg), poorly controlled (140–160/90–100 mm Hg), and very poorly controlled (>160/100 mm Hg) groups according to their resting BP. The mean age[±standard error of the mean] of the patients was 68±13 years, and 885 (43.4%) were men. The prevalence of well‐controlled hypertension (HTN) was 47.2%, poorly controlled HTN was 29.5%, and very poorly controlled HTN was 23.3%. Evidence of ischemia was seen in 19.8% and 19.3% of the well‐controlled and poorly controlled groups, respectively. The very poorly controlled group had the lowest incidence of ischemia (14.3%) (P<.05) compared with the other two groups. Symptoms that mimic ischemic heart disease in hypertensive patients may be partly explained by poorly controlled BP. Quality of care might be improved by optimally controlling BP in patients with angina symptoms prior to ordering diagnostic testing associated with radiation exposure and cost.
Hypertension (HTN) is defined as a persistent systolic blood pressure (BP) of at least 140 mm Hg and/or diastolic BP of at least 90 mm Hg, or BP that is controlled to guideline‐recommended levels using antihypertensive medication.1 Data from the National Health and Nutrition Examination Survey indicated that 50 million or more Americans had high BP that warranted some form of treatment between 1988 and 19942 and a repeat analysis from 1999 to 2000 showed that at least 65 million adults had HTN, a 3.7‐percentage point increase in HTN prevalence.1 As many as two thirds of individuals with HTN in the United States are either untreated or undertreated2, 3, 4 and suboptimal BP is the number one attributable risk for death throughout the world.2
HTN is a powerful risk factor for fatal and nonfatal cardiovascular (CV) events.3 Zhang and colleagues5 showed that patients with not‐at‐goal HTN bear a heavy burden of CV disease. These patients had an estimated 10‐year risk of CV events of 25% and a 10‐year risk of CV death of 6.8%±6.6%. Achieving adequate BP control can reduce morbidity and mortality from CV diseases. A 5‐mm Hg reduction in systolic BP is estimated to result in a 14% overall reduction in mortality due to stroke, a 9% reduction in mortality due to coronary heart disease, and a 7% decrease in all‐cause mortality.2
We sought to examine the degree of BP control in hypertensive patients referred for cardiac stress testing. We hypothesized that poor BP control would correlate with higher risk findings on stress myocardial perfusion imaging as manifested by a higher incidence of ischemia.
Methods
We retrospectively analyzed 2039 consecutive patients with the diagnosis of HTN referred to New York Hospital Medical Center of Queens/Weill Cornell Medical College nuclear cardiology laboratory for stress testing from January 2007 through July 2010.
BP was measured using an IntelliVue MP70 (Royal Philips Electronics, the Netherlands) noninvasive BP monitor. It uses an oscillometric technique and the device meets the Association for the Advancement of Medical Instrumentation Standards. The device was operated manually by an experienced stress laboratory nurse. BP was measured twice after the patient had rested for at least 5 minutes in a quiet comfortable room. The average of both readings was used for these analyses. Patients' BP was categorized as follows: well‐controlled (<140/90 mm Hg), poorly controlled (140–160/90–100 mm Hg), and very poorly controlled (>160/100 mm Hg).
The incidence of ischemia was defined as the presence of at least one reversible perfusion defect on stress/rest single‐photon emission computed tomography scan. Both exercise and pharmacologic stress studies were included. Standard single and dual isotope protocols using Thallium and Technetium‐99m Sestamibi were used.
Statistical Analysis
Data were analyzed using unpaired, two‐tailed Student t tests and multiple regression analysis (SPSS software package, version 10; SPSS Inc, Armonk, NY). When not in absolute numbers, data were reported as mean±standard deviation. A P value <.05 was considered significant.
Results
Patients had a mean age of 68±13 years, 885 (43.4%) were men, 742 (36.4%) had diabetes, 1189 (58.3%) had dyslipidemia, and 767 (37.6%) had a history of coronary heart disease (Table). We found that 47.2% of the study population had BP categorized as well‐controlled, 29.5% as poorly controlled, and 23.3% as very poorly controlled. Among the well‐controlled HTN group, 19.8% had evidence of ischemia while 19.3% of the poorly controlled group had ischemia. The very poorly controlled group had the lowest incidence of ischemia (14.3%) (P<.05) compared with the well‐controlled and poorly controlled BP groups (Figure).
Table 1.
Baseline Characteristics According to Degree of Blood Pressure Control
| Well‐Controlled HTN | Poorly Controlled HTN | Very Poorly Controlled HTN | P Value | |
|---|---|---|---|---|
| No. | 962 | 602 | 475 | – |
| Age, y | 70±12 | 71±13 | 73±12 | NS |
| Men, No. (%) | 415 (43.1) | 261 (43.3) | 198 (41.7) | NS |
| Dyslipidemia, No. (%) | 561 (58.3) | 341 (56.7) | 305 (64.2) | NS |
| Diabetes, No. (%) | 350 (36.4) | 195 (32.4) | 148 (31.2) | NS |
| History of CAD, No. (%) | 362 (37.6) | 220 (36.5) | 196 (41.2) | NS |
Abbreviations: CAD, coronary artery disease; HTN, hypertension; NS, not significant.
Figure 1.
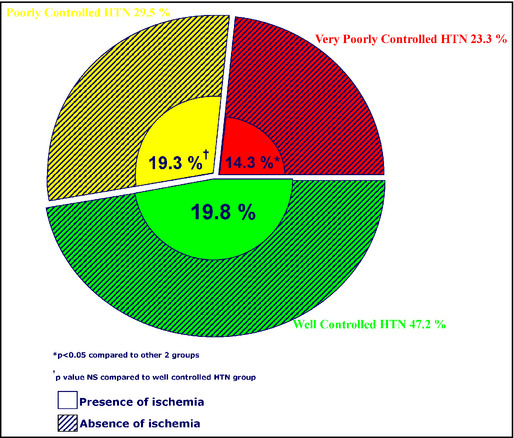
Study population according to blood pressure control and presence of ischemia.
Discussion
HTN is an important risk factor for the development of coronary artery disease6, 7, 8; therefore, the development of chest pain in a patient with HTN is commonly ascribed to coronary atherosclerosis.9 As many as two thirds of individuals with HTN in the United States have suboptimal BP control either because of undertreatment or absence of treatment.3 In the present study, we found that more than half of the patients referred for stress testing had uncontrolled HTN, and almost one quarter had BP >160/100 mm Hg. We hypothesized that, as a result of being at higher risk for CV disease, patients with uncontrolled HTN would be more likely to have an abnormal and high‐risk stress test as manifested by a higher incidence of ischemia on stress myocardial perfusion imaging. However, in contrast to our hypothesis, patients with very poorly controlled HTN had a lower incidence of ischemia compared with patients with poorly controlled and well‐controlled HTN. One possible explanation for this finding is that patients with very poorly controlled HTN may be experiencing symptoms mimicking ischemic heart disease but are caused by their markedly elevated BP.
Several studies have reported on the pathophysiology of HTN‐induced angina symptoms in the absence of evidence of ischemia on perfusion imaging.10, 11, 12 Pichard and colleagues12 suggested that severe left ventricular (LV) hypertrophy, usually seen in individuals with uncontrolled HTN, is associated with a reduction in coronary vascular reserve (CVR). The study was conducted on the premise that increased blood flow occurs in ventricular hypertrophy and the increase in flow may be obtained at the expense of the existing CVR. The CVR was studied by analyzing the hyperemic reaction to selective injection of contrast into coronary arteries in 25 patients. The authors found that the hyperemic response correlated with LV mass (r=0.51, P<.01) and speculated that the decrease in the vascular reserve capacity may be related to the ischemic component of hypertrophic heart disease. This finding was supported by previous studies reported by Brush and colleagues,9 They demonstrated that LV hypertrophy caused by HTN is associated with a limitation in CVR and such an abnormality can lead to the precipitation of myocardial ischemia and therefore may account, at least in part, for the presence of angina in hypertensive patients. Although we did not measure LV mass in our study, the reduction in CVR can still be applied to hypertensive patients in our study population, as reduction in CVR has also been shown in hypertensive patients with no evidence of LV hypertrophy.9
Brush and colleagues9 found that angina symptoms in hypertensive patients without epicardial coronary disease may be caused by myocardial ischemia as a result of abnormally elevated coronary artery resistance at the microvascular level. Several studies have confirmed the presence of a structurally based increase in flow resistance in patients with HTN caused by increased arterial pressure.10, 13, 14, 15 With elevated BP, endothelial cells are exposed to an increased transmural force and increased circumferential stretch, both of which are signals that can alter endothelial cell phenotype.16, 17 Histological studies have shown increased vascular muscle mass caused by hyperplasia within small arteries in hypertensive patients leading to an increased media to lumen ratio contributing to flow resistance.18, 19 Abnormal resistance to flow in angiographically normal coronary arteries may explain some clinically typical angina‐like symptoms in these uncontrolled hypertensive patients.20
As reported by Stakos and colleagues,21 another plausible cause of angina‐like symptoms in uncontrolled hypertensive patients with no evidence of ischemia is aortic stiffness. Alterations in collagen turnover are seen in hypertensive patients through a process known as vascular remodeling, contributing to aortic stiffness,22, 23, 24 which has been shown to cause angina‐like pain in hypertensive patients with normal coronary arteries.21 A sudden rise in aortic systolic pressure as a result of aortic stiffness increases aortic wall tension, which stimulates aortic wall pain fibers resulting in chest pain.25 It was postulated that aortic stiffness resulted in reduced aortic diastolic BP and reduction in subendocardial blood flow, causing some myocardial ischemia that may mimic angina symptoms.21 In addition, aortic stiffness has been shown to reduce CVR.26 A combination of all of these factors may be responsible for angina‐like symptoms experienced by patients with uncontrolled HTN. This may partly explain the low incidence of ischemia seen in the very poorly controlled HTN group because their symptoms, necessitating cardiac stress test, are most likely not caused by coronary artery disease.
Conclusions
More than half of patients referred for stress testing in our study population had uncontrolled HTN, and almost one quarter had BP >160/100 mm Hg. This represents suboptimal treatment in a group of comorbid patients with suspected or known CV disease. As discussed above, the poorly controlled BP may at least partially explain the symptoms of ischemic heart disease in hypertensive patients. Quality of care might be improved by optimally controlling BP in patients with angina symptoms prior to ordering diagnostic testing associated with radiation exposure and cost.
Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
J Clin Hypertens (Greenwich). 2015:709–712. DOI: 10.1111/jch.12586. © 2015 Wiley Periodicals, Inc.
References
- 1. Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the united states 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. [DOI] [PubMed] [Google Scholar]
- 2. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 3. Wang TJ, Vasan RS. Epidemiology of uncontrolled hypertension in the United States. Circulation. 2005;112:1651–1662. [DOI] [PubMed] [Google Scholar]
- 4. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988‐2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Lelong H, Kretz S, et al. Characteristics and future cardiovascular risk of patients with not‐at‐goal hypertension in general practice in France: the AVANT'AGE study. J Clin Hypertens (Greenwich). 2013;15:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calhoun DA, Booth JN, Oparil S, et al. Refractory hypertension: determination of prevalence, risk factors, and comorbidities in a large, population‐based cohort. Hypertension. 2014;63:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorrentino M, Bakris GL. Diagnosis, prevention, and treatment of hypertensive heart disease. In: Evidence‐Based Cardiology Consult. New York, NY, Springer; 2014:51–58. [Google Scholar]
- 8. Yano Y, Stamler J, Garside DB, et al. Isolated systolic hypertension in young and middle‐aged adults and 31‐year risk for cardiovascular mortality: the Chicago Heart Association detection project in industry study. J Am Coll Cardiol. 2015;65:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brush JE, Cannon RO, Schenke WH, et al. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med. 1988;319:1302–1307. [DOI] [PubMed] [Google Scholar]
- 10. Crea F, Lanza GA, Camici PG. Mechanisms of coronary microvascular dysfunction. In: Coronary Microvascular Dysfunction. New York, NY: Springer; 2014:31–47. [Google Scholar]
- 11. Galderisi M, Capaldo B, Sidiropulos M, et al. Determinants of reduction of coronary flow reserve in patients with type 2 diabetes mellitus or arterial hypertension without angiographically determined epicardial coronary stenosis. Am J Hypertens. 2007;20:1283–1290. [DOI] [PubMed] [Google Scholar]
- 12. Pichard AD, Gorlin R, Smith H, et al. Coronary flow studies in patients with left ventricular hypertrophy of the hypertensive type: evidence for an impaired coronary vascular reserve. Am J Cardiol. 1981;47:547–554. [DOI] [PubMed] [Google Scholar]
- 13. Chen W, Srinivasan SR, Li S, et al. Gender‐specific influence of NO synthase gene on blood pressure since childhood: the Bogalusa Heart Study. Hypertension. 2004;44:668–673. [DOI] [PubMed] [Google Scholar]
- 14. Juonala M, Järvisalo MJ, Mäki‐Torkko N, et al. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the cardiovascular risk in young Finns study. Circulation. 2005;112:1486–1493. [DOI] [PubMed] [Google Scholar]
- 15. Mulvany MJ. Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol. 2012;110:49–55. [DOI] [PubMed] [Google Scholar]
- 16. Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise‐induced changes in endothelial cell phenotype. J Appl Physiol (1985). 2008;104:588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Padilla J, Jenkins NT, Laughlin MH, Fadel PJ. Blood pressure regulation VIII: resistance vessel tone and implications for a pro‐atherogenic conduit artery endothelial cell phenotype. Eur J Appl Physiol. 2014;114:531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gobé G, Browning J, Howard T, et al. Apoptosis occurs in endothelial cells during hypertension‐induced microvascular rarefaction. J Struct Biol. 1997;118:63–72. [DOI] [PubMed] [Google Scholar]
- 19. Antonios TF. Microvascular angina in essential hypertension. In: Chest Pain With Normal Coronary Arteries. New York, NY: Springer; 2013:121–126. [Google Scholar]
- 20. De Bruyne B, Hersbach F, Pijls NHJ, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “normal” coronary angiography. Circulation. 2001;104:2401–2406. [DOI] [PubMed] [Google Scholar]
- 21. Stakos DA, Tziakas DN, Chalikias G, et al. Chest pain in patients with arterial hypertension, angiographically normal coronary arteries and stiff aorta: the aortic pain syndrome. Hellenic J Cardiol. 2013;54:25–31. [PubMed] [Google Scholar]
- 22. Stakos DA, Tziakas DN, Chalikias GK, et al. Associations between collagen synthesis and degradation and aortic function in arterial hypertension. Am J Hypertens. 2010;23:488–494. [DOI] [PubMed] [Google Scholar]
- 23. Chatzikyriakou SV, Tziakas DN, Chalikias GK, et al. Serum levels of collagen type‐I degradation markers are associated with vascular stiffness in chronic heart failure patients. Eur J Heart Fail. 2008;10:1181–1185. [DOI] [PubMed] [Google Scholar]
- 24. McNulty M, Mahmud A, Spiers P, Feely J. Collagen type‐I degradation is related to arterial stiffness in hypertensive and normotensive subjects. J Hum Hypertens. 2006;20:867–873. [DOI] [PubMed] [Google Scholar]
- 25. Vlachopoulos C, Alexopoulos N, Tsiamis E, Stefanadis C. Aortic pain associated with deteriorated aortic elastic properties: an early manifestation of aortopathy. Hellenic J Cardiol. 2006;47:176–179. [PubMed] [Google Scholar]
- 26. Nemes A, Forster T, Csanady M. Reduction of coronary flow reserve in patients with increased aortic stiffness. Can J Physiol Pharmacol. 2007;85:818–822. [DOI] [PubMed] [Google Scholar]


